Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista mexicana de ciencias agrícolas
versión impresa ISSN 2007-0934
Rev. Mex. Cienc. Agríc vol.7 no.7 Texcoco sep./nov. 2016
Research note
Culture medium and inhibitors of ethylene in the coffee somatic embryogenesis
1Campo Experimental Rosario Izapa- INIFAP. Tuxtla Chico, Chiapas. México. Tel: 01 (962) 1100271 C. P. 30870. (lopez.pablo@inifap.gob.mx; iracheta.leobardo@inifap.gob.mx).
2Facultad de Agronomía-Universidad Autónoma de Nuevo León. Francisco Villa S/N. Col. Ex-Hacienda El Canadá. Gral. Escobedo, Nuevo León, México. C. P. 66054 Tel. 1340-4399. (ojedacz@yahoo.com.mx).
3Research and Development Center Nestlé, 101, Avenue Gustave-Eiffel, Notre-Dame-D'Oé. B. P. 49716, 37097, Tours Cedex 2. France. Tel: (0033) 02 4762 8383. (Jean-Paul.Ducos@rdto.nestle.com).
The combination of salts, hormones and the gaseous environment in vitro could affect the expression of somatic embryogenesis. The effect of the combination of two culture media and inhibitors of ethylene induction of embryogenic callus of Coffea canephora P., and their ability to proliferate and form embryos was studied. five leaf explants of C. canephora P. genotypes were established in two different media, 23A8 [Yasuda salts with BAP (1.12 mg L-1)]; and T1B/T2B sequence consisting of a first crop T1B [50% of MS salts with 2iP (2 mg L-1), 2, 4-D (0.5 mg L-1) and AIB (1 mg L-1) ]; followed by T2B [50% of MS salts with BAP (4 mg L-1) and 2, 4-D (1 mg L-1) ]. These media were supplemented with AgNO3 (6.8 mg L-1) and Na2S2O3 (6.3 mg L-1). Using T1B/T2B led greater callus proliferation (up to 248 mg), but low percentages of embryogenic callus (less than 29%) and less differentiated than those obtained in the middle 23A8 (160 mg). In the multiplication phase were obtained calluses from 1 to 2.5 mg and multiplication rate of 10 to 24 times the initial biomass they. In differentiation, embryogenic potential of 25 000 to 137 000 embryos per gram of inoculated callus was observed. At each stage the effect of ethylene inhibitors genotype dependent on interaction with the culture medium.
Keywords: Coffea canephora P.; AgNO3; embryogenic potential; multiplication rate; Na2S2O3
La combinación de sales, hormonas y el ambiente gaseoso in vitro podrían afectar la expresión de la embriogénesis somática. Se estudió el efecto de la combinación de dos medios de cultivo e inhibidores de etileno sobre la inducción de callo embriogénico de Coffea canephora P., y su capacidad para proliferar y formar embriones. Se establecieron explantes foliares de cinco genotipos de C. canephora P., en dos medios diferentes, el 23A8 [sales de Yasuda con BAP (1.12 mg L-1)]; y la secuencia T1B/T2B consistente en un primer cultivo en T1B [50% de sales de MS con 2iP (2 mg L-1), 2, 4-D (0.5 mg L-1) y AIB (1 mg L-1) ]; seguido del T2B [50 % de sales de MS con BAP (4 mg L-1) y 2, 4-D (1 mg L-1) ]. Estos medios se suplementaron conAgNO3 (6.8 mg L-1) y Na2S2O3 (6.3 mg L-1). El uso de T1B/T2B propició mayor proliferación de callos (hasta248 mg), pero bajos porcentajes de callo embriogénico (menores a 29 %) y menor grado de diferenciación que los obtenidos en el medio 23A8 (con 160 mg). En la fase de multiplicación se obtuvieron callos de 1 a 2.5 mg y una tasa de multiplicación de 10 a 24 veces la biomasa inicial. En la de diferenciación, se observó un potencial embriogénico de 25 mil a 137 mil embriones por gramo de callo inoculado. En cada etapa el efecto de los inhibidores de etileno dependió del genotipo en interacción con el medio de cultivo.
