Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista mexicana de ciencias agrícolas
Print version ISSN 2007-0934
Rev. Mex. Cienc. Agríc vol.7 n.8 Texcoco Nov./Dec. 2016
Investigation note
Preliminary study for in vitro propagation of Cedrus atlantica through axillary buds
1Institución Universitaria Colegio Mayor de Antioquia. Carrera 78 No 65-46 Robledo, Medellín, Colombia. Institución Universitaria Colegio Mayor de Antioquia. Carrera 78 No 65-46 Robledo, Medellín, Colombia (alejita.montes@hotmail.com).
2Centro de Desarrollo de Productos Bióticos del Instituto Politécnico Nacional. CEPROBI-IPN. Calle CEPROBI 8. Col. San Isidro. Yautepec, Morelos. México CP. 62730. Tel. +527353942020 (gsepulvedaj@ipn.mx; sevangel@ipn.mx).
Cedrus atlantica is an ornamental plant used to decorate parks and gardens. In order to micropropagate quality material of this plant, an in vitro multiplication protocol was implemented. For this, the axillary buds were used as explants which were disinfected with sodium hypochlorite 10% for 15 min, treatment with which obtained 83% aseptic material. Explants culture was performed in the basal medium McCown’s Woody Plant (WPM) added with activated carbon (1g L-1) and cysteine (25 mg L-1), which allowed to obtain crops without oxidation features. In the multiplication stage with the use of benzyl amino purine (5 mg L-1) obtained up to 5 shoots per explant, which showed an increase of 1.4 cm. However, indole butyric acid at 2.5 and 5 mg L-1 did not induce root formation in vitro shoots.
Keywords: cedar; bud; micropropagation; woody plants
Cedrus atlantica es una planta ornamental utilizada para la decoración de parques y jardines. Con el fin de micropropagar material de calidad de esta planta, se implementó un protocolo de multiplicación in vitro. Para ello, como explantes se usaron las yemas axilares que se desinfestaron con hipoclorito de sodio al 10% por 15 min, tratamiento con lo que se obtuvo un 83% de material aséptico. El cultivo de los explantes se realizó en el medio basal McCown’s Woody Plant (WPM) adicionado con carbón activado (1 g L-1) y cisteína (25 mg L-1), lo que permitió obtener cultivos sin rasgos de oxidación. En la etapa de multiplicación con el uso de la bencil amino purina (5 mg L-1) se obtuvieron hasta 5 brotes por explante, que presentaron un crecimiento de 1.4 cm. Sin embargo, el ácido indol butírico a 2.5 y 5 mg L-1 no indujo la formación de raíces en los brotes in vitro.
Palabras clave: cedro; brotación; micropropagación; plantas leñosas
Introduction
Cedrus atlantica is a species belonging to the family pinacea known as true cedar. The plant was introduced to Mexico as an ornamental plant. The tree can reach 30 to 40 m high (Pijut, 2000), its wood possesses excellent characteristics to be used in construction, crafts and industry. The essential oil of this plant has antifungal activity against Penicilluim digitatum (Chebli et al., 2004), antimicrobial against Acinetobacter baumannii, Aeromonas veronii, Candida albicans, Enterococcus faecalis, Escherichia coli and cytotoxic against various cancer lines (Barrero et al., 2005).
C. atlantica propagation is by seed; however, seed production is irregular and seeds are scarce. An alternative to multiply tree species difficult to reproduce is the use of plant tissue culture. The characteristics of in vitro culture for plant multiplication are: a) use of small segments of the plant, which are the starting point to initiate the culture; b) a large number of seedlings are obtained in a short time and with phenotypic and genotypic characteristics similar to the mother plant; c) growth is continuous throughout the year and is not affected by climatic variations; d) little space and maintenance is required for propagation than traditional methods; and e) the sanitary quality of plants is ensured (Evans, 2003).
The in vitro propagation system have been successfully used for the multiplication of several ornamental species, but its application in tree species is limited due to oxidation problems present in the explants; so it is required to implement the disinfection and multiplication of plants protocols (Orellana, 1998). Particularly for the Pinaceae family there is a history of the use of these techniques for the propagation of some species such as Pinus maximartinezii (Zacarias et al., 2006 Paz et al., 2009), Pinus pseudostrobus and Pinus jaliscana (Rebolledo et al., 2006); Pinus roxburghii (Arya et al., 2000); Picea chihuahuana (López et al., 2000); but only in vitro propagation of Cedrus deodara is documented (Rebolledo et al., 2006).
