Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista mexicana de ciencias agrícolas
Print version ISSN 2007-0934
Rev. Mex. Cienc. Agríc vol.7 n.8 Texcoco Nov./Dec. 2016
Articles
Experimental evaluation of seed germination and emergence in purging nut from Totonacapan
1Universidad Autónoma Chapingo- Departamento de Fitotecnia y Preparatoria Agrícola, km 38.5 Carretera México-Texcoco, Chapingo, México. Tel. 595 952 16 14.
2Colegio de Postgraduados- Campus Montecillos, km 36.5. Carretera México-Texcoco. 56230, Montecillo, Texcoco, Estado de México.
Purging nut, Jatropha curcas L. (Euphorbiaceae) has different purposes: food, medicine, stake, shade and biofuels; despite the growing importance of the species worldwide, there is no accurate information relevant to its propagation. Aiming to evaluate: 1) germination; 2) emergence-time and percentage; 3) release of cotyledon leaves; and 4) seedlings type ten days after sowing (DAS); seeds from six accessions belonging to six elevation layers (191, 347, 542, 545, 610 and 630 masl), at three temperature (25, 30 and 35 °C) and two testa conditions (with and without) were used. At 30 °C germination occurred in 3.8 days, in accession A-630 masl germination, emergence and leave release was rapid (3.6, 4.6 and 7.4 days), while the highest percentage in the indicated variables was in A-347 (97.7, 96.3, 95.5%) and larger seedlings (16.7 cm) also to A-630, compared with other temperature and accessions collected in other elevations. Regarding to testa, germination, emergence and leave release occurred faster (3.8, 5.5 and 7.8 days) when removed; however, the highest germination percentage was in seeds with testa (88.5%). The results provide useful information to define technology leading to improve agronomic criteria for efficient cultivation.
Keywords: Jatropha curcas; biofuel; emergence; germination; initial growth
El piñón mexicano, Jatropha curcas L. (Euphorbiaceae), es utilizada para diferentes propósitos: alimento, medicina, tutor, sombra y biocombustibles. No obstante la creciente importancia de la especie a nivel mundial, aún se carece de información precisa pertinente a su propagación. Teniendo como objetivo evaluar experimentalmente: 1) la germinación; 2) emergencia -tiempo y porcentaje-; 3) liberación de hojas cotiledonares; y 4) el porte de plántulas a diez días después de siembra (DDS). Se utilizaron semillas de seis accesiones, provenientes de seis estratos de elevación (191, 347, 542, 545, 610 y 630 msnm), en tres niveles de temperatura (25, 30 y 35 °C) y dos condiciones de testa (con y sin). A 30 °C la germinación se presentó en 3.8 días, en la accesión A-630 msnm la germinación, emergencia y liberación de hojas fue rápida (3.6, 4.6 y 7.4 días), mientras que el mayor porcentaje en las variables indicadas se presentó en A-347 (97.7, 96.3, 95.5%) y las plántulas de mayor porte (16.7 cm), también de A-630, comparado con los otros niveles de temperatura y accesiones colectadas en otras elevaciones. En relación con la testa, la germinación, emergencia y liberación de las hojas ocurrió más rápido cuando se eliminó ésta (3.8, 5.5 y 7.8 días); sin embargo, el mayor porcentaje de germinación se observó en las semillas con testa (88.5%). Los resultados aportan información útil en la definición de la tecnología conducente al perfeccionamiento de los criterios agronómicos para su cultivo eficiente.
Palabras clave: Jatropha curcas; biocombustible; crecimiento inicial; emergencia; germinación
Introduction
Currently there are several major problems associated with fossil fuels such as pollution in the extraction process and its use (Adams et al., 1999; Di Toro et al., 2007), besides its exhaustion from natural deposits. Based on the above, the need to count with renewable energy sources is a priority. Plant species such as Jatropha curcas L., can prove to be important for the quantity and quality of oil content in its seeds, has excellent possibilities of use as domestic and industrial fuels (Foidl et al., 1996; Francis et al., 2005; Toral et al., 2008; Verma and Gaur, 2009).
J. curcas, commonly known as Mexican pinion, purging nut, naxtli (Nahuatl), Chu'ta (Totonaco) or tempate, belongs to the Euphorbiaceae family, fast growing and can reach 4 to 8 m (Heller, 1996) or more in fertile and moist soils. It is native to Mexico and Central America, and grows in most tropical and subtropical regions (Heller, 1996), areas with average annual rainfall of 250 - 1200 mm (Openshaw, 2000; Achten et al., 2008). In Mexico, it is found in ten states, among which are: Chiapas, Veracruz, Oaxaca, Morelos, Puebla, Yucatán and Hidalgo (CONAFOR, 2010), since climatic conditions in some of these regions are suitable for their development (SEMARNAT, 2010).
