Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista mexicana de ciencias agrícolas
Print version ISSN 2007-0934
Rev. Mex. Cienc. Agríc vol.7 n.8 Texcoco Nov./Dec. 2016
Articles
Estimation of genetic parameters and selection of S1 lines in a segregating population of tropical maize
1Universidad Autónoma del estado de Morelos-Facultad de Ciencias Agropecuarias. Av. Universidad Núm. 1001, Col. Chamilpa, Cuernavaca, Morelos. CP. 62209. Tel. 777 329 7981.
2Universidad Autónoma del Estado de México.
In Mexico 7.5 million hectares of corn are grown, with an average grain yield of 3.2 t ha-1, a strategy to increase yield is the use of improved varieties. The objectives of this study were 1) estimate genetic parameters in a segregating population of tropical maize; 2) select S1lines based on grain yield and earliness; and 3) determine the phenotypic and genetic correlation between traits. The germplasm under study consisted of 193 segregating S1 lines, parents and a control, S1 lines come from the F2 population from crossing Ac7643 x B-39. The lines were evaluated under irrigation using an alpha lattice design with two replications during the spring-summer 2013 and autumn-winter 2013/2014 cycle, in Ayala, Morelos. The variables recorded were days to male (FM) and female silking (FF), floral synchrony (SF), plant height (AP) and cob (AM) and grain yield (RG). The results indicate that the coefficient of additive genetic variation for RG was 17% and for FM 4%; heritability for the same traits was 0.42 and 0.80, respectively. The expected genetic gain for RG was 0.143 t ha-1 and for FM -0.62 d. The LUM-80 line turned with positive transgressive inheritance in RG. Phenotypic and genetic correlations between RG and the rest of the traits were low. The high degree of genetic variability present in the segregating population allows continuing with the breeding program.
Keywords: genetic gain; heritability; transgressive inheritance
En México se cultivan 7.5 millones de hectáreas con maíz, con un rendimiento de grano promedio de 3.2 t ha-1, una estrategia para incrementar el rendimiento es el uso de variedades mejoradas. Los objetivos del presente estudio fueron: 1) estimar los parámetros genéticos en una población segregante de maíz tropical; 2) seleccionar líneas S1 con base en el rendimiento de grano y precocidad; y 3) determinar la correlación fenotípica y genética entre caracteres. El germoplasma de estudio fueron 193 líneas S1 segregantes, los progenitores y un testigo, las líneas S1 provienen de la población F2 de la cruza de Ac7643 x B-39. Las líneas se evaluaron bajo riego usando un diseño látice alfa con dos repeticiones, en los ciclos primavera-verano 2013 y otoño-invierno 2013/2014, en Ayala, Morelos. Las variables registradas fueron días a floración masculina (FM) y femenina (FF), sincronía floral (SF), altura de planta (AP) y mazorca (AM), y rendimiento de grano (RG). Los resultados indican que el coeficiente de variación genético aditivo para RG fue de 17% y en FM de 4%; la heredabilidad para los mismos caracteres resultó de 0.42 y 0.80, respectivamente. La ganancia genética esperada para RG fue de 0.143 t ha-1 y para FM de -0.62 d. La línea LUM-80 resultó con herencia transgresiva positiva en RG. Las correlaciones fenotípicas y genéticas entre RG y el resto de caracteres fueron bajas. El alto grado de variabilidad genética presente en la población segregante permite continuar con el programa de mejoramiento genético.
Palabras clave: ganancia genética; heredabilidad; herencia transgresiva
Introduction
In Mexico 7.5 million hectares are cultivated with corn, an average yield of 3.2 t ha-1 (SIAP-SAGARPA, 2013). A strategy to increase grain yield of corn nationwide is the use of improved varieties, which can be free, synthetic or hybrid pollination (Hallauer and Miranda, 1988). In the formation of open-pollinated varieties, intra-population breeding schemes are used by recurrent selection, where the main objective is to increase the frequency of favorable alleles in the population (Hallauer, 1981). Knowledge of the magnitude of genetic parameters of the selection base population, allows designing the best strategy to follow in breeding programs (Rovaris et al., 2011).
The selection base populations can be formed from the cross between contrasting inbred lines, from crosses between improved varieties, through crossing a group of lines or populations (gene pool), or may be native maize populations (panmictic populations). Germplasm combinations derived from native populations x improved varieties can also be germplasm base for breeding programs in maize (Dzib-Aguilar et al., 2011).
