Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista mexicana de ciencias agrícolas
versión impresa ISSN 2007-0934
Rev. Mex. Cienc. Agríc vol.7 no.8 Texcoco nov./dic. 2016
Essays
Iodine biofortification in plants for human consumption
1Universidad Autónoma Agraria Antonio Narro-Departamento de Horticultura. Calzada Antonio Narro 1923, Saltillo, Coahuila, México. CP. 25315.
2Universidad Autónoma de Nuevo León- Facultad de Ciencias Biológicas. Ave. Pedro de Alba s/n, Ciudad Universitaria, San Nicolás de los Garza, Nuevo León. CP. 66450.
This paper describe health problems due to insufficient iodine intake, thus the alternatives that have been used to alleviate this global problem, both traditional and iodization of table salt to new trends of iodine biofortification in the main plants for human consumption. The mechanisms of absorption, volatilization and transport of iodine in plants, distinguishing between marine and terrestrial species are included. The results of some biofortification studies are also mentioned, citing the different forms of application and the differences obtained between cropping systems in soil and soilless.
Keywords: fortification with iodine; haloperoxidase; iodization; iodine absorption; iodine deficiency disorder
En el presente trabajo se describe la problemática de salud debido al consumo insuficiente del yodo, así como las alternativas que han sido empleadas para mitigar este problema global, tanto las tradicionales como la yodatización de sal de mesa hasta las nuevas tendencias de biofortificación con yodo en las principales plantas de consumo humano. Se incluyen los mecanismos de absorción, volatilización y transporte del yodo en las plantas, distinguiendo entre especies marinas y terrestres. Se mencionan asimismo los resultados obtenidos en algunos estudios de biofortificación, citando las diferentes formas de aplicación y las diferencias obtenidas entre los sistemas de cultivo en suelo y sin suelo.
Palabras clave: absorción de yodo; desorden por deficiencia de yodo; fortificación con yodo; haloperoxidasas; yodatización
Introduction
Inadequate consumption of micronutrients through food causes mineral malnutrition in humans. So far 11 trace elements that are essential for proper growth and development of humans have been determined (Fraga, 2006). Some of these elements are required in such small amounts that its deficiency can become rare or even unknown (Stein, 2009). According to the World Health Organization (WHO) the most common nutritional deficiencies are iron (Fe), zinc (Zn), iodine (I) and Vitamin A (Burlingame, 2013) (Figure 1).
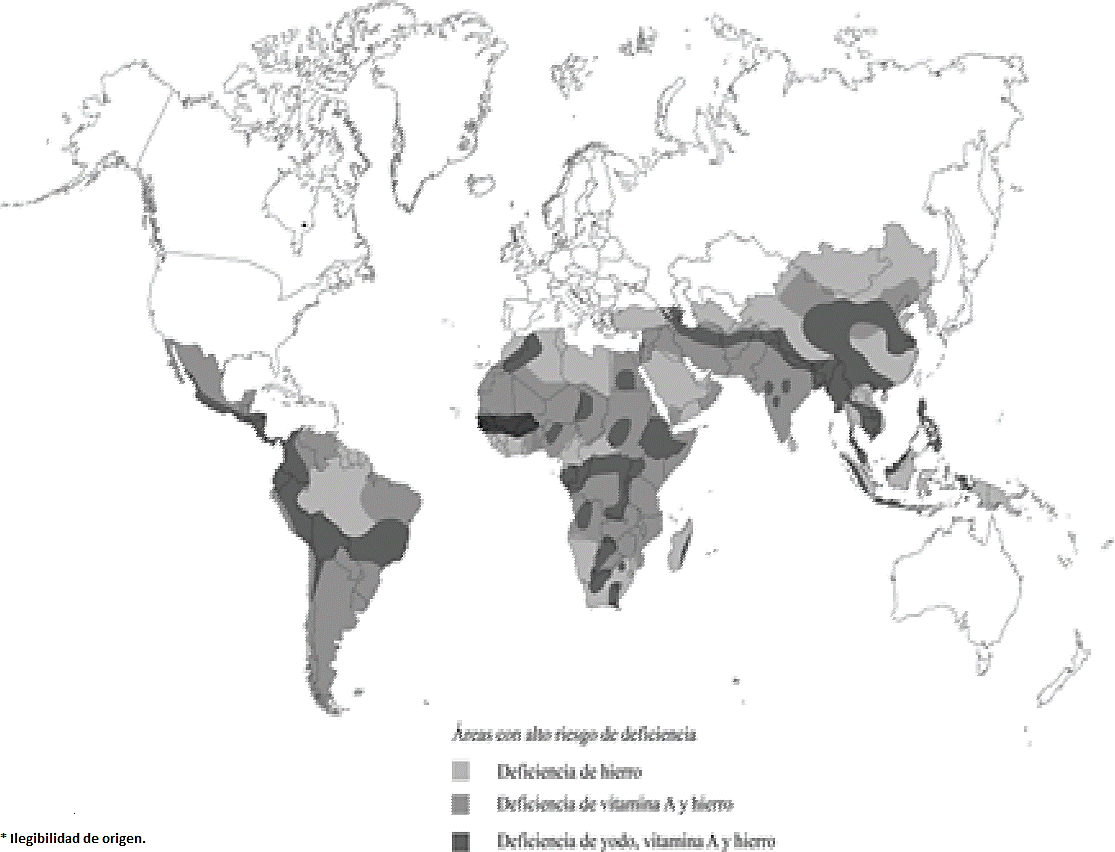
Figure 1 Most common nutritional deficiencies (Modified from Burlingame, 2013. Frontiers in Agricultural Sustainability: studying the protein supply chain to improve increase dietary quality).