Palabras clave: Coffea canephora P.; AgNO3; Na2S2O3; potencial embriogénico; tasa de multiplicación
Introduction
Several factors can affect the somatic embryogenesis in coffee, such as the combination of salts, presence or absence of some hormones and the gaseous environment of the containers in vitro (Kumar et al., 2009; López-Gómez et al, 2010). Generally, it is possible to induce somatic embryogenesis in Coffea canephora Robusta variety, only the presence of the cytokinin 6-benzylaminopurine (BAP) in the induction medium. However, in some clones no good results are obtained with this medium and in some cases the development of embryogenic callus is a long process, ranging from six to ten months depending on the genotype (López-Gómez et al., 2010; López-Gómez et al, 2011).
In C. arabica, the presence of some auxin is always necessary for obtaining embryogenic callus (Pierson et al., 1983; Van Boxtel and Bertouly, 1996). On the other hand, the gaseous environment plays a very important role in the culture in vitro. The atmosphere of the culture vessels may contain high concentrations of carbon dioxide (CO2) and ethylene (C2H4). The first is known for its positive effect on germination phase ex vitro (Ducos et al., 2009). The second is a component that affects the morphogenic response in vitro.Kumar et al. (2009), they showed that inhibits ethylene secondary embryogenesis in robusta coffee. Some studies have been based on the application of ethylene inhibitors (AgNO3, cobalt chloride and Na2S2O3) or inhibitors of ethylene synthesis (salicylic acid) to suppress their negative effect (Kumar et al., 2007).
Some reports describe the effect of inhibitors on the induction of in vitro organogenesis (Giridhar et al., 2003) and direct embryogenesis robusta coffee (Giridhar et al., 2004), but its effects are unknown indirect somatic embryogenesis in as for embryogenic callus induction and its effect for multiplication and differentiation phases.
The aim of this work was to study the effect of the combination of two culture media and ethylene inhibitors on induction of embryogenic callus of C. canephora P., and their ability to multiply and differentiate.
Materials and methods
This work was conducted at the Center for Research and Development Nestle, Tours, France (NR&DC-T) the young leaves are used and fully expanded genotypes of C. canephora P., Robusta variety (FRT06, FRT07, FRT09, FRT22 and FRT23), selected for their high performance. All they obtained from the collection of NR&DC-T, which were disinfested and established in vitro according to the methodology of Ducos et al. (2007). The explants were incubated in the dark at 25 °C, subcultured every 30 days and placed under the same conditions.
The Six treatments consisted genotype were taken: 1) the 23A8 [Yasuda et al. (1985)) salts, Gamborg (2002) vitamins, 1.12 mg L-1 of 6-benzylaminopurine (BAP) and 30 g L-1 sucrose]; 2) I 23A8 supplemented with 6.8 mg L-1 AgNO3; 3) I 23A8 supplemented with 6.3 mg L-1 of Na2S2O3; 4) sequence T1B/T2B means (Van Boxtel and Berthouly 1996): the T1B medium (used for a month) composed of 50% of Murashige and Skoog (1962), more vitamins Gamborg (2002), 2 mg L-1 of 2-isopentenyladenine (2iP), 0.5 mg L-1 of 2,4-dichlorophenoxyacetic (2, 4-D), 1 mg L-1 of indolbutyric acid (AIB), 400 mg L-1 of extract malt, 100 mg L-1 of hydrolysed casein and 30 g L-1 of sucrose; T2B (employed for two months) comprised 50% of the salts and vitamins of Murashige and Skoog (1962), 4 mg L-1of BAP, 60 mg L-1 of adenine sulfate, 1 mg L-1 of 2 , 4-D, 800 mg L-1 of malt extract, 200 mg L-1 of hydrolysed casein and 30 g L-1 of sucrose; 5) sequence T1B/T2B media supplemented with 6.8 mg L-1 of AgNO3; and 6) the T1B/T2B supplemented with 6.3 mg L-1 of Na2S2O3. All media were gelled with 8 g L-1 of agar and adjusted to pH 5.6. In the fourth month they eliminated and Na2S2O3and AgNO3 in the sixth month of in vitro setting were evaluated percentage and fresh weight of embryogenic callus (friable tissue, yellow and grainy) (Ducos et al., 1999). Each treatment consisted of four replications of three Petri dishes, a Petri dish with 50 mL of culture medium and inoculated with fifteen explant explant consisted of 3 mm2 sheet.