Corn hybrid was one of the first examples of genetic theory successfully applied in food production, the genetic basis of heterosis remains unknown (Duvick, 2001). The basis for commercial hybrids formation are inbred lines of good combining ability; however, the success of obtaining outstanding lines, depends on the level of genetic variability present in the segregating population and of gene frequency of alleles of interest (Borel et al., 2013).
In breeding programs it is desirable to count with genetic knowledge of base populations to facilitate selection of top lines. To choose the plant breeding scheme, it is necessary to know the type of inheritance or gene specific action for quantitative traits of agronomic interest (Peyman et al., 2009). The main objective of breeding programs is to increase grain yield; there are other selection criteria for traits in maize genotypes that can be useful to plant breeders, such as: i) greater permanence of green leaves; ii) reduced spike size; and iii) erect leaves (Chapman and Edmeades, 1999; Edmeades et al., 1999).
The estimation of genetic parameters in segregating maize populations provides information on the genetic variation present in the population and supports the progress of the selection process. The proportion of the genetic variance that is heritable is broke down in an additive portion associated with the average effect of gene effects, a portion of dominance, due to intralocus allelic interaction and to epistasis (Hallauer, 1981). Additive variance measures the amount of variation present in the population which is due to additive gene effects, while the coefficient of genetic additive variation allows quantifying the extent of genetic variation that can be exploited by recurrent selection (Rovaris et al., 2011). The estimate of heritability is essential to predict the selection response in a population; such response is defined as a change in the population mean of the next generation. Broad-sense heritability was determined for plant height in S1lines from seven corn populations and had an expression ranged of 0.58 to 0.8 (Garbuglio et al., 2009).
The estimate of the association among traits is useful in plant breeding because it allows estimating the selection effect in two or more traits and performs indirect selection based on a trait easy to measure. The main cause of the genetic correlation between traits is pleiotropy and a transient cause genetic linkage, especially in populations derived from highly divergent lines (Falconer and Mackay, 1996). The purpose of developing S1lines from segregating populations is to exploit the effect of transgressive inheritance in traits of agronomic interest, which would allow selecting lines that show greater phenotypic expression than that from its best parent, leading to identify inbred outstanding lines. The objectives of this research were: 1) to estimate genetic parameters in a segregating population of tropical maize; 2) select S1lines based on grain yield and earliness in male flowering; and 3) determine the phenotypic and genetic correlation between traits.
Materials and methods
The genetic material was constituted by 193 S1lines, plus Ac7643, B39 and Ac7729/TZSRW. Tropical inbred lines Ac7643 and Ac7729 / TZSRW were provided by the International Maize and Wheat Improvement Center (CIMMYT) and the sub-tropical B39 line by the National Institute of Forestry, Agriculture and Livestock (INIFAP). The S1 lines come from an F2 generation from the initial cross between the lines Ac7643 x B39, performed in the spring-summer 2010, in the maize breeding program from the Escuela de Estudios Superiores of Xalostoc (EESuX) belonging to the Universidad Autonoma del Estado de Morelos.
In the experimental field of EESuX during the springsummer 2012 cycle, F1 generation was planted on a field of 500 m2, at the time of flowering a self-pollination of 120 F1 plants was performed, and at harvest 80 cobs were chosen based on their health, were shelled and mixed to form the F2 population. Finally, the F2 seed was sown in the autumnwinter 2012-2013 cycle in a field of 700 m2 and 250 not selected plants were self-pollinated to derive the 193 S1lines, which make up the segregating population under study in this research.
The evaluation of the 196 lines was conducted under irrigation conditions during the spring-summer 2013 and autumn-winter 2013-2014 cycles in the experimental field from EESuX, located in Ayala, Morelos, with geographic location of 18° 46’ 01’’ north latitude and 98° 58’ 96’’ west longitude, an altitude of 1 218 masl, annual rainfall of 885 mm and warm tropical sub-humid climate (INEGI, 2014). The experimental design used in both agricultural cycles was a simple alpha lattice of 14 x 14 with two replications. The experimental unit was composed of a groove 5 m long, 0.8 m between rows and 0.25 m between plants. The response variables were: male (FM) and female (FF) flowering, both were determined by the days elapsed since emergence until 50% plus one of the plants in the experimental unit was in anthesis and stigmata emergence, respectively. Flowering synchrony (SF) was determined by the difference in days over FM and FF. Plant (AP) and cob (AM) height were expressed in cm and consisted in measuring the length from the knot of adventitious roots to the knot from flag leaf and insertion knot of the main cob, respectively. Grain yield (RG) was determined at 15.5% moisture, an adjustment per covariance for each environment between RG and number of harvested plants was performed, and it was expressed in t ha-1.