Among the causes of mineral malnutrition are irregular distribution of elements in soils and impoverishment of agricultural soils due to intensive production and lack of good management (Vir, 2012).
One of the most studied elements due to their metabolic importance in mammals is iodine. The deficiency of this element occurs in many parts of the world but it is more pronounced in mountainous and plain areas, this caused by the uneven distribution of iodine in Earth's crust (FAO, 2009). It is estimated that 2 000 million people live on an insufficient intake of this element (Mottiar, 2013), causing the so-called iodine deficiency disorders (IDD) (Weng et al., 2008). IDD refer to all disorders associated with low iodine intake and can be prevented by ensuring adequate intake of the element (WHO, 2001). IDD occurs when iodine intake and assimilation is lower than the amount required by the thyroid gland to synthesize enough amounts of hormones thyroxine and triiodothyronine. The most known IDD for its clear symptomatology is goitre, however, in recent decades has been recognized the presence of less obvious disorders from the point of view of symptoms, such as the adverse impact of inadequate iodine intake in mental and physical development in children as well as in adult productivity (Lazarus, 2015).
In addition to the importance of iodine intake, justified by the essentiality for the proper functioning of thyroid gland, it has been recently shown a different function to the aforementioned associated with carcinogenesis and its treatment in various cell lines, showing that iodine is able to act in two ways: as an antioxidant and as malignant cell antiproliferative (Aceves and Anguiano, 2009). At low concentrations iodine can directly neutralize hydroxyl radicals (OH-) and additionally in its oxidized form (I2) competes with reactive oxygen species (ROS) by various cellular components, significantly reducing the lipoperoxidation. At high concentrations iodine acts as a mild oxidant, dispelling the mitochondrial membrane potential and promoting apoptosis in cancer cells (Arroyo et al., 2006; Shrivastava et al., 2006; Torremante and Rosner, 2011).
Daily requirement of iodine, according to the recommended dietary allowances (RDA) (WHO, 2011; Andersson et al., 2011) are shown in Table 1.
Table 1 Recommended daily intake of iodine.
| Cantidad diaria | Edad |
| 90 µg | Infantes (0-59 meses) |
| 120 µg | Niños (6-12 años) |
| 150 µg | Adultos (mayores de 12 años) |
| 200 µg | Embarazo y lactancia |
There have been numerous attempts to mitigate the deficit in iodine consumption, mainly through universal iodization of table salt since the 1920s (De Caffarelli et al., 1997; Zimmermann, 2009; Charlton et al., 2013). However, over the years it has been proven that this technique alone is insufficient to ensure total iodine requirement (De Benosit et al., 2008). Additionally, in most industrialized countries consumption of table salt is decreasing (Hetzel and Standbry, 1980) plus it has been shown that iodine in table salt volatilizes (Laillou et al., 2015). Moreover, iodine consumption in organic forms is considered more appropriate (Weng et al., 2014), as it has been also evidenced cases of endemic goiter associated to excessive consumption of inorganic iodine (Zhao et al., 2000). Because of this, it is necessary to increase the use of techniques such as crop bio-fortification to achieve adequate iodine intake, either as complement or as an alternative to iodization of table salt. The aim of this paper is to review the advances on iodine application in plants and crop bio-fortification with this element.
Availability of iodine in food
The largest reservoir of iodine is in the sea; from there is mobilized to terrestrial areas through volatile chemical forms produced by marine seaweed or by marine aerosols carried by winds which explains why remote soil from the ocean are usually short of iodine and therefore plants grow in these soils would have low concentration of the element (Zimmermann, 2009).
Iodine is not considered as an essential element for terrestrial plants, although several studies showed that these absorb and accumulate iodine (Mackowiak and Grossl, 1999; Zhu et al., 2003). This apparent non-essentiality explains why iodine is not contemplated in mineral fertilization programs of crops, despite the fact that it has been shown that the contribution of inorganic iodine salts can increase stress tolerance in plants (Leyva et al., 2011; Gupta et al., 2015). Not to include fertilizers with iodine for crops causes that the content of this element in plant and animal foods depends exclusively on the natural availability of the element in soil and water. For this reason, to date, the main source of iodine for most of the world's population is the contribution of inorganic salts (iodide and potassium iodate: KI and KIO3) in table salt, adding 20 to 40 mg of iodine per kg of salt following the guidelines from WHO/UNICEF/International Council for the Control of iodine Deficiency Disorders (WHO, 2007; FAO, 2009; Zimmermann, 2009).