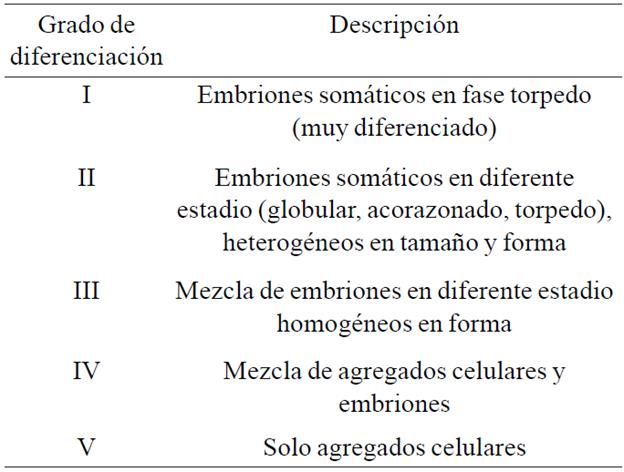
After six months of induction and treatments where there were sufficient callus, embryogenic callus were selected and transferred to 10 mL of liquid multiplication medium (23A8 without agar) in Erlenmeyer flasks 25 mL with a density of 10 g L-1. The flasks were placed on an orbital shaker (110 rpm) at 25 °C in darkness. At this stage the fresh weight (mg), multiplication rate was evaluated, [Tm=(Pf (week 6) - Pf (week 0)) / Pf (week 0)] and the degree of differentiation of the suspension according to the criteria described in Table 1.
Table 1 Criteria for evaluating the degree of differentiation of the suspension in the multiplication phase in liquid medium of C. canephora genotypes.
Furthermore embryogenic callus were selected and placed in 100 mL of liquid medium for differentiation (23A8 without agar and BAP) in 250 mL of Erlenmeyer flasks capacity, with a density of1 g L-1. The flasks were placed on orbital shakers (110 rpm) at 25 °C with 16 hours photoperiod light (0.1 to 0.6 kilolux) and 8h dark. For this step were evaluated the number of embryos produced per flask to know the embryogenic potential [ number of embryos g-1 of callus= number embryos per flask (number of embryos in 1 g of sample x total weight of embryos per flask g) / initial inoculum density (g)]. The changes were made to fresh medium according to suggested by Ducos et al. (2003). The three repetitions per treatment, which was a repetition Erlenmeyer flask with the aforementioned density were established. All variables were analyzed by ANOVA and mean comparison was carried out with the Tukey test with statistical significance 0.05, with the help of statistical program SAS version 9.0 (SAS Institute 2002).
Results and discussion
At six months of induction, explants began to produce different types of morphogenic response. One was the formation of embryogenic callus (Figure 1a). The was also possible to observe direct embryogenesis expressed in the formation of groups of embryos directly explant (Figure 1b), production of aqueous, necrotic and nodular callus (Figure 1 c, d, e and f); and late embryogenic callus formation from the aqueous, necrotic and nodular callus (Figure 1 e, f g h) and explant no response (Figure 1i). About Ducos et al. (2007)) mentioned undesirable embryo formation directly explant, as this type of response (direct embryogenesis) limits the potential of somatic embryogenesis for mass propagation scheme.

Figure 1 Different morphogenic response of robusta coffee genotypes. a) embryogenic callus on the FRT06 in 23A8; b) direct embryogenesis FRT07 in 23A8-Na2S2Oa; c) in necrotic callus FRT07 in T1B/T2B; d) aqueous and embryogenic callus of 23A8-FRT06 in Na2S2O3; e) embryogenic callus from necrotic callus of FRT22 in T1B/T2B- Na2S2O3; f) embryogenic callus from necrotic callus of FRT07 in T1B / T2B-AgNO3; g) callus from nodular embryogenic callus FRT22 in the medium 23A8- Na2S2O3; h) embryogenic callus of FRT23 in T1B/T2B- Na2S2O3; and i) explant without morphogenic response. Bai= 1 mm.