The variables measured were tested for normality and it was necessary to normalize only the variable SF, through transformation:
Results and discussion
The analysis of variance (Table 1) in the sources of variation of environments and lines detected statistical differences (p≤ 0.01 and p≤ 0.05) in all the traits, while for lines x environments interaction no significant differences were observed for plant height and cob traits. The coefficients of variation ranged between 2.50 and 24.20%, these values correspond to male flowering and grain yield, respectively. The statistiwcal differences between environments can be attributed to the effects of temperature and relative humidity, since the experiments were conducted in the same locality, but in different agricultural cycles, where temperatures ranged from 21.8 to 19.5 °C and 20.0 to 23.4 °C, respectively; while relative humidity ranged from 78.1 to 74.7 and 54.2 to 62.3%, respectively (SEMARNAT, 2015). Due to random fluctuation of the environment, it is necessary to perform the agronomic evaluation of maize through different environments, which would allow a more accurately estimate of the genetic components value and separate environmental-genetic effect (Gutiérrez et al., 2004; Alejos et al., 2006).
Table 1 Mean squares of the variables measured in Cd. Ayala, Mor. Spring-summer 2013 and autumn-winter 2013-2014.
| FV | GL | FM | FF | SF | AP | AM | RG |
| (d) | (d) | (trans.)† | (cm) | (cm) | (t ha-1) | ||
| A | 1 | 5907.02** | 3915.18** | 0.249** | 11393.53** | 11006.26** | 40.34** |
| R/A | 2 | 13.43* | 36.86** | 0.02** | 8391.82** | 3450.03** | 1.38** |
| B/A x R | 52 | 7.29** | 12.99** | 0.004** | 329.96** | 91.54** | 0.24** |
| Líneas (L) | 195 | 51.98** | 58.26** | 0.004** | 1032.44** | 446.38** | 0.48** |
| L x A | 195 | 10.36** | 11.19** | 0.004** | 33.01NS | 12.48NS | 0.28** |
| Error | 338 | 3.35 | 3.93 | 0.002 | 104.12 | 37.06 | 0.09 |
| CV (%) | 2.5 | 2.62 | 3.95 | 6.18 | 9.73 | 24.2 | |
| Media | 73.25 | 75.53 | 1.25 | 165.08 | 62.56 | 1.28 | |
| R2 | 0.95 | 0.94 | 0.74 | 0.89 | 0.91 | 0.87 |
FV= fuente de variación; GL= grados de libertad; FM= floración masculina; FF= floración femenina; SF= sincronía floral; AP= altura de planta; AM= altura de mazorca; A= ambientes; R= repeticiones; B= bloques, *, ** significativo al 0.05 y 0.01 de probabilidad, respectivamente; †= valores transformados por ln(SF + 10)0.5; RG= rendimiento de grano.
Statistical differences observed between lines, is the result of genetic variability of the germplasm, which was derived from multiple allelic combinations in a segregating population (Ribaut et al., 1996, Agrama and Moussa, 1996). The presence of genotype-environment interaction was present in four of the six measured traits, which can be explained by the high number of genotypes from the segregating population and the differential effect that environmental factors produce on genotypes (Fehr, 1993).
Table 2, shows the estimates of genetic parameters for segregating population under study; this table shows that the estimate of phenotypic variance for all the tested traits showed a greater magnitude in relation to the estimation of the additive variance. The values of the coefficients of additive genetic variation were expressed in amplitude of 4to 17%, these values were related to the traits from male flowering and grain yield respectively. Traits heritability had values from 4% (floral synchrony) to 97% (plant height). Based on the selection pressure (20%) in the segregating population, genetic gain estimator (∆G) for critical traits selection, was -0.62 d in male flowering and 0.143 t ha-1 for grain yield, which establishes that the select lines tend to reduce their male flowering.