Availability of iodine in the soil
The available iodine concentration in the soil is in function of the distance to the ocean as well as soil composition from mother material. Mountainous areas, valleys and plains from the interior of continents show low concentrations of iodine (Aston and Brazier, 1979). It has been noted that iodine is unique among the elements in consideration to its geochemical behavior. Most elements available in the soil come from the erosion of the lithosphere, and generally this is not the case for iodine (Fuge and Johnson, 1986). This element shows a wide range of soil concentrations from <0.1 to 150 mg kg-1. It is considered that iodine concentration in soil is generally higher than that found in soil bedrock. Geochemical agree on the above because the largest supply of iodine in the soil comes from the atmosphere which in turn this receives it from marine environment (Fuge, 2013).
Among the edaphic factors that modify the availability and iodine absorption in soil are: amount of organic matter, which allows higher iodine absorption by plants, especially when it is in the form of iodate (IO3-) (Seki et al., 1984). This effect seems to depend on the ability of humic substances to adsorb iodine (process apparently mediated by microorganisms) decreasing its volatilization (Bostock, 2003), although in the absence of organic matter seems to be great iodine volatilization activity as CH3I through microbial activity (Amachi et al., 2003). This fact may partly explain the apparent negative relationship between clay content of the soil and iodine uptake by plants, while soil pH does not appear to exert any effect in the range of 5.4 to 7.6 (Shinonaga et al., 2001).
Iodine content in plants
Iodine concentration normally found in plant tissue ranges from 0.1 to 1 µg g-1, reaching up to 3 µg g-1 or more (Benton Jones, 1998). In a study conducted by Shinonaga et al. (2001) established that iodine concentration in grain cereal from inland agricultural areas in Europe was particularly low, with values of 0.002 to 0.03 µg g-1, compared to soils near the ocean. Little is known about how this element is accumulated in terrestrial plants, however, in a research conducted in pumpkin plants was deduced through a study with electron microscopy that the inorganic and organic iodine applied directly to the substrate, most of iodine accumulated in the root, while another small portion was transported to the aerial parts to be stored in the chloroplasts (Weng et al., 2008a), fact that may partly explain its role of inducing tolerance to certain types of stress since a significant volume of the antioxidant cellular machinery is found in chloroplasts.
Fertilization with iodine for biofortification in soilless crop systems
Iodine content in plants generally increases when applying iodine as iodide, iodate or other chemical forms (Weng et al., 2008b). The best results were obtained by applying iodine compounds in the nutrient solution or foliar spraying. In each plant species the results are variable in the sense that the response is modified versus the concentration and the distribution changes in plants organs. For example, in a study conducted in spinach under hydroponics different chemical forms of iodine as acid iodine acetic, iodide and iodate were applied and proved that concentrations greater than 0 and up to 1 mg L-1, iodine absorption rate increased linearly against the concentration, maintaining a concentration ratio of 1:1 in roots and leaves. The following accumulation order was shown with the different iodine forms CH2ICOO-> I-> IO3-.
Additionally it was found that even with these low concentrations there was an increase in biomass (Weng et al., 2008c). Meanwhile Blasco et al. (2008) demonstrated that applying iodine concentrations ≤5.1 mg L-1 iodide (I-) in hydroponic lettuce obtained an adequate accumulation of foliar iodine, which was 900 µg-1 g dry tissue, amount that is sufficient to meet the daily demand for iodine intake in humans (150 µg day-1) (Charlton et al., 2013) when consumed between 17 and 200 g of fresh tissue of lettuce per week, as this concentration does not exceed the maximum recommended amount which is 2 mg per day. However at concentrations above 5.1 mg L-1 iodide a significant reduction in biomass was present, thing that did not happen applying the same concentrations of iodate.
Zhu et al. (2003) studied iodine absorption in spinach plants under hydroponic culture at concentrations of 0.127, 1.27, 6.35 and 12.7 mg L-1 of iodide and iodate, finding that with 12.7 mg L-1 iodide there were no negative effects on plant growth, while with the same iodate concentration occurred only a small effect on plant biomass; however low concentrations, such as 0.127 mg L-1 of iodide and iodate resulted in adequate accumulation of this element, showing 3 and 1.8 µg I g-1 fresh weight, which is considered adequate.
Voogt et al. (2010) conducted another biofortification experiment with iodine as iodide and iodate in lettuce plants, applied very low concentrations of these chemical forms, which ranged between 0.013 and 0.129 mg L-1, finding that none of concentrations showed toxic effect on lettuce plants and with a concentration of 0.129 mg L-1 iodide obtained an accumulation of 0.653 and 0.764 µg I g-1 fresh tissue, five times lower than the concentration obtained by applying iodate.