Depending on the analysis process, seeds may contain 35 to 40% oil (Kaushik et al., 2006), 27 to 32% protein (Makkar et al., 1998b), can be obtained up to 63% dry flour (Makkar et al., 1997). For these and other features, it is spreading for commercial and industrial purposes, including flour for animal feed (Aderibigbe et al., 1997). It also has medicinal properties (Duke, 1985; Kumar and Sharma, 2008). In some regions from Mexico, as in Totonacapan, the genotypes distributed there show domestication by its lack or low toxicity and used as human food (Makkar et al., 1998a). Previously toasted, the seeds are eaten alone, incorporated in the preparation of regional dishes, such as pipián, or accompanied with pot beans.
For their adaptive and easy growth characteristics, this species is used in soil remediation, to prevent erosion, reforestation of degraded areas (Ratree, 2004), as well as hedge row, stake for vanilla plant and shade for the same species or in coffee plantations (Kumar and Sharma, 2008), among other uses. Although J. curcas shows wide potential to increase its culture through somatic multiplication, which is relatively quick; still there are no accurate protocols covering the ideal conditions for seed germination, situation which among other aspects has limited their breeding, since, as is known, to achieve this purpose is essential to have genetic diversity arising from sexual reproduction (by botanical seed).
In addition to the above, J. curcas seed is processed to produce biodiesel, reason why is spreading massively, both vegetatively and by seed. However, according to several researchers and producers, the use of seeds to obtain seedling is the most common, especially on large plantations, as seedlings obtained by this process are more robust, longlived and resistant to disease and drought, due to the root system generated by these structures are more efficient than generated by twigs Saturnino et al. (2005).
Germination. The properties of J. curcas seeds are highly influenced by environmental conditions (Ginwal et al., 2005), besides having inhibitory substances, reason why must be dried to 5% moisture to increase the germination rate in short time. The germination rate of J. curcas ranges from 0 to 65% (Moncaleano-Escandon et al., 2013). Some authors mention that this heterogeneity is caused by exogenous dormancy induced by testa, being restricted to seed imbibition. In addition, germination and emergence can vary from days to months, demonstrating the physiological process mentioned beforfe, which apparently also influences the presence of caruncle. Another characteristic that needs proper management is storing, since the seeds lose their viability and germination potential, in periods as short as 6 months without environmental control, being able to restrict the viability period if temperature and humidity are increased (Moncaleano-Escandon et al., 2013).
Due to the above background, in this paper was raised as a general objective, to evaluate the responses of six different materials (genotypes), adapted to six different elevations, to pre-germination treatments indicated.
Materials and methods
The experiment was established in the laboratory “Banco Nacional de Germoplasma Vegetal” (BANGEV), located in the Department of Plant Science at the Autonomous University Chapingo. The tested plant material corresponded to six accessions from the Sierra Norte of Puebla and Veracruz state (Table 1), grown in the region called Totonacapan. The seeds used were previously dried to 5% in their tissues, for which silica gel with humidity indicator was used, comparing this level of dehydration through the method commonly known stove. The reduction of moisture in seed was conducted prior to storage for a period of six months in the cold room from BANGEV, at -18 °C and 15% RH.
Table 1 Accessions evaluated and stored in the National Plant Germplasm Bank.
| Accesiones | Paraje y municipio | Altitud (m) | Ubicación geográfica |
| A-191 | Martínez de la Torre, Ver. | 191 | 20º02’12’’ N, 97º05’44’’ O |
| A-347 | Tlapacoyan, Ver. | 347 | 19º58’36’’ N, 97º12’30’’ O |
| A-542 | San Rafael, Huehuetla, Pue. | 542 | 20º04’38’’ N, 97º38’12’’ O |
| A-545 | Tuzamapan, Pue. | 545 | 20º03’54’’ N, 97º34’39’’ O |
| A-610 | Ecatlán, Jonotla, Pue. | 610 | 20º03’30’’N, 97º33’51’’ O |
| A-630 | Zozocolco de Hidalgo, Ver. | 630 | 20º05’40’’ N, 97º40’37’’ O |
Seed preparation. Scarification was performed manually partially removing testa. Once testa was removed, the seeds were treated with a fungicidal solution (3 g L-1 Captan), for ten minutes, in order to inhibit the development of fungi in the structure.
Experiment establishment. The experiment was carried out in a germination chamber, Seedburo, model MPG3000, where trays with Peat moss® at 80% humidity at temperatures: 25°, 30° and 35 °C were introduced. Once the seeds are placed in each well, were covered with a substrate layer of 1 cm, to keep moisture.
Data collection. The variables evaluated were: 1) days to germination; 2) germination rate; 3) days to emergence; 4) emergence rate; 5) days to release of cotyledon leaves; and 6) seedling size ten days after planting.