Table 2 Genetic parameters from measured variables in Ayala, Morelos in spring-summer 2013 and autumn-winter 2013-2014.
| Parámetro estimado | FM | FF | SF | AP | AM | RG |
| (d) | (cm) | (t ha-1) | ||||
| σ2A | 10.40±2.62 | 11.77±2.94 | 0.03±0.16 | 249.86±52.17 | 108.47±22.53 | 0.05±0.02 |
| σ2F | 12.99±2.62 | 14.56±2.94 | 0.74±0.15 | 258.11±52.01 | 111.59±22.49 | 0.12±0.02 |
| CVa (%) | 4.4 | 4.54 | 7.27 | 9.57 | 16.65 | 17.47 |
| h2 | 0.80±0.23 | 0.81±0.23 | 0.04±0.38 | 0.97±0.19 | 0.97±0.19 | 0.42±0.34 |
|
|
0.71 | 0.72 | -0.38 | 0.95 | 0.96 | 0.16 |
|
|
0.86 | 0.86 | 0.33 | 0.98 | 0.98 | 0.59 |
| ∆G | -0.62 | -0.79 | -0.34 | 17.46 | 9.21 | 0.14 |
| µPS | 73.24 | 75.52 | 2.28 | 164.98 | 62.54 | 1.26 |
| µLS | 72.69 | 74.82 | 2.13 | 175.9 | 68.91 | 1.77 |
σ2A = varianza aditiva; σ2F = varianza
fenotípica; CVA= coeficiente de variación genético
aditivo; h2= heredabilidad; FM= floración masculina;
FF= floración femenina; SF= sincronía floral; AP= altura de
planta; AM= altura de mazorca; RG= rendimiento de grano.
∆G= ganancia genética. µPS= media de
la población segregante; µLS= media de líneas
seleccionadas;
The components of additive and phenotypic variance were in accordance with expectations, because, phenotypic variance is comprised by the genetic effect, the deviation attributable to the environment and by the component genotype x environment interaction, so in genetic studies, it is expected that phenotypic variance magnitudes exceeds additive variance; a similar behavior of these parameters was found in a study designed to assess the genetic effects in populations derived from crosses between commercial corn hybrids (Gutiérrez et al., 2004).
The coefficient of additive genetic variation (CVA) in this study, overall revealed a major amount of the additive component in the segregating population, mainly in grain yield (17.47%), ear (16.65%) and plant (9.57%) height traits. Based on coefficients of additive genetic variation, quantified in a synthetic variety of tropical maize for the same characters, RG= 6.12%; AM= 3.13%; AP= 2.25% (Rovaris et al., 2011), can be concluded that this segregating population has a high additive genetic variation, which guarantees a substantial genetic gain per selection cycle. Furthermore, the amount of genetic variation decreases through the process of breeding population, as observed in a Brazilian corn population, where the original population showed a coefficient of genetic variation of 15.3%, while in the third selection cycle this coefficient decreased to 7.1% (Paterniani, 1967).
Regarding to the results of heritability, most of the characters (except in floral synchrony) had high estimates in this parameter, so in the population under selection predominate additive genetic effects (Rafiq et al., 2010), as it was demonstrated in the values from the coefficients of additive genetic variation. The heritability for grain yield in this study was 0.42, a similar estimate was obtained for the same trait (h2= 0.43) in a synthetic variety (Rovaris et al., 2011) and contrasts with the high heritability (h2= 0.88) reported for recombinant inbred lines of tropical maize (Ferreira et al., 2015), the results show that estimates of genetic parameters are specific to the population under study. The estimated heritability for male flowering (0.80) in the present study, is very similar to that value reported with recombinant lines 0.83 (Ferreira et al., 2015) and higher than that determined in a synthetic variety of maize 0.55 (Rovaris et al., 2011).
The genetic gain (∆G) value for yield grain estimated in this study predicts an increase of 143 kg and a reduction of -0.62 d in male flowering per selection cycle, results in line with the objectives outlined in this study. Since there is no significant difference between this value and from the original population, changes is not expected in days to flowering in the population from cycle one. Finally, the expected genetic gain for plant height in selected segregating lines is 17.46 cm, a similar trend for the same trait in genetic gain (11.65 cm) in a intrapopulation selection cycle based on half sibs families in maize (Afonso et al., 2012).
On the other hand, intrapopulation breeding programs, additive genetic variance contributes significantly in the selection response in the population, so heritability estimate is essential to predict the response of a population to selection. Therefore, the existence of a high additive genetic variation justifies the continuing with the population breeding program (Rovaris et al., 2011).