Today there is a growing interest in iodine supplementation in the food chain through uptake and accumulation of this element in plants for human consumption (Nestel, 2006), the positive results described above, mainly in horticultural species on soilless systems are important and suggest that are good candidates for bio-fortification programs with iodine, however, the real challenge is crop bio-fortification in extensive soil systems, since it is what would allow a truly comprehensive coverage of the target population.
Fertilization with iodine for crop biofortification in soil
The addition of iodine to the soil allows to broaden the spectrum of plant species for biofortification (Cui and Song, 2003), including also medicinal vegetables and even staple crops (Yuita, 1992) normally grown in field. In the soil can coexist various chemical forms of iodine, among the most abundant are iodate (IO3) and iodide (I-) (Borst Pauwels, 1962). Some studies related to application of iodine to the soil have shown that plants can tolerate higher concentrations of iodate than iodide (Dai et al., 2006; Smolen et al., 2012) and that very low concentrations of iodine (0.02-0.2 mg kg-1) regardless of the form, are beneficial for different crops, especially for halophytic (Borst Pauwels, 1961; Dai et al., 2006; Smolen and Sandy, 2012). Dai et al. (2006) conducted the application of iodide and potassium iodate directly to the soil to biofortify lettuce plants, noting that the highest accumulation of this element was obtained with iodate treatments.
Contrary to what happened when applying iodide and potassium iodate through hydroponics in lettuce plants and rice (Mackowiak and Grossl 1999; Zhu et al., 2003). Studies performed in rice have shown that these have the ability to reduce iodate to iodide, fact that seems to confer metabolic flexibility compared to iodine (Kato et al., 2013). Moreover, Weng et al. (2003) found that when applying kelp and diatomaceous earth to the soil managed to increase the availability of iodine for vegetables. The same authors found that the iodine absorption by vegetables was a direct function of the concentration from the element in the soil, up to a limit that was different for each plant species. In a recent study Weng et al. (2014) demonstrated that it is possible to use seaweed compost incorporated into the soil as a mechanism of fertilization with iodine.
The origin of the differences in iodine or iodate absorption in soil and nutrient solutions are unknown. They are attributed in part to the substantial volatilization of Iodine from the plant-soil system to the atmosphere mediated by different microorganisms (Muramatsu et al., 1995; Fuge, 1996). However, there is evidence that the synthesis of volatile forms of iodine, called organoiodine, mainly from the chemical form iodide (Keppler et al., 2003) this fact might seem contradictory since iodide is the chemical form that plants absorb more easily from the soil. Iodine content in irrigation water can be a source of this element for plants; however it is unlikely that water contains adequate levels of iodine in regions where the soil has low concentrations of the element. The application of iodine as potassium iodate in irrigation water (0.010 to 0.08 mg L-1 for four weeks) was effective for four years to increase the concentration of iodine in soil and plants in regions from China with severe iodine deficiency (Jiang et al., 1998).
In another study Caffagini et al. (2011) conducted the application of iodide and iodate through irrigation at concentrations of 500 and 1 000 mg L-1 and 500, 1 000, 2 000 and 5 000 mg L-1, respectively, in barley, corn, tomato and potato; finding favorable results only in tomato fruits and potato tubers reaching concentrations of 272 to 6 245 µg I per 100 g of fresh weight and from 527 to 5 375 µg I per 100 g of fresh weight respectively. Iodine concentration achieved in tomato fruits of treated plants with iodide at 500 mg L-1 was seven times higher than that achieved with the application of iodate at 500 mg L-1 (3 900-527 µg 100 g-1). Similarly for potato tubers, where the achieved concentration with the application of iodide at 500 mg L-1 was seven times higher than the concentration achieved with the application of iodate at 500 mg L-1 (1 875 versus 272 µg I per 100 g fresh weight), which confirmed the greater ease of absorption from iodide against iodate.
One way to avoid the complexity of the phenomena of absorption and transport of iodine when applied to the soil, is to apply it through foliar spraying as iodide and iodate (Zanirato and Mayerle, 2009), finding good results when compared to soil applications (Lawson et al., 2015). In another recent study Tonacchera et al. (2013) achieved to increase iodine concentration in potato, carrot, tomato and lettuce applying iodine as foliar spray. The results indicated that the edible parts of the plants reached up to 30% of the recommended daily intake (RDA) of iodine without affecting other aspects of food quality.
Mechanisms of iodine absorption and transport
Marine plants are immersed in a medium rich in iodine, therefore constitute the simplest model to study the phenomenon of iodine absorption. According to the proposed mechanism Küper et al. (1998), iodine absorption in brown algae occurs through the pass of iodide present in the apoplast through the cell wall through enzyme iodine peroxidase dependent of vanadium (V-IPO) (Truesdale et al., 1995; Colin et al., 2003; Winter and Moore, 2009) which catalyzes the oxidation of iodide to hypoiodous acid (HIO), as shown in reaction 1:
Additionally hypoiodous acid can react spontaneously with iodide maintaining equilibrium of reaction in aqueous medium as shown in reaction 2 (Truesdale et al., 1995).