Days to germination: days from sowing to seed germination, it was considered germinated seed when it showed a growth of the taproot, with an approximate length of 1 cm. Germination rate: was calculated by counting the germinated seeds compared to seeds placed initially in the experiment. Days to emergence: days from sowing to seedling emergence with cotyledon leaves. It was considered emerged plant that whose cotyledon leaves could be seen on the substrate surface. Emergence rate: was determined counting the number of seedlings that emerged in relation to total amount of seed placed in the experiment. Days to release of cotyledon leaves: this stage was determined in those seedlings in which where observed fully developed cotyledon leaves. Percentage of seedlings that developed true leaves: is the seedling ratio achieving to release true leaves, taking into account that the leaves were complete and able to photosynthesize, from the total seeds placed in the experiment. Seedling size to ten days from emergence: this variable was determined by measuring the plants from the neck to the apex thereof.
Experimental design. The design was randomized complete blocks with three replications. The evaluated factors were: temperature, with three levels, 25, 30 and 35 ° C, testa treatment with two levels: without testa “ST”, with testa “CT” and six accessions: A-191, A-347, A -542, A-545, A-610 and A-630.
Data analysis. The results were analyzed using the SAS statistical package, with the GLM procedure and means comparison Tukey (α= 0.05).
Results and discussion
According to the analysis of variance performed to the experiment data, statistically significant differences (α= 0.05) were observed in different variables, by effect of pre-germination, temperature and accessions (Table 2 and 3). By pre-germination treatment there was an effect on variables days to germination (Table 2), days to leave release and seedling size at 10 days (Table 3). By temperature, there were differences in: days to germination, days to emergence (Table 2), days to release of cotyledon leaves, and seedling size at 10 days after germination (Table 3). Among accessions, differences were observed in all variables. In factors interaction, there were only statistical difference in seedlings size in interaction TEMP x TES (Table 3).
Table 2 Analysis of variance of days to germination and emergence and germination and emergence rate.
| FV | GL | DGER | P | PGER | P | DEMER | P | PEMER | P |
| ACC | 5 | 10.2 | <.0001* | 8.25 | <.0001* | 11.07 | <.0001* | 4.89 | 0.0007* |
| TES | 1 | 11.41 | 0.0012* | 0.18 | 0.6703 | 0.07 | 0.7939 | 2.03 | 0.1587 |
| TEMP | 2 | 24.16 | <.0001* | 0.1 | 0.9045 | 43.7 | <.0001* | 0.94 | 0.394 |
| TEMP*ACC | 10 | 0.45 | 0.9145 | 1.09 | 0.384 | 1.65 | 0.111 | 1.55 | 0.139 |
| ACC*TES | 5 | 0.88 | 0.4972 | 1.65 | 0.1589 | 1.13 | 0.3532 | 0.78 | 0.5692 |
| TEMP*TES | 2 | 0.11 | 0.8917 | 0.95 | 0.3923 | 0.68 | 0.5101 | 0.04 | 0.9587 |
| TEMP*ACC*TES | 10 | 0.66 | 0.76 | 0.76 | 0.6649 | 1.11 | 0.3652 | 0.72 | 0.7046 |
| Error | 72 | 0.384 | 214.12 | 0.538 | 262.962 | ||||
| CV | 13.85 | 16.87 | 11.4 | 19.5 |
Significativa (p≤ 0.05). FV= fuente de variación; GL= grados de libertad; DGER= días a germinación; PGER= porcentaje de germinación; DEMER: días a emergencia; PEMER= porcentaje de emergencia; ACC= accesión; TES= condición de testa; TEMP= nivel de temperatura; CV= coeficiente de variación.
Table 3 Analysis of variance of days to release of cotyledon leaves, seedlings rate of plants that released true leaves and plant size.
| FUENTE | GL | DLH | P | PLH | P | ALTP | P |
| ACC | 5 | 7.64 | <.0001* | 4.03 | 0.0028* | 9.8 | <.0001* |
| TES | 1 | 6.23 | 0.0148* | 1.43 | 0.2359 | 99.7 | <.0001* |
| TEMP | 2 | 35.33 | <.0001* | 1.13 | 0.3291 | 10.29 | 0.000* |
| TEMP*ACC | 10 | 1.55 | 0.4405 | 1.63 | 0.1166 | 1.46 | 0.1727 |
| ACC*TES | 5 | 0.19 | 0.9649 | 0.81 | 0.5454 | 1.95 | 0.0959 |
| TEMP*TES | 2 | 1.09 | 0.3432 | 0.7 | 0.4999 | 3.41 | 0.0383* |
| TEMP*ACC*TES | 10 | 1.48 | 0.1635 | 1.16 | 0.3299 | 1.41 | 0.1949 |
| Error | 72 | 0.542 | 259.259 | 5.171 | |||
| CV | 8.36 | 19.13 | 15.48 |
Significativa (p≤ 0.05). FV= fuente de variación; GL= grados de libertad; DLH= días a liberación de hojas cotiledonares; PLH= porcentaje de plántulas que liberaron hojas cotiledonares; ALTP= porte media de las plántulas. ACC= accesión; TES= condición de testa; TEMP= nivel de temperatura; CV= coeficiente de variación.