Traits average under study in this work (Table 3) correspond to 39 lines selected, based on the highest grain yield. The amplitude for this trait in this group of lines was from 1.60 to 3.36 t ha-1; these values corresponded to lines LUM-36 and LUM -80, respectively. The average of the selected lines showed an increase of 40% compared to the average of grain yield from the segregating population. Days to male flowering in selected lines ranged from 67 to 77 d and plant height had a phenotypic expression range of 118-233 cm. In the group of selected lines it was observed that for grain yield there were lines above average (1.77 t ha-1) and only LUM-80 exceeded in grain yield to the best parent (B39) and control (Ac7729). For the trait male flowering, second selection criteria in this study, it was found that 15% of the selected lines had an equal or lower flowering than the early parent (B39), with a male flowering of 67 days. Regarding plant height, 46% of the selected lines showed a lower height than the lowest parent (B39, 160 cm), the rest equaled or exceeded the taller parent (Ac7643, 181 cm). For grain yield and plant height traits, it was observed that segregating lines outperformed the average values of the parents (Figure 1a and 1b), indicating the presence of transgressive inheritance.
Table 3 Selected lines based on grain yield in Ayala, Morelos, spring-summer 2013 and autumn-winter 2013/2014.
| Línea | RG | FM | FF | SF | AP | AM |
| (t ha-1) | (d) | (cm) | ||||
| LUM-80 | 3.36 | 68 | 69 | 1 | 233 | 87 |
| LUM-18 | 2.10 | 70 | 72 | 2 | 188 | 81 |
| LUM-73 | 2.08 | 75 | 77 | 2 | 199 | 86 |
| LUM-97 | 2.00 | 69 | 72 | 3 | 180 | 72 |
| LUM-21 | 1.97 | 69 | 71 | 2 | 194 | 84 |
| LUM-87 | 1.95 | 69 | 70 | 1 | 183 | 67 |
| LUM-51 | 1.92 | 67 | 69 | 1 | 151 | 50 |
| LUM-123 | 1.87 | 68 | 69 | 1 | 188 | 72 |
| LUM-76 | 1.86 | 75 | 76 | 1 | 171 | 68 |
| LUM-134 | 1.85 | 76 | 79 | 3 | 189 | 83 |
| LUM-145 | 1.82 | 69 | 71 | 2 | 193 | 87 |
| LUM-166 | 1.81 | 75 | 77 | 2 | 196 | 72 |
| µLS | 1.77 | 73 | 75 | 2 | 176 | 69 |
| RLS | 1.56-3.36 | 67-77 | 69-82 | 1-5 | 118-233 | 41-88 |
| AC7729 | 2.27 | 76 | 79 | 3 | 173 | 66 |
| AC7643 | 1.47 | 79 | 82 | 3 | 181 | 70 |
| B39 | 2.64 | 67 | 68 | 2 | 160 | 58 |
| DSH (5%) | 1.01 | 6 | 6 | 4 | 33 | 20 |
RG= rendimiento de grano; FM = floración masculina; FF= floración femenina; SF= sincronía floral; AP= altura de planta; AM = altura de mazorca; µLS= pomedio de líneas selectas; RLS= rango de líneas seleccionadas.