In previous reports, it is documented that micropropagation is through organogenesis, with multiplication rates of 3 to 29 shoots per explant, it is also frequent the use of antioxidants to prevent tissue oxidation, as is the use of ascorbic acid and activated carbon. It is also reported that it is possible to obtain embryogenesis in cultures of Pinus cubensis (Cantillo et al., 2011); however, although embryogenic callus and embryos are obtained in developmental stages I and II, it was not possible to complete the propagation process.
Previous studies show that it is technically feasible to obtain in vitro plants mainly from the genus Pinus, however for Cedrus atlantica are not reported in vitro cultures. So in this work was raised as a target, to implement a protocol for the establishment of in vitro culture of Cedrus atlantica and it multiplication by adventitious buds that serve for micropropagation of this species.
Materials and methods
Vegetative material
C. atlantica plants of 50 cm height were provided by the Nursery El Solitario from Cuautla, Morelos, Mexico. These plants were kept in quarantine in the nursery from CEPROBI-IPN in Yautepec, State of Morelos, Mexico. The plants were applied with an antifungal and antibacterial solution every two day for 15 days. The solution contained Agri-Mycin® 1.5 g L-1 and Benlate® 0.8 g L-1.
Establishment of axenic culture
Disinfestation of C. atlantica was conducted by the methodology reported for Pinus pseudostrobus and Pinus jaliscana (Rebolledo et al., 2011); which consisted on using 80 plant explants formed by segments of axillary buds of 1 cm. The material was washed in soapy water for 15 min; Explants were treated with 50% ethyl alcohol for 30 seconds and three rinses with sterile distilled water; afterwards explants were immersed in reagent grade sodium hypochlorite (Hycel, HY57011019000) 10% v/v for 15 min, with a drop of Tween 20. The explants were rinsed three times with sterile distilled water.
The culture medium used to grow the explants was the basal medium McCown’s Woody Plant WPM (Lloyd and McCown, 1981) supplemented with 2.7 g L-1 phytagel. The pH of the medium was adjusted to 5.7 and poured in Gerber type flasks with 30 ml of the medium. The material was sterilized in an autoclave at 120 °C and 15 lbs for 20 min. In total 25 bottles, each with 3 explants was planted. The cultures were incubated at a temperature of 25 ± 3 oC with a photoperiod of 16 h light: 8 h dark with light intensity of 11.14 µmol m-2 s-1. After 5 days of culture, the determination of contamination percentage was performed: considering the relationship contaminated explants on total explants planted.In addition oxidation frequency of explants was documented.
Induction of multiple bud outbreaks
To induce multiple outbreaks, apical buds obtained after the disinfestation process were used. Explants were inoculated in the culture medium WPM supplemented with sucrose 3%, activated carbon 1 g L-1 and L-cysteine 25 mg L-1, with phytagel 2.7 g L-1. The effect of the cytokinin benzyl amino purine (BAP) in concentrations of 3 and 5 mg L-1 was tested. Three explants were cultured per flask with three replicates per treatment. After 21 days, the morphometric parameters measured were: explant growth and number of outbreaks.
Results and discussion
Disinfection and in vitro establishment
The disinfestation treatment in explants of apical buds from C. atlantica used in this work consisted on washing with sodium hypochlorite and alcohol, allowed to obtain 83% of explants without pollution, only 17% of explants showed fungal contamination after the 5th day (Figure 1). In the unpolluted apical buds there was no oxidation and only polluted explants showed oxidation. Figure 1 shows the mother plant of C. atlantica used, as well as the different stages of culture establishment.