When algae are subject to low levels of oxidative stress, I2 formed in the reaction (2) can cross the cell membrane, subjected to a reduction in the cytoplasm and then stored as iodide in the vacuole, where will be mobilized at times of oxidative stress. Instead, when algae are subjected to a high level of oxidative stress I2 will volatilize into the atmosphere (Le Blanc et al., 2006; Kupper et al., 2008). The same fate may suffer HIO, because it can accumulate in vacuoles or react with organic compounds forming mainly methyl iodide (CH3I) which will volatilize (Le Barre et al., 2008) (Figure 2).
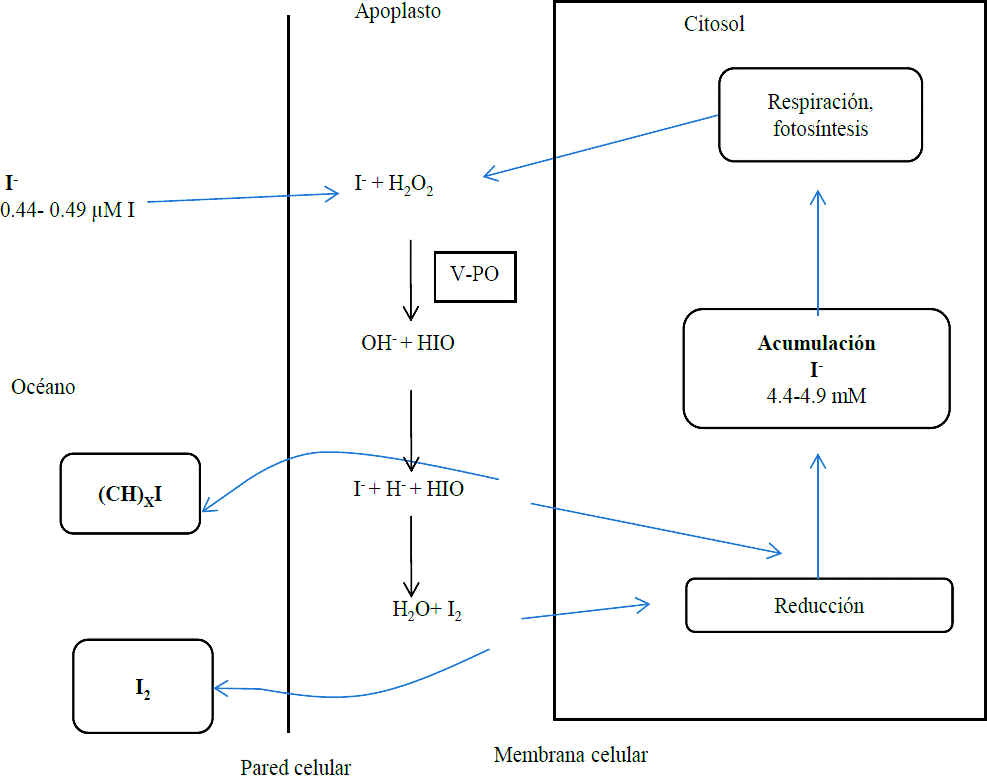
Figure 2 Diagram of the absorption and volatilization mechanism of iodine by marine plants; (Modified from Le Blanc et al., 2006).
Weng et al., (2013) performed a series of experiments with cabbage plants and concluded that the iodine absorption through the root occurs in a different way to that observed in marine plants. In cabbage iodide transport occurs via coupled transporters to a proton bomb, thus through anion channels and the same has been observed in other terrestrial species (White and Broadley, 2009). By contrast, in marine plants it seems to operate only the mechanism associated to proton pump (Weng et al., 2013). Subsequent mobilization of iodine from the root to the stems and leaves occurs through the xylem (Herret et al., 1962; Mackowiak and Grossl, 1999; Zhu et al., 2003; Kato et al., 2013) but as it occurs with other elements seems to be a subsequent redistribution of iodine via phloem (Landini et al., 2011).
The assimilation and transport processes of iodine start to elucidate and it promises to obtain great benefit in terms of nutritional quality of vegetables. However, even in the absence of such knowledge a practical way to quantify iodine uptake by plants is the so-called transfer factor (TF) which is calculated by the following equation:
The disadvantage of this factor (TF) is that it does not indicate anything about the factors that modify it, however, if the goal is to detect soil types or management factors that increase the uptake of iodine or decrease its loss by volatilization, then TF will be very useful as an indicator. For example Cl- has a high TF, due to its flow in the plant shows high correlation with xylem water flow, it has been calculated a TF = 785 value for Cl- in Lolium perene (Ashworth and Shaw, 2006). In contrast TF values for iodine are usually very low: 0.00034 for forage (IAEA, 1994), 0.0005-.02 for cereals in Austria (Shinonaga et al., 2001), 0.024-0.19 for corn, beets and pumpkin (Sheppard et al., 1993) and 0.01-0.03 for radishes and lettuce (Kashparov et al., 2005).