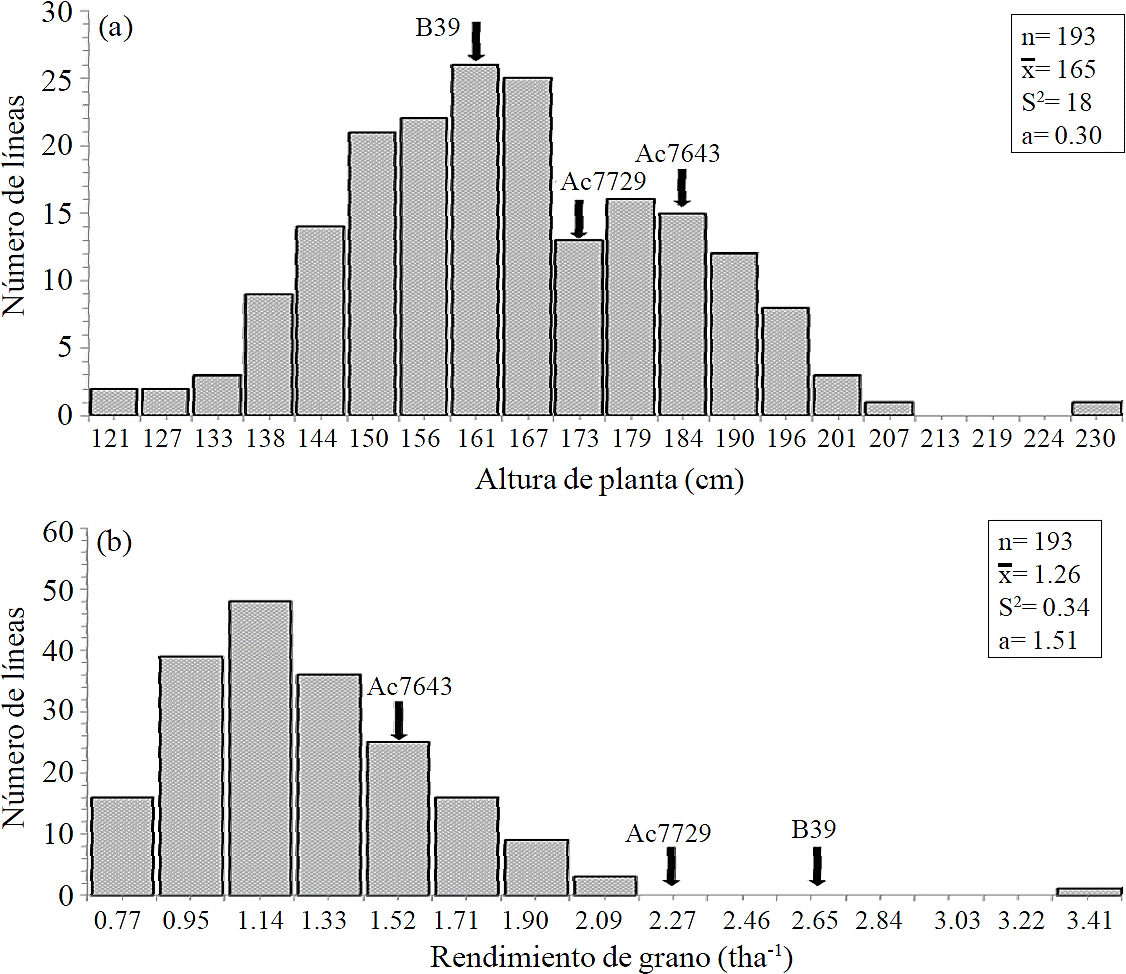
Figure 1 Frequency distribution for plant height (a) and grain yield (b) traits evaluated in Cd. Ayala, Morelos in s-s 2013 and a-w 2013-2014 (n= sample size;
This type of inheritance is explained by deviations caused by loci with non-additive gene action, such as epistasis and over-dominance (Falconer and Mackay, 1996); also, transgressive segregation can be caused by a set of alleles from the parents, who have opposite effects (Lynch and Walsh, 1998). The presence of transgressive inheritance has been reported in other studies with segregating lines, as is the case for grain yield in maize under water stress (Agrama and Moussa, 1996), and floral synchrony (Ribaut et al., 1996). One of the advantages of transgressive inheritance is that it enables the breeder to obtain highly outstanding genotypes in the trait under selection, as in the case of LUM-80 line, which exceeded the best parent and control for grain yield.
In the analysis of phenotypic and genotypic association between traits, Table 4, when considering grain yield and phenotypic correlation, only associations with plant height (0.33**) and cob (0.27**) were found. These values are considered low, although the correlation values vary with the germplasm used for its estimation, such as that reported for inbred lines of maize, where the estimate of phenotypic correlation between grain yield and plant height was 0.34**, and cob height of 0.51** (Ramírez et al., 1998).
Table 4 Coefficients of phenotypic r (f) and genetic r (g) correlation between traits in 193 S1 lines, parents and control in Cd. Ayala, Morelos, during spring-summer 2013 and autumn-winter 2013-2014.
| Carácter | FF | SF | AP | AM | RG | |
| FM | r(f) | 0.97** | 0.16NS | -0.19** | 0.13NS | -0.12NS |
| r(g) | 0.99** | 0.26** | 0.13** | 0.12** | -0.08** | |
| FF | r(f) | 0.36** | -0.2** | 0.11NS | -0.13NS | |
| r(g) | 0.41** | -0.16** | 0.17* | -0.11** | ||
| SF | r(f) | -0.06NS | -0.03NS | -0.12NS | ||
| r(g) | -0.05NS | -0.02NS | -0.23NS | |||
| AP | r(f) | 0.81** | 0.33** | |||
| r(g) | 0.8** | 0.39NS | ||||
| AM | r(f) | 0.27** | ||||
| r(g) | 0.31NS |
RG= rendimiento de grano; FM = floración masculina; FF= floración femenina; SF= sincronía floral; AP= altura de planta; AM = altura de mazorca; *, **significativo y altamente significativo, respectivamente.