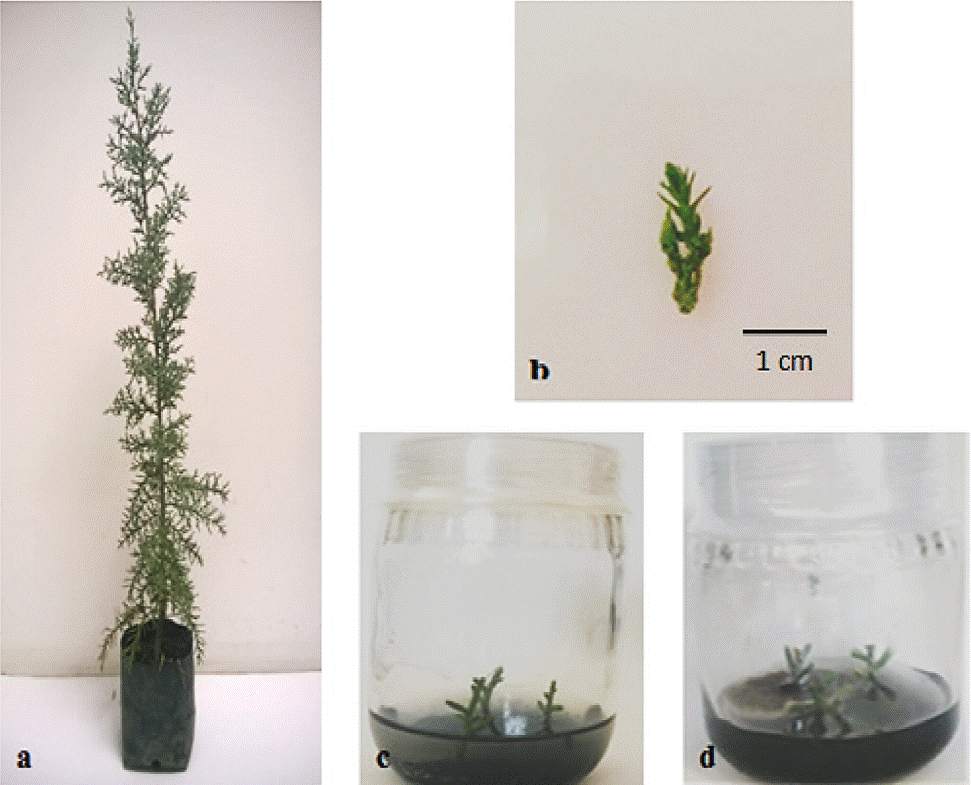
Figure 1 Establishment of in vitro culture of Cedrus atlantica: mother plant (a) explants of apical buds; (b) in vitro cultures without pollution; and (c) contaminated cultures by fungus (d).
The disinfestation method of apical buds of C. atlantica can be considered satisfactory, considering that the tissue did not show damage and contamination rate was 17%. This result is consistent with that reported by Rebolledo et al. (2006), who tested the same disinfectant agents and immersion times but in seeds of Pinus jaliscana and Pinus pseudostrobus. For other pinus species also reported the use of other disinfecting agents with which obtained satisfactory results such as mercuric chloride (Tamta and Palmi 2004; Oliveira et al., 2011) and hydrogen peroxide (Arya et al., 2000; Zacarias et al., 2006; Robledo et al., 2009).
In micropropagation of woody species, one factor limiting the development of the technique is the cut or tissue injury, since the oxidation thereof is generated. Therefore, during the establishment of their in vitro cultures an aspect to control is the development of the oxidation process. Choosing a suitable culture medium and the use of antioxidants are two important considerations for the survival of apical bud explants of in vitro cultures. For apical buds of C. atlantica, the culture medium WPM along with activated carbon and cysteine, turned adequate to prevent explant oxidation. This result is consistent with that reported for Cedrus deodara (Tamta and Palni, 2004) where explant oxidation was avoided using the WPM medium in combination with ascorbic acid. It is also consistent with that reported by Rebolledo et al. (2006), who also used the WPM medium for Pinus pseudostrobus and Pinus jaliscana, in combination with ascorbic and citric acid. Also in other studies using mediums such as Murashige and Skoog MS (1962) and Schenk and Hildebrandt SH (1972), widely used in vitro plant cultures for non-woody species is reported. For Pinus cubensis is reported the used the MS medium without antioxidants agents (Cantillo et al., 2011) and for Picea chihuahuana the SH medium supplemented with ascorbic and citric acid was used (López et al., 2000).
Multiplying by multiple sprouting of Cedrus atlantica
Table 1 show that the presence of BAP in the culture medium stimulated sprouting in C. atlantica in both concentrations evaluated (3 and 5 mg L-1). However, no significant differences were observed in the number of outbreaks. It was also observed that BAP stimulate shoot elongation, showing up to 1.53 cm in length, but with the two concentrations of BAP used, no significant difference in the buds size were observed (Figure 2).
Table 1 Effect the benzyl amino purine (BAP) in multiple sprouting of Cedrus atlantica.
| BAP (mg L-1) | Núm. de brotes | Longitud del brote (cm) |
| 0 | 2.77 ± 0.83 a* | 0.08 ± 0.16 a |
| 3 | 4.44 ± 0.88 b | 1.53± 0.22 b |
| 5 | 4.88 ± 0.92 b | 1.43 ± 0.92 b |
*Letras diferentes en cada columna indican que existen diferencias significativas. Prueba de Tukey con un nivel de significancia de p< 0.05.