In treatments whose base medium was the 23A8 the formation of embryogenic callus was given from the explant in treatments with the average 23A8, but in treatments with T1B/T2B sequence (in the presence of auxin), the formation of embryogenic callus It came from a necrotic callus that formed early, as in the case of genotype FRT07 in the middle T1B/T2B supplemented with AgNO3 (Figure 1f). This confirms the findings of Ducos et al. (2009) and López-Gómez et al. (2010), who mentioned that it is possible to obtain embryogenic callus only with the presence of BAP as a growth regulator; however, some authors in studies with different genotypes of robusta coffee mentioned that the presence of auxin (González, 2003) is necessary, and even a balance between auxin and cytokinin, which favor the formation of callus with embryogenic potential (Van Boxtel and Berthouly, 1996).
In Table 2, it can be seen that there are significant differences between treatments for each variable, with the exception of biomass for FRT07 and FRT22 genotypes as well as in the percentage of embryogenic callus for genotype FRT22. In comparing the percentage of embryogenic callus obtained in the means 23A8 and T1B/T2B, it can be seen that the second half led to a drastic reduction in the morphogenic response for FRT09 and FRT07 genotypes. Conversely, this same medium favored the weight of embryogenic callus FRT23 genotype. For FRT06 and FRT22 genotypes hereby tended to decrease the frequency of embryogenic callus, however, it increased weight. This type of response has been observed in various species using inductors callogenesis new crops of agricultural interest (Montes et al, 2000).
Table 2 Percentage of embryogenic callus (CE) and fresh weight (PFS) in semisolid medium of coffee genotypes obtained from the induction means; fresh weight in liquid medium (PFL), multiplication rate (TDM), degree of differentiation of the suspension (GD) OF cell lines under multiplication in liquid media and number of embryos (ES) per gram of callus for embryogenic potential callus differentiation stage in the presence of inhibitors and ethylene.

† Medias con letras iguales no son estadísticamente diferentes según Tukey (p≤ 0.05). ¶ En estos tratamientos no fue posible establecer la multiplicación y diferenciación en medios líquidos por no contar con suficiente callo embriogénico.
The concerning the effect of inhibitors of ethylene, the response was different and depended on the genotypes and the environment. For genotype FRT06, you could see that the presence of AgNO3 inhibited the production of embryogenic callus in both media. Overall, Na2S2O3 in both media had a positive effect, since in most genotypes, interacting with any of the media tested favored formation of embryogenic callus and therefore to obtaining somatic embryos. The FRT07 for genotype, the favored AgNO3 biomass production, but only when was added to the sequence T1B/T2B means; the latter confirms the results obtained by other authors, who recorded the effect of AgNO3, will depend on the genotype, species and especially in interaction with other regulators such as auxin and carbon sources (Fuentes et al, 2000; Giridhar et al, 2004). The FRT09 for genotype inhibitors embryogenesis not favored in any of the media tested.
The FRT22 genotype for both the frequency of embryogenic callus as biomass, no effect was detected by the presence of inhibitors of ethylene. And finally, for genotype FRT23, production of embryogenic callus was better with the sequence T1B/T2B media supplemented with Na2S2O3. This response can be attributed to the use concentration of ethylene inhibitors, since according to Wang et al. (2002) the inhibitory effect of ethylene action byAgNO3 depend on the number of silver ions to replace copper ions need to have functional ethylene receptors in plant cells.
In the multiplication phase, no significant differences were detected in terms of biomass, multiplication rate and quality of the suspension for FRT06, FRT07, FRT09 and FRT22 genotypes. The multiplication rate was 10 to 20 after six weeks of culture. These results are consistent with the results of Ducos and Pétiard (2003), who mentioned that the multiplication of biomass is given by a factor of two to three every two weeks.
In the case of genotype FRT23, statistical analysis showed that biomass and multiplication rate of callus obtained from the T1B/T2B sequence added with Na2S2O3 were greater than the other treatments; however, this suspension was mainly composed of embryos in different state, such material received a rating of "II" so cannot be considered as a cell suspension. About Ducos and Pétiard (2003) note that a cell suspension in the multiplication phase must consist of compact and a size of approximately 0.3 to 1.5 mm cell aggregates. In other eases, it was observed that suspensions were composed of a mixture of cell aggregates and embryos, or only aggregates; the latter type of response is desired as they allow to establish a mass propagation scheme either to restart a multiplication cycle or induce differentiation to obtain embryos.