At 30 °C obtained the best response to germination, with lower time (3.8 days), while at 25 °C and 35 °C, germination response was up to 4.6 days (Figure 1A). As for germination rate, the best response was recorded at 25 °C (88.7%), followed by temperatures of 30 and 35 °C, with 87.7 and 87.2%, respectively (Figure 2A).
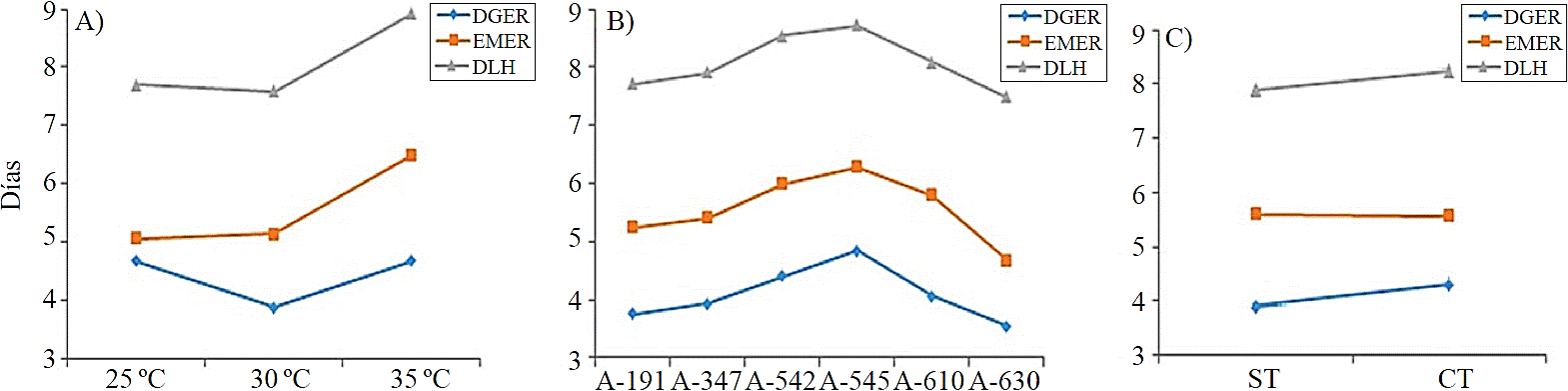
Figure 1 Days to germination, emergence and release of cotyledon leaves by the effect of temperature (A), accessions (B), and testa removal (C).
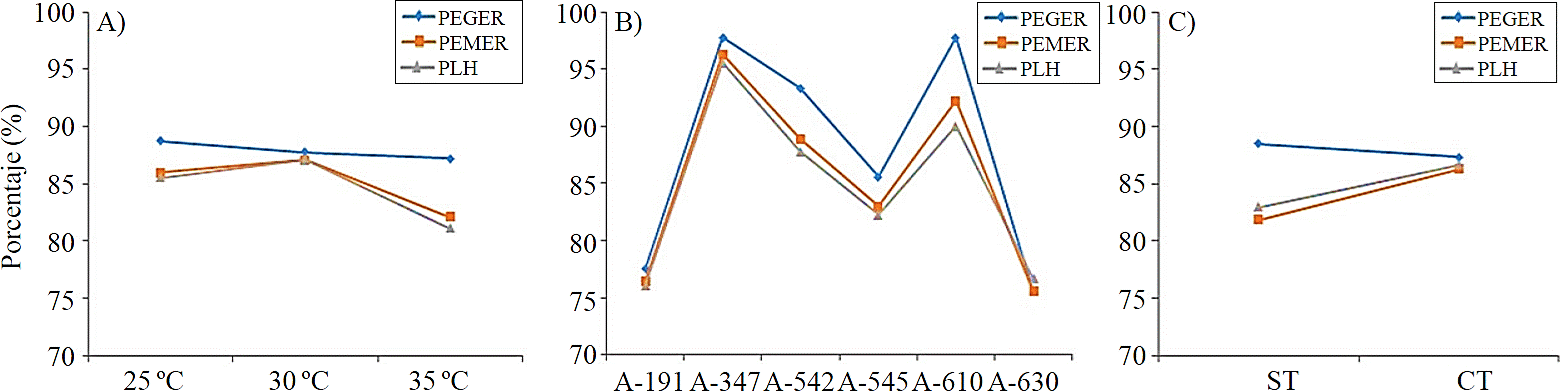
Figure 2 Germination, emergence and release of cotyledon leaves rate by the effect of temperature (A), accessions, (B) and testa (C).