The genetic correlation between grain yield and the rest of the traits, showed a statistically significant association just for male flowering (-0.08**) and female (-0.11**). Higher genetic correlations have been reported for the same traits, as in the case of a group of mestizos corn, where the correlation between grain yield and male and female flowering were -0.76** and -0.71** respectively (Jawaharlal et al., 2011). In contrast to the same trait, has been obtained positive and high genetic correlations in single corn hybrids between grain yield and male flowering (0.85*) and female 0.8* (Kumar et al., 2013).
Overall, both phenotypic and genotypic correlations estimated in this study are relatively low, which may result from the effect of environment on the polygenic system that regulates the expression of these traits. This can be supported to some extent, by the fact that the environment reduces the correlation between phenotype and genotype (Falconer and Mackay, 1996). However, it is necessary to know the degree of association between traits and more relevant genetic correlation, because it largely determines the true association between traits, as it excludes environmental effect (Rafiq et al., 2010).
Conclussion
The determination of genetic parameters in a segregating population allowed to elucidate that there is a high additive genetic variation; this allows greater genetic gain per selection cycle. The transgressive segregation observed for grain yield, allowed to select the line LUM-80 with outstanding grain yield, similar in days to flowering to the early parent. Phenotypic and genetic correlations from grain yield with the rest of the variables to be of low magnitude would not allow performing indirect selection for grain yield.
Literatura citada
Afonso, D. A.; Vieira, M. G.; Oliveira, D. L. R.; Gonçalves, C. L. and Gomes, G. E. E. 2012. Genetic parameters and predictive genetic gain in maize with modified recurrent selection method. Chilean J. Agric. Res. 72(1):33-39. [ Links ]
Agrama, H. A. S. and Moussa M. E. 1996. Mapping QTLs in breeding for drought tolerance in maize (Zea mays L.). Euphytica. 91:89-97. [ Links ]
Alejos, G.; Monasterio, P. y Rea, R. 2006. Análsis de la interacción genotipo-ambiente para el rendimiento de maíz en la región maicera del estado Yaracuy, Venezuela. Agron. Trop. 56(3):369-384. [ Links ]
Betrán, F. J.; Ribaut, J. M.; Beck, D. and González, de L. D. 2003. Genetic diversity, specific combining ability, and heterosis in tropical maize under stress and nonstress environments. Crop Sci. 43:797-806. [ Links ]
Borel, J. C.; Patto, R. M. A.; Rezende, F. de C. V. and Barbosa, A. A. de F. 2013. Genetic and phenotypic parameters in common bean segregant populations from intra and inter-gene pool crosses of elite lines. Euphytica. 193:39-47. [ Links ]
Chapman, S. C. and Edmeades, G. O. 1999. Selection improves drought tolerance in tropical maize populations. II. Direct and correlated responses among secondary traits. Crop Sci. 39:1315-1324. [ Links ]
Dzib-Aguilar, L. A.; Segura, J. C.; Ortega, R. y Latournerie, L. 2011. Cruzas dialélicas entre poblaciones nativas de maíz de yucatán y poblaciones mejoradas. Trop. Subtrop. Agroecosys. 14:119-127. [ Links ]
Duvick, D. N. 2001. Biotechnology in the 1930s: the development of hybrid maize. Nature Rev. of Genet. 2: 69-74. [ Links ]
Edmeades, G. O.; Bolaños, J.; Chapman, S. C.; Lafitte, H. R. and Bänziger, M. 1999. Selection improves drought tolerance in tropical maize populations: I. Gains in biomass, grain yield, and harvest index. Crop Sci. 39:1306-1315. [ Links ]