However, it has been reported that it is possible to significantly increase TF of iodine, by increasing the concentration of this element in the soil. Dai et al. (2004) obtained that when applying iodine at a concentration of 5 mg kg-1, TF from edible parts of different vegetables increased from 0.1 to 10. The values increased in the following order carrot =onion <celery <spinach outbreak < spinach leaf. Because of the problems described above, and not only to uneven distribution of iodine in the soil, have been proposed application techniques to apply iodine to soil, water or foliar spray and has been proven the effectiveness of these practices to achieve improved iodine concentration in the main plants for human consumption.
Conclusions
Nutritional health of about 2*109 people provides a huge incentive for the development of new techniques for biofortification with iodine for the main plants for human consumption.
The constant sources of information allows to confirm that the fortification strategy has enabled a breakthrough in the fight against the deficiency in iodine intake; however, it is required to extend this strategy now including crop bio-fortification, and for it, is key the elucidation of the mechanisms related to the absorption, volatilization and accumulation of this element by the plants.
Literatura citada
Aceves, C. and Anguiano, B. 2009. Is iodine an antioxidant and antiproliferative agent for the mammary and prostate glands? In: Preedy, V. R.; Burrow, G. N. and Watson, R. R. (Eds.). Handbook of iodine. San Diego, California: Elsevier. 249-257 pp. [ Links ]
Amachi, S.; Kasahara, M.; Hanada, S.; Kamagata, Y.; Shinoyama, H.; Fujii, T. and Muramatsu, Y. 2003. Microbial participation in iodine volatilization from soils. Environ. Sci. Technol. 37(17):3885-3890. [ Links ]
Andersson, M.; Karumbunathan, V. and Zimmermann, M. 2012. Global iodine status in 2011 and trends over the past decade. J. Nutr. 142:744-750. [ Links ]
Aston, S. R. and Brazier, P. H. 1979. Endemic goitre, the factors controlling iodine deficiency in soils. Sci. Total Environ. 11(1):99-104. [ Links ]
Benton Jones, J. 1998. Plant Nutrition Manual, CRC Press, UK. 7-10 p. [ Links ]
Blasco, B.; Ríos, J. J.; Cervilla, L. M.; Sanchez-Rodríguez, E.; Ruiz, J. M. and Romero, L. 2008. Iodine biofortification and antioxidant capacity of lettuce: potential benefits for cultivation and human health. Ann. Appl. Biol. 152(3):289-299. [ Links ]
Borst, P. G. W. F. H. 1962. An investigation into the effects of iodide and iodate on plant growth. Plant Soil. 16(3):284-292. [ Links ]
Bostock, A. C.; Shaw, G. and Bell, J. N. 2003. The volatilization and sorption of (129) I in coniferous forest, grassland and frozen soils. J. Environ. Radio. 70(1-2):29-42. [ Links ]
Burlingame, B. 2013. The role of agriculture in diet: quantity versus quality. Conference frontiers in agricultural sustainability: studying the protein supply chain to improve dietary quality. The New York Academy of Sciences. [ Links ]
Charlton, K. E.; Jooste, P. L.; Steyn, K.; Levitt, N. S. and Ghosh, A. 2013. A lowered salt intake does not compromise iodine status in Cape Town, South Africa, where salt iodization is mandatory. Nutrition. 29(4):630-634. [ Links ]
Cui, X.; Sang, Y. and Song, J. 2003. Residual of exogenous iodine in forest soils and its effect on some wild-vegetable plants. J. Appl. Ecol. 14(10):1612-1616. [ Links ]
Dai, J. L.; Zhu, Y. G.; Zhang, M. and Huang, Y. Z. 2004. Selecting iodineenriched vegetables and the residual effect of iodate application to soil. Biol. Trace Elem. Res. 101(3):265-276. [ Links ]
De Benoist. B.; McLean, E.; Andersson, M. and Rogers, L. 2008. Iodine deficiency in 2007: global progress since 2003. Food and Nutrition Bulletin. 29(3):195-202. [ Links ]
Food and Agriculture of the United Nations (FAO). 2009. The state of food insecurity in the world, Rome: Electronic Publishing Policy and Support Branch Communication Division. ftp://ftp.fao.org/docrep/fao/012/i0876e/i0876e_flyer.pdf. [ Links ]
Fraga, C. A. 2005. Relevance, essentiality and toxicity of trace elements in human health molecular. Mol. Aspects Med. 26(4-5):235-244. [ Links ]