The best response for seed germination was recorded at 25 and 30 °C. This results are consistent with those reported by Windauer et al. (2012) who studied the responses of seed germination of J. curcas at different temperatures associated with different water potential, noting that at 25 °C seeds have the highest germination rate (82%) and seedlings with good initial development, compared with treatment at 30 °C. Although for the latter, germination and emergence occurred faster and seedlings suffered elongation. Trindade-Lessa et al. (2015), in studies on seeds of Enterolobium contortisliquum (Vell) Morong, found that germination was higher at temperatures from 25 to 30 °C (> 80%), while at 40 °C it was detrimental to seedling initial growth, showing that temperature control is important during germination, because stimulates metabolic activity and availability of some substances for hypocotyl to start growing, also a stable temperature aids to promote uniform imbibition of seeds, favoring uniform germination (Murcia et al., 2006). However, it is important to consider that in other genres, the highest germination rate is obtained under fluctuating temperature conditions, as in the case of some species of Bursera sp. (Bonfil-Sanders et al., 2008).
The effect of the pre-germinative treatment showed significant differences (p≤ 0.05) in seed with testa, germination was at 4.2 days, while seed without testa germination was at 3.8 days (Figure 1C). However, the highest germination rate obtained was in seeds with testa (88.5%) (Figure 2C), nevertheless, in seed without testa, there were no statistically significant differences in emergence, appearing at 5.5 days in both conditions (Figure 1C); while seedling rate emerged from seeds with testa was higher (87.2%) and 83.8% in those without testa (Figure 2C).
The separation of testa from seed or scarification, is a treatment often used in fruit or species with seeds of hard testa that prevents free water absorption, since in seeds with these features germination is delayed or it is presented but very uneven. Pavón et al. (2011), by studying germination and establishment of Mimosa aculeaticarpa (Willd) recorded that seeds which were scarified showed higher germination rate, and even better results when this treatment is combined with temperatures of 25 to 30 °C. However, sometimes pre-germinative treatments have little effect on speed or percentage of this process, but can lead to homogeneous germination (Brevis, 2003).
Seeds from low elevation sites (191 masl) germinated at 1.1 days and those from higher elevation (630 masl) germinated at 3.6 days on average; while accessions from intermediate elevations (350- 600 mals), germination occurred between 4 to 4.8 days (Figure 1B).
Germination rate between accessions, showed significant differences, having: A-630 was 75.5%, A-347 and A-610 had germination 97.7%, A-545 and A-542, percentages of 85.5 and 93.3%, respectively (Figure 2B). However, these data also involved different collection sites.
Accessions showed statistically significant differences in both days to germination (4 to 5 days), and rate (75 to 100%). This may be because the conditions where seeds were collected, i.e., the conditions under which are the mother plants are contrasting (Baskin and Baskin, 2001; Windauer et al., 2012). The results obtained here coincide with those reported by Hernández-Verdugo et al. (2010), assessing wild pepper seeds collected in different environments in which detected differences in germination patterns due largely to environmental conditions of growth of the mother plant.
Seedling emergence also showed significant effect (p≤ 0.05), primarily on accessions and temperature. For this variable, the best levels of temperature were 25 °C and 30 °C, as seedlings emerged at 5 and 5.1 days after sowing, respectively, whereas at 35 °C, was at 6.4 days (Figure 1A). Germination rate were 88.1 and 82.1% at 30 °C and 35 °C, respectively (Figure 2A), this is consistent with that reported by Herrera and Elizaga (1995), studying the germination of seed from China (Impatiens balsamica), where the germination rate index increases to the optimum temperature where it stabilizes and then begins to decline. In relation to the emergence among accessions, there were statistically significant differences, showing: accession A-630 emerged at 4.6 days, followed by A-347 and A-191 at 5.4 and 5.2 days, respectively; while A-545, emerged at 6.2 days (Figure 1B).
Regarding emergence rate, accessions A-347 and A-610 recorded 96.3 and 92.2%, respectively, being these accessions with the highest values for this variable; where A-630 and A-191 are the accessions with the lowest rate, 75.5 and 76.4%, respectively (Figure 2B).
Seed germination is an important process in the propagation of a plant that should lead to the establishment of a seedling and to achieve it, should end with emergence. Emergence in many cases is related to seed vigor; i.e. the amount of reserves contained (Windauer et al., 2012), the response to the environment or conditions that are provided (Baskin and Baskin, 2001; Pavón et al., 2011) and health thereof. Gally et al. (2004), performing test with soybean seeds determined that seedling emergence increases by treating them with fungicides, because many of these structures germinate even if this have little vigor, but if infected by any pathogen, the seedling does not emerge. In this regard Vázquez et al. (2011) reported differences in emergence and early seedling development in three maguey species. Godínez-Ibarra et al. (2007), in studies on nurseries of Fagus grandifolia Ehrh., observed that germination and seed emergence does not guarantee the survival of seedlings, since these can be invaded by diseases, consumed by herbivores or affected by adverse environment.