Falconer, D. S. and Mackay, T. F. C. 1996. Introduction to quantitative genetics. 4t h Ed. Longman Group Ltd, London, England. 312-321 pp. [ Links ]
Fehr, W. R. 1993. Principles of cultivar development theory and technique. Macmillian Publishing Company. New York, USA. 1:219-245. [ Links ]
Ferreira, M. F.; Moreira, G. L. J.; Teixeira, G. C.; Souza, J. C.; Oliveira, G. P. E. and Netto, P. S. 2015. Genetic control of traits related to phosphorus use efficiency in tropical maize. Crop Breed. Appl. Biotechnol. 15:59-65. [ Links ]
Garbuglio, D. D.; De Miranda Fihlo, J. B. y Cella, M. 2009. Variabilidade genética em famílias S1 de diferentes populações de milho. Acta Sci. Agron. Maringá. 31(2):209-213. [ Links ]
Gutiérrez R., E.; Espinoza B., A.; Palomo G., A.; Lozano G., J. J. y Antuna G., O. 2004. Aptitud combinatoria de híbridos de maíz para la Comarca Lagunera. Rev. Fitotéc. Mex. 27(1):7-11. [ Links ]
Hallauer, A. R. and Miranda, J. B. 1988. Quantitative genetics in maize breeding. 2th Ed. Iowa State University Press. Ames, Iowa. 468 p. [ Links ]
Hallauer, A. R. 1981. Selection and breeding methods. In: plant breeding II. Frey, K. J. (Ed.). Iowa State University Press. Ames. Iowa. 497 p. [ Links ]
INEGI. 2014. Anuario estadístico y geográfico de Morelos. Instituto Nacional de Geografía e Informática. México. 475 p. [ Links ]
Jawaharlal, J.; Reddi, G. L. and Kumar. R. S. 2011. Genetic variability and character association studies in maize. Agric. Sci. Digest. 31(3):173-177. [ Links ]
Kumar, B.; Razdan, A.K. and Gupta, B. B. 2013. Heterosis and character association for grain yield and its component traits in single cross maize hybrids under mid hills of Jammu and Kashmir, India. Agric. Sci. Digest. 33(3):198-202. [ Links ]
Lynch, M. and Walsh, B. 1998. Genetics and analysis of quantitative traits. Sinauer Associates, Inc. Sunderland, MA, USA. 477- 478. [ Links ]
Rafiq, Ch.; Rafique, M.; Hussain, A. and Altaf, M. 2010. Studies on heritability, correlation and path analysis in maize (Zea mays L.). J. Agric. Res. 48(1):35-38. [ Links ]
Paterniani, E. 1967. Selection among and within half-sib families in a Brazilian population of maize (Zea mays L.). Crop Sci. 7:212-216. [ Links ]
Peyman, S. D. Hamid. M. Ali and M. Mohammad. 2009. Genetic and genotype x enviroment interaction effects for appearance quality of rice. Agric. Sci. China. 8(8):891‐901. [ Links ]
Ramírez, J. L.; Ron, J.; Sánchez, J.; Carcía, A. y Maya, J. 1998. Aptitud combinatoria general y correlaciones fenotípicas entre líneas y mestizos de maíz. Agron. Mesoam. 9(2):69-76. [ Links ]
Ribaut, J. M.; Hoisington, D. A.; Deutsch, J. A.; Jiang, C. and González de León. D. 1996. Identification of quantitative trait loci under drought conditions in tropical maize. 1. Flowering parameters and the anthesis-silking interval. Theor. Appl. Genet. 92:905-914. [ Links ]
Rovaris, S. R. S.; Araújo, P. M.; Garbuglio, D. D.; Prete, C. E. C.; Zago,V. S. and da Silva, L. J. F. 2011. Estimates of genetic parameter in maize commercial variety IPR 114 at Paraná State, Brazil. Acta Scientiarun. Agron. Maringá. 33(4):621-625. [ Links ]
SAGARPA. 2013. Servicio de Información Estadística Agroalimentaria y Pesquera. Subsistema de Información Agrícola. Versión en CD. [ Links ]
SEMARNAT. 2015. Instituto Mexicano de Tecnología del Agua. Red de estaciones meteorológicas del estado de Morelos y Fundación Porduce. Versión en CD. [ Links ]
Statistical Analysis System Institute (SAS). 1999. SAS User’s Guide. Statistics. Versión 8.2. SAS Inst. Cary, N. C. 102 p. [ Links ]
Walsh, B. 2001. Quantitative Genetics. Encyclopedia of Life Sciences. Nature publishing group. 1-7 pp. [ Links ]
Received: August 2016; Accepted: October 2016











 text in
text in 