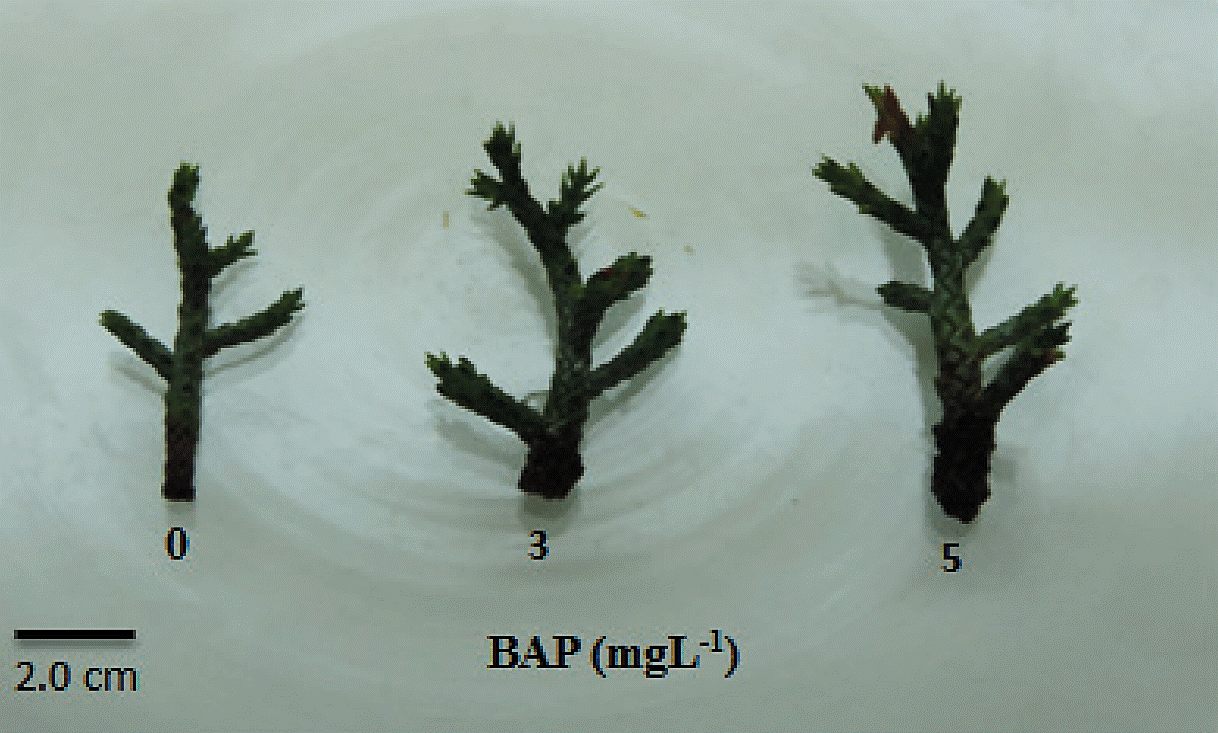
Figure 2 Effect of benzyl amino purine (BAP) in multiple sprouting from apical buds of Cedrus atlantica.
The effect of BAP on multiple sprouting obtained in this work is consistent with the reports of other authors in plants of the pinacea family. López et al. (2000) reported for Picea chihuhuana, that with the addition of 3.5 mg L-1 of kinetin seven shoots per explant are produced; Oliveira et al. (2011) reported that 0.4 mg L-1 of benzyl adenine (BA) can generate three shoots per explant in vitro cultures of Pinus taeda.
However, the number of outbreaks obtained from C. atlantica in this work is below those reported for Pinus maximartinezzi, where it was reported that the addition of BA at a concentration of 16 mg L-1 produced 29 outbreaks per explant Robledo et al. (2009); while BA at a concentration of 1.1 mg L-1 also produces from 7 to 50 outbreaks per explant of Cedrus deodara (Tamta and Palni, 2004). It is evident the importance of cytokinins in the metabolism of pinacea to induce the multiple sprouting process.
Rooting
In this study the concentrations of indole butyric acid (IBA) were used for C. atlantica were not optimal, because it did not formed root, only the presence of callus was observed in explants cultured (Figure 3). This result is consistent with that reported in the literature, which shows that the induction of in vitro rooting are low compared to ex vitro treatments. In addition, Hartmann et al. (2002) consider that the rooting stage often represents the critical and difficult phase in micropropagation protocols of most woody species. In this regard Paz et al. (2009) reported that in P. maximartinezii, only 3% succeeded in inducing root formation in shoots with IBA concentration of 3 mg L-1. An alternative to improve the rooting process of C. atlantica seedlings could be the use of IBA in combination with NAA. This considering the results reported for Pinus cubensis (Oliveira et al., 2011) who report that the combination of 1.8 mg L-1 naphthaleneacetic acid (NAA) and 2.03 mg L-1 IBA promotes the formation of 47% of roots, without callus formation.