The release of cotyledon leaves was in the first week after planting, with statistically significant differences (p≤ 0.05) between treatments. By effect of temperature, days to release of cotyledon leaves were recorded at 7.5, 7.7 and 8.9 days after sowing, at temperatures of 30°, 25° and 35 °C, respectively (Figure 1A).
As for release rate of cotyledon leaves, at 30 °C recorded the highest value 87.2%, followed by 25 °C and the lowest 35 °C (Figure 2A). Among the accessions, there were statistically significant differences (p≤ 0.05), in which accession A-630 released their leaves in less time (7.4 days), while A-545 did it in 8.7 days (Figure 1B).
In relation to the previous variable, the effect of scarification in this showed statistical differences (Table 3), having that seeds without testa released the leaves before (7.8 days) than seeds with testa (8.2 days) (Figure 1C). However, after several days to release of the cotyledon leaves and seedlings size is no longer related at all with the characteristics of the seed (Vázquez et al., 2011) and seedling survival will depend on other factors mainly environmental. On the other hand, when a young plant emerges and establishes it increases the probability of survival. Whereas, if seed is less vigorous germination and emergence takes longer, seedling competition for light and space is stronger and possible presence of diseases, which hinder further development (Godínez-Ibarra et al., 2007); although sometimes seedlings size in the first days is also influenced by factors such as temperature, which can change the relationship between root and aerial parts (Murcia et al., 2006). Zitacuaro and Aparicio (2004) found striking differences in seedling size and diameter of Pinus oaxacana Mirov from three localities in Mexico. Genetic differences are one of the factors that promote diversity in seedling size at its initial development. Alba et al. (2003) report differences in growth of P. oaxacana from different environments, consistent with that observed in this study.
In variable seedling size at 10 days after sowing, recorded means from 11 to 16 cm, by effect of temperature, testa removal and accessions, finding statistically significant differences; both in simple factors and interaction TEMP x TES. Considering temperature, at 30 °C obtained an average size of 15.5 cm at ten days after planting, whereas at 35 °C was 13.1 cm. These results are consistent with those reported by Murcia et al. (2006), when testing sunflower germination at different temperatures recorded seedlings with larger size and dry weight at low temperatures, whereas at higher temperatures had lower weight.
In the interaction 35 °C and seed without testa, average plant height was 19.6 cm, unique outstanding interaction. When considering accessions, the highest average size was 16.7 cm, corresponding to A-630, and the lowest 11.7 cm for A-610. In seeds with testa, seedlings recording higher growth, with an average of 16.3 cm and 12 cm were those derived from seed without testa, this may be because sometimes the availability of reserves from cotyledons is transferred to the formation of structures or used for respiration (Simon and Meany, 1965).
Conditions such as temperature, humidity, light, nutrients, growth period, defoliation, fruit location on the tree, among others interact with plant genome, resulting in different values, related to seed size, color, shape, vigor and germination potential. These variations on growth conditions of seed make them to have different responses in germination, emergence and early seedling growth and from this conditions depend its survival; since part of the conditions of its origin depend the reserves that such seed possesses (Baskin and Baskin, 2001; Ginwal et al., 2005). Therefore affects germination rate, days to emergence, growth (Cordazzo, 2002) and initial size of the seedlings (Moegenburg et al., 1996).
The environment in many cases determines seed behavior and this reflects in germination, emergence and early seedling development, thus in dormancy after harvest (Windauer et al., 2012). The dynamic response of plants to variations in environmental conditions it is observed in adaptability, necessary to develop under different environments, similarly to survive the changes in climatic conditions, thus express the genetic diversity of the species (Ginwal et al., 2005).
Conclusions
According to experiment results, it´s deduced that Jatropha curcas seeds are highly influenced in the germination, emergence and growth process, by effect of temperature and pregerminative treatment, deducing following.
The right temperature to propagate J. curcas seed is from 25 to 30 °C, since germination and emergence is faster and occurs at a higher rate.
The pre-germinative treatment (testa removal) stimulates germination and emergence, as it allows water absorption without limitations. However, untreated seeds (seeds with testa) increase germination and emergence rate.