Fuge, R. 2013. Soil and iodine deficiency. Essentials of medical geology, part II Springer Netherlands. 417-432 pp. [ Links ]
Fuge, R. 1996. Geochemistry of iodine in relation to iodine deficiency disease. In: Appleton, J. D.; Fuge, R. and Mccall, G. J. H. (Eds.). Environmental geochemistry and health. Geological Society Special Publication, London. 201-211 pp. [ Links ]
Fuge, R. and Jhonson, C. C. 1986. The geochemistry of iodine -a review. Environ. Geochem. Health. 8(2):31-54. [ Links ]
Gartner, R.; Rank, P. and Ander, B. 2010. The role of iodine and deltaiodolactone in growth and apoptosis of malignant thyroid epithelial cells and breast cancer cells. Hormones. 9(1):60-6. [ Links ]
Gupta, N.; Bajpai, M. S.; Majumdar, R. S. and Mishra, P. K. 2015. Response of Iodine on antioxidant levels of Glycine max L. Grown under Cd Stress. Adv. Biol. Res. 9(1):40-48. [ Links ]
Hetzel, B. S. and Stanbury, J. B. 1980. Endemic goiter and endemic cretinism: iodine nutrition in health and disease. Wiley Medical Publication, Wiley, New York. 606 p. [ Links ]
IAEA. 1994. Handbook of parameter values for the prediction of radionuclide transfer in temperate environments. Technical Report Series No. 364. International Atomic Energy Agency, Vienna. 160 p. [ Links ]
Jiang, X. M.; Cao, X. Y.; Jiang, J. Y.; Tai, M.; James, D. W. and Rakeman, M. A. 1998. Dynamics of environmental supplementation of iodine: four years’ experience of iodination of irrigation water in Hotien, Xinjiang, China. Arch. Environ. Health. 52(6):399-408. [ Links ]
Juvenal, G. J.; Thomas, L.; Oglio, R.; Pisarev, M. A.; Rossich, L. and Salvarredi, L. 2011. Thyroid: iodine beyond the thyronines. Curr. Chem. Biol. 5(3):163-167. [ Links ]
Kashparov, V.; Colle, C.; Zvarich, S.; Yoschenko, V.; Levchuc, S. and Lundin, S. 2005. Soil-to-plant halogens transfer studies: 1. Root uptake of radioiodine by plants. J. Environ. Radioac. 79(2):187-204. [ Links ]
Kato, S.; Wachi, T.; Yoshihira, K.; Nakagawa, T.; Ishikawa, A. and Takagi, D. 2013. Rice (Oryza sativa L.) roots have iodate reduction activity in response to iodine. Front. Plant Sci. 4:1-11. [ Links ]
Keppler, F.; Borchers, R.; Elsner, P.; Fahimi, I.; Pracht, J. and Scholer, H.F. 2003. Formation of volatile iodinated alkanes in soil: results from laboratory studies. Chemosphere. 52(2):477-483. [ Links ]
Kupper, F.C.; Carpentere, L.; McFiggans, G.; Palmere, G.; Waiteh, T. and Bonebergb, E. 2008. Iodide accumulation provides kelp with an inorganic antioxidant impacting atmospheric chemistry. Proceedings of National Academy of Science. 105(19):6954-6958. [ Links ]
La Barre, S.; Potin, P.; Leblanc, C. and Delage, L. 2010. The halogenated metabolism of brown algae (Phaeophyta), its biological importance and its environmental significance. Marine Drugs. 8(4):988-1010. [ Links ]
Laillou, A.; Mam, B.; Oeurn, S. and Chea, C. 2015. Iodized salt in Cambodia: trends from 2008 to 2014. Nutrients. 7(6):4189-4198. [ Links ]
Landini, M.; Gonzali, S. and Perata, P. 2011. Iodine biofortification in tomato. J. Plant Nutr. Soil Sci. 174(3):480-486. [ Links ]
Lazarus, J. H. 2015. The importance of iodine in public health. Environmental geochemistry and health. 1-14 pp. [ Links ]
Lawson, P. G.; Daum, D.; Czauderna, R.; Meuser, H. and Härtling, J. W. 2015. Soil versus foliar iodine fertilization as a biofortification strategy for field-grown vegetables. Frontiers Plant Sci. 6:450. [ Links ]
Leyva, R.; Sánchez, R. S.; Ríos, J. J.; Rubio, W. M.; Romero, L.; Ruiz, J. M. and Blasco, B. 2011. Beneficial effects of exogenous iodine in lettuce plants subjected to salinity stress. Plant Sci. 181:195-202. [ Links ]
Mottiar, Y. 2013. Iodine biofortification through plant biotechnology. Nutrition. 29(11-12):1431-1433. [ Links ]
Nestel, P.; Bouis, H.; Meenakshi, J. V. and Pfeiffer, W. 2006. Biofortification of staple food crops. J. Nutr. 136(4):1064-1067. [ Links ]
Núñez, A. R. E.; Cajero, J. M. and Aceves, C. 2011. Peroxisome proliferator activated receptors. Role of isoform gamma in the antineoplasic effect of iodine in mammary cancer. Current Cancer Drug Target 11(7):775-786. [ Links ]
Roti, E. and Uberti, E. 2011. Iodine excess and hyperthyroidism. Thyroid. 11(5):493-500. [ Links ]