Conclusion
This study reports a protocol to obtain in vitro culture of C. atlantica from axillary buds. The culture medium used is WPM with addition of activated carbon and cysteine with which an in vitro culture is obtained without oxidation features and with potential for the generation of outbreaks in the presence of benzyl amino purine. Future studies are designed to induce root formation.
Literatura citada
Arya, S. and Kalia, R. 2000. Induction embryogenesis in Pinus roxburghii sarg. Plant Cell Reports. 19:775-780. [ Links ]
Barrero, A.; Quilez, J.; Herrador, M.; Arteaga, J; Akssira, M.; Benharref, A. and Dakir, M. 2005. Abietane diterpenes from the cones of Cedrus atlantica. Phytochemistry. 66:105-111. [ Links ]
Cantillo, R.; Igarza, J. y Ochoa, A. 2011. Propagación in vitro de plantas de Pinus cubensis griseb. Biotecnol. Vegetal. 11:3-13. [ Links ]
Chebli, B.; Achouri, M.; Hasssani, I. and Hmamouchi, P. 2003. Antifungal activity of essential oils from several medicinal plants against four postharvest citrus pathogens. Phytopathol. Mediterranean. 42:251-256. [ Links ]
Evans, D. E.; Coleman, J. O. D. and Kearns, A. 2003. Plant cell culture. Bios Scientific Publisher. New York, USA. 19-29 pp. [ Links ]
Hammer, K.; Carson, C. and Rile, Y. T. 1999. Antimicrobial activity of essential oils and other plant extracts. J. Appl. Microbiol. 86:985-990. [ Links ]
Hartmann, H.; Kester, D.; Davies, and Geneve, R. 2002. Plant propagation: principles and practices. Prentice Hall. 7th Ed. 880 p. [ Links ]
López, A.; Olguín, L.; Márquez, J.; Chávez, V. and Bye, R. 2000. Adventitious bud formation from mature embryos of Picea chihuahuana Martínez, an endangered Mexican spruce tree. Ann. Bot. 86:921-927. [ Links ]
Mccown, B. and Lloyd, G. 1981. Woody plant medium (WPM) a revised mineral formulation for micro-culture of woody plant species. HortSci. 16:453. [ Links ]
Murashige, T. and Skoog, E. 1962. A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol. Plantarum. 15:473-497. [ Links ]
Oliveira, L.; Lopes, P.; Quoirin, M.; Soares, H.; Amano, E. and Rioyei, A. Micropropagation of Pinus taeda L. from juvenile material. 2011. Tree Foresty Sci. Biotechnol. 1:96-101. [ Links ]
Orellana, P. 1998. Introducción a la propagación masiva. In: propagación y mejora genética de plantas por biotecnología. Instituto de Biotecnología de Plantas, Santa Clara. Cuba. Pérez-Ponce J. Ed. 125-133 pp. [ Links ]
Pijut, P. 2000. Cedrus- the true cedars. J. Arboric. 26:4. [ Links ]
Schenk, R. and Hildebrandt, A. 1972. Medium and techniques for induction and growth of monocotyledonous and dicotyledonous plant cell cultures. Canadian J. Bot. 50:199-204. [ Links ]
Rebolledo, V.; Aparicio, A. y Cruz, H. 2006. Estudio preliminar para la propagación in vitro de dos especies de pinos. Foresta Veracruzana. 8:27-32. [ Links ]
Robledo, A.; Villalobos, V. y Santacruz, A. 2009. Inducción eficiente de brotes adventicios en cotiledones de Pinus maximartinezii Rzedowski. 2009. Acta Bot. Mex. 89:47-62. [ Links ]
Tamta, S. and Palni, L. 2004. Studies on in vitro propagation of Himalayan cedar (Cedrus deodara) using zygotic embryos and stem segments. Indian J. Biotechnol. 3:209-215. [ Links ]
Zacarias, M.; Luna, H.; Morales, L.; Verde, M.; Torres, T.; Pereyra, B.; Iracheta, L.; Saénz, E.; Salazar, R. y Cárdenas, E. 2006. Multiplicación in vitro del piñón azul Pinus maximartinezii (Rzedowski). Inter. Bot. Exp. 75:109-113. [ Links ]
Received: October 2016; Accepted: December 2016











 text in
text in 