Literatura citada
Achten, W. M. J.; Verchot, L.; Franken, Y. J.; Mathijs, E.; Singh, V. P.; Aerts, R. and Muys, B. 2008. Jatropha bio-diesel production and use. Bio. Bioen. 32:1063-1084. [ Links ]
Adams, R. H.; Domínguez, V. y García, L. 1999. Potencial de la biorremediación de suelo y agua impactados por petróleo en el trópico mexicano. Terra. 17:159-174. [ Links ]
Aderibigbe, A. O.; Johnson, C. O. L. E.; Makkar, H. P. S.; Becker, K. and Foidl, N. 1997. Chemical composition and effect of heat on organic matter -and nitrogen-degradability and some antinutritional components of Jatropha meal. Animal Feed Science Technology. 67:223-243. [ Links ]
Alba, L. J.; Mendizábal, H. L. y Aparicio, R. A. 2003. Estudio de germinación y plántulas de tres poblaciones de Pinus oaxacana Mirov de México. Foresta Veracruzana. 5(1):33-38. [ Links ]
Baskin, C. C. and Baskin, J. M. 2001. Seeds: ecology, biogeography, and evolution of dormancy and germination. Academic Press. San Diego, CA, USA. [ Links ]
Bonfil-Sanders, C.; Cajero-Lázaro, I. y Evans, R. Y. 2008. Germinación de semillas de seis especies de Bursera del Centro de México. Agrociencia. 7(42):827-834. [ Links ]
Brevis, A. P. 2003. Efecto de tratamiento pregerminativo sobre la germinación de semillas de Eucryphia glutinosa (Poepp. et Endl.) Baillon. Bosque. 2(24):79-84. [ Links ]
CONAFOR. 2010. A3. Plantaciones forestales comerciales.- municipios elegibles para las plantaciones de Jatropha curcas. In: convocatoria de reglas de operación 2010: Términos de Referencia. http://www.conafor.gob.mx/portal2/index.php?option=com_content&task=view&id=454&Itemid=527. [ Links ]
Cordazzo, C. V. 2002. Effect of seed mass on germination and growth in three dominant species in southern Brazilian coastal dunes. Braz. J. Bot. 3(62):427-435. [ Links ]
Di Toro, D. M.; McGrath, J. A. and Stubblefield, W. A. 2007. Predicting the toxicity of neat and weathered crude oil: toxic potential and the toxicity of saturated mixtures. Environ. Toxicol. Chem. 26:24-36. [ Links ]
Duke, J. A. 1985. Medicinal plants. Science. 229:1036-1039. [ Links ]
Foidl, N.; Foidl, G.; Sanchez, A.; Mittelbach, M. and Hackel, S. 1996. Jatropha curcas L. as a source for the production of biofuel in Nicaragua. Bio. Technol. 58:77-82. [ Links ]
Francis, G.; Edinger, R. and Becker, K. 2005. A concept for simultaneous wasteland reclamation, fuel production, and socio-economic development in degraded areas in India: need, potential and perspectives of Jatropha plantations. Natural Res. Forum. 29:12-24 [ Links ]
Gally, T.; Pantuso, F. y González, B. 2004. Emergencia de plántulas de soya (Glycine max (L.) de semillas tratadas con fungicidas en tres periodos agrícolas. Rev. Mex. Fitopatol. 3(22):377-381. [ Links ]
Ginwal, H. S.; Phartyal, S. S.; Rawat, P. S. and Srivastava, R. L. 2005. Seed source variation in morphology, germination and seedling growth of Jatropha curcas Linn. in Central India. Silvae Genetica. 2(54):76-80. [ Links ]
Godínez-Ibarra, O.; Ángeles-Pérez, G.; López-Mata, L.; García-Moya, E.; Valdez-Hernández, J. I.; De Los Santos-Posadas, H. y TrinidadSantos, A. 2007. Lluvia de semillas y emergencia de plántulas de Fagus grandifolia subesp. mexicana en la Mojonera, Hidalgo, México. Rev. Mex. Biod. 1(78):117-128. [ Links ]
Heller, J. 1996. Physic nut. Jatropha curcas L. Promoting the conservation and use of underutilized and neglected crops. Institute of Plant Genetics and Crop Plant Research, Gatersleben/ International Plant Genetic Resources Institute, Rome. [ Links ]
Hernández-Verdugo, S.; López-España, R. G.; Porras, F.; Parra-Terra za, S.; Villarreal-Romero, M. y Osuna-Enciso, T. 2010. Variación en la germinación entre poblaciones y plantas de chile silvestre. Agrociencia. 6(44):667-677. [ Links ]
Herrera, J. y Alizaga, R. 1995. Efecto de la temperatura sobre la germinación de la semilla de china (Impatiens balsamina). Agron. Costarric. 19:79-84. [ Links ]
Kaushik, N.; Roy, S. y Biswas, G. C. 2006. Screening of Indian Jatropha curcas for selection of high oil yielding plants. Indian J. Agrof. 2(8):54-57. [ Links ]