Seki, R.; Takahashi, T. and Ikeda, N. 1984. Adsorption behavior of radioactive iodide and iodate in soil. Radioisotopes. 33(2):51-54. [ Links ]
Sheppard, S. C.; Evenden, W. G. and Amiro, D. 1993. Investigation of the soil-to-plant pathway for I, Br, Cl and F. J. Environ. Radio. 21(1):9-32. [ Links ]
Shinonaga, T.; Gerzabek, M. H.; Strebl, F. and Muramatsu, Y. 2001. Transfer of iodine from soil to cereal grains in agricultural areas of Austria. Sci. Total Environ. 267(1-3):33-40. [ Links ]
Smolen, S. and Sady, W. 2012. Influence of iodine form and application method on the effectiveness of iodine biofortification, nitrogen metabolism as well as the content of mineral nutrients and heavy metals in spinach plants (Spinacia oleracea L.). Sci. Hortic. 142:176-183. [ Links ]
Stein, A. 2009. Global impacts of human mineral malnutrition. Plant Soil. 335(1-2):133-154. [ Links ]
Tonacchera, M.; Dimida, A.; De Servi, M.; Frigeri, M.; Ferrarini, E. and De Marco, G. 2013. Iodine fortification of vegetables improves human iodine nutrition: in vivo evidence for a new model of iodine Prophylaxis. J. Clinical Endocr. Metab. 98(4):694-697. [ Links ]
Torremante, P. E. and Rosner, H. 2011. Antiproliferative effects of molecular iodine in cancers. Current Chemical Biology. 5(13):168-176. [ Links ]
Truesdale, V.W.; Luther, G.W. and Canosa, M. C. 1995. Molecular iodine reduction in seawater: an improved rate equation considering organic compounds. Marine Chem. 48(2):143-150. [ Links ]
Vir, S. C. 2002. Current status of iodine deficiency disorders (IDD) and strategy for its control in India. Indian J. Ped. 69(7):589-596. [ Links ]
Voogt, W.; Holwerda, H. T. and Khodabaks, R. 2010. Biofortification of lettuce (Lactuca sativa L.) with iodine: the effect of iodine form and concentration on growth, development and iodine uptake of lettuce grown in water culture. J. Sci. Food Agric. 90(5):906-913. [ Links ]
Weng, H. X.; Hong, C. L.; Yan, A. L.; Pan, L. H.; Qin, Y. C. and Bao, L.T. 2008. Mechanism of iodine uptake by cabbage: effects of iodine species and where it is stored. Biol. Trace Elem. Res. 125(1):59-71. [ Links ]
Weng, H. X.; Liu, H. P.; Li, D. W.; Ye, M.; Pan, L. and Xia, T. H. 2014. An innovative approach for iodine supplementation using iodine-rich phytogenic food. Environ. Geo. Health. 36(4):815-828. [ Links ]
Weng, H. X.; Yan, A. I.; Hong, C. L.; Xie, L. L.; Yong, W. B. and Qin, Y. C. 2008b. Increment of iodine content in vegetable plants by applying iodized fertilizer and the residual characteristics of iodine in soil. Biol. Trace Elem. Res. 123(1-3):218-228. [ Links ]
Weng, H. X.; Yan, A. I.; Hong, C. L.; Xie, L. L.; Qin, Y. C. and Cheng, C. H. 2008c. Uptake of different species of iodine by water spinach and its effect to growth. Biol. Trace Elem. Res. 124(2):184-194. [ Links ]
Weng, H. X.; Weng, J. K.; Yong, W. B.; Sun, X. W. and Zhong, H. 2003. Capacity and degree of iodine absorbed and enriched by vegetable from soil. J. Environ. Sci. 15(1):107-111. [ Links ]
Weng, H.; Hong, C.; Yan, A. and Ji, Z. 2013. Biogeochemical transport of iodine and its quantitative model. Sci. China Earth Sci. 56(9):1599-1606. [ Links ]
White, P. J. and Broadley, M. 2009. Biofortification of crops with seven mineral elements often lacking in human diet- Iron, zinc, copper, calcium, magnesium, selenium and iodine. New Phytologist 182(1):49-84. [ Links ]
Winter, J. M. and Moore, B.S. 2009. Exploring the chemistry and biology of Vanadium-dependent Haloperoxidases. J. Biol. Chem. 284(28):18577-18581. [ Links ]
Zanirato, V. and Mayerle, M. 2009. Method for enriching crops with iodine, and crops thus obtained. International Patent PCT/EP2009/050142. [ Links ]
Zhao, J.; Wang, P.; Shang, L.; Kevin, M.; Sullivan, K. M.; Van der Haar, F. and Maberly, G. 2000. Endemic goiter associated with high iodine intake. Am. J. Public Health. 90(10):1633-635. [ Links ]
Zhu, Y. G.; Huang, Y. Z.; Hu, Y. and Liu, Y. X. 2003. Iodine uptake by spinach (Spinacia oleracea L.) plants grown in solution culture: effects of iodine species and solution concentrations. Environ. Inter. 29(1):33-37. [ Links ]
Zimmermann, M. B. 2009. Iodine deficiency. Endocrine Reviews. 30(4):376-408. [ Links ]
Received: May 2016; Accepted: August 2016











 texto en
texto en 