Kumar, A. and Sharma, A. 2008. An evaluation of multipurpose oil seed crop for industrial uses (Jatropha curcas L.): A review. Industrial Crops and Products. Elsevier. 28:1-10. [ Links ]
Makkar, H. P. S.; Becker, K. and Schmook, B. 1998a. Edible provenances of Jatropha curcas from Quintana Roo state of Mexico and effect of roasting on antinutrient and toxic factors in seeds. Plant Foods Human Nutrition. 52:31-36. [ Links ]
Makkar, H. P. S.; Aderibigbe, A. O. and Becker, K. 1998b. Comparative evaluation of non-toxic and toxic varieties of Jatropha curcas for chemical composition, digestibility, protein degradability and toxic factors. Food Chem. 2(62):207-215. [ Links ]
Makkar, H. P. S.; Becker, K.; Sporer, F. and Wink, M. 1997. Studies on nutritive potential and toxic constituents of different provenances of Jatropha curcas. J. Agric. Food Chem. 8(45):3152-3157. [ Links ]
Moegenburg, S. M. 1996. Sabal palmetto seed size-causes of variation, choices of predators, and consequences for seedlings. Oecologia. 106: 539-543. [ Links ]
Moncaleano-Escandon, J.; Silva, B. C. F.; Silva, S. R. S.; Granja, J. A. A.; Alves, M. C. J. L. and Pompelli, M. F. 2013. Germination responses of Jatropha curcas L. seeds to storage and aging. Industrial Crops Products. 44:084-090. [ Links ]
Murcia, M.; Del Longo, O.; Argüello, J.; Pérez, M. A. y Peretti, A. 2006. Evaluación del crecimiento de plántulas de cultivares de girasol con diferentes proporciones de ácidos oleico/linoleico en respuesta a la baja temperatura. Rev. Bras. Sementes. (2)28:95-101. [ Links ]
Openshaw, K. 2000. A review of Jatropha curcas: an oil plant of unfulfilled promise. Bio. Bioen. 19:1-15. [ Links ]
Pavón, N. P.; Ballato-Santos, J. y Pérez-Pérez, C. 2011. Germinación y establecimiento de Mimosa aculeaticarpa var. Biuncifera (Fabaceae-Mimosoideae). Rev. Mex. Biod. 2(82):653-661. [ Links ]
Ratree, S. 2004. A preliminary study on physic nut (Jatropha curcas L.) in Thailand. Pak. J. Biol. Sci. 9(7): 1620-1623. [ Links ]
Saturnino, H. M.; Pacheco, D.; Kakida, J.; Tominaga, N. e Gonzales, N. P. 2005. Cultura do Pinhão Manso (Jatropha curcas L.). Informe Agropecuario. Belo Horizonte. 229(26):44-78. [ Links ]
Secretaría de Medio Ambiente y Recursos Naturales (SEMARNAT). 2010. Ficha técnica Jatropha. http://www.semarnat.gob.mx/pfnm2/fichas/jatropha_curcas.htm. [ Links ]
Simon, E. W. and Meany, A. 1965. Utilization of reserves in germinating Phaseolus seeds. Plant Physiology, Washington. 40:1136-1139. [ Links ]
Toral, O. C.; Iglesias, J. M.; Montes de Oca, S.; Sotolongo, J. A.; García, S. y Torsti, M. 2008. Jatropha curcas L., una especie arbórea con potencial energético en Cuba. Pastos y Forrajes. 3(31):191-207. [ Links ]
Trindade-Lessa, D. F.; Nobre-de Almeida, J. P.; Lobo-Pinheiro, C.; Melo-Gomes, F. y Medeiros-Filho, S. 2015. Germinación y crecimiento de plántulas de Entorolobium contortisiliquum en función del peso de la semillas y las condiciones de temperatura y luz. Agrociencia. 3(49):315-327. [ Links ]
Vázquez, D. E.; García, N. J. R.; Peña, V. C. B.; Ramírez, T. H. M. y Morales, R. V. 2011. Tamaño de la semilla, emergencia y desarrollo de la plántula de maguey (Agave salmiana Otto ex Salm-Dyck). Rev. Fitotec. Mex. 3(34):167-173. [ Links ]
Verma, K. C. and Gaur, A. K. 2009. Jatropha curcas L.: Substitute for conventional energy. World J. Agric. Sci. 5(5):552-556. [ Links ]
Windauer, L. B.; Martinez, J.; Rapoport, D. and Benech-Arnold, R. 2012. Germination responses to temperature and water potential in Jatropha curcas seeds: a hydrotime model explains the difference between dormancy expression and dormancy induction at different incubation temperatures. Ann. Bot.109:265-273. [ Links ]
Zitacuaro, C. F. H. and Aparicio, R. A. 2004. Variación de altura y diámetro de plántulas de Pinus oaxacana Mirov de tres poblaciones de México. Foresta Veracruzana. 6:21-26. [ Links ]
Received: September 2016; Accepted: November 2016











 text in
text in 