As differentiation could be observed that the embryogenic potential ranged from 25 to 137 000 embryos per gram of callus inoculated (Table 2). These results are similar to those obtained by Ducos et al. (2007), who mentioned that the embryogenic potential of robusta coffee callus is on average 50 000 embryos per gram of callus.
The concerning the origin of corns, no significant differences for FRT06, FRT07, FRT09 and FRT22 genotypes were detected. However, in the genotype FRT06 it could be seen that the embryogenic callus from the T1B/T2B and T1B/ T2B with Na2S2O3 sequence showed a greater potential calluses from the middle 23A8 (Witness). On the other hand, for genotype FRT23 were found significant differences and the best treatment for this genotype led the calluses from the middle 23A8 added with Na2S2O3 (a little more than 137 000 embryos per gram of embryogenic callus inoculated). About Kumar et al. (2007) mention that the effect of Na2S2O3 is unclear and appears to be associated with this as a component of the medium can promote the endogenous synthesis of auxin. So a study to assess endogenous levels of this regulator in the presence and absence of Na2S2O3, could help confirm or refute this hypothesis.
Conclusions
It was possible to determine the effect of ethylene inhibitors in interaction with two culture media. The embryogenic callus obtained from treatments showed ability to proliferate in liquid medium, the embryogenic potential sufficient and to obtain an acceptable number of embryos per gram of callus. In each phase of somatic embryogenesis coffee, the effect of ethylene inhibitors genotypes depended on interaction with the culture medium. This allowed suggest additional means of culture in each genotype evaluated for propagation by somatic embryogenesis.
Literatura citada
Ducos, J. P.; Gianforcaro, M.; Florin, B.; Pétiard, V. and Deshayes, A. 1999. A technically and economically attractive way to propagate elite Coffea canephora (Robusta) elones: in vitro somatie embryogenesis. In: XVIII Colloquium Sci. Int. Coffee. Helsinki. 295-301 pp. [ Links ]
Ducos, J. P.;Alenton, R.; Reano, J. F.; Kanehanomai, C.; Deshayes, A. and Pétiard, V. 2003.Agronomie performance of Coffea canephora P. trees derived from large-scale somatic embryo production in liquid medium. Euphytica. 131(2):215-223. [ Links ]
Ducos, J. P. and Pétiard, V. 2003. Propagation de clones de Robusta (Coffea canephora P.) par embryogenése somatique en milieu liquide. In: El Hadrami, I.; Daayf, F. (Eds.). Biotechnologies Végétales: de la Structure des Génomes ál'Amélioration des Plantes, El Watanya, Marrakeeh, Maroeeo. 142-159 pp. [ Links ]
Ducos, J. P.; Labbe, G.; Lambot, C.; Pétiard, V. and Towill, L. 2007. Pilot seale process for the production of pre-germinated somatic embryos of selected robusta (Coffea canephora) clones. In Vitro Cellular and Developmental Biology - Plant. 43:(6) 652-659. [ Links ]
Ducos, J. P.; Prévot A.; Lambot C. and Pétiard V. 2009. Positive effect of the CO2 released by eommereial substrates on the ex vitro germination rates of coffee somatic embryos. Acta Hortic.(ISHS). 812:329-336. [ Links ]
Fuentes, S. R. L.; Calheiros, M. B. P.; Manettifilho, J. and Vieira, L. G. E. 2000. The effects of silver nitrate and different carbohydrate sources on somatic embryogenesis in Coffea canephora. Plant Cell Tissue Organ Culture. 60(1):5-13. [ Links ]
Gamborg, O. L. 2002. Plant Tissue Culture. The Technology. Part 1. Exegeties Ltd. Edington. 547 p. [ Links ]
Giridhar, P.; Indu, E. P.; Vijaya Ramu, D. and Ravishankar, G. A. 2003. Effect of silver nitrate on in vitro shoot growth of Coffee. Tropieal Sei. 43(3):144-146. [ Links ]
Giridhar, P.; Indu, E. P.; Vinod, K.; Chandrashekar, A. and Ravishankar, G. A. 2004. Direct somatic embryogenesis from Coffea arabica L. and Coffea canephora P. ex Fr., under the influence of ethylene action inhibitor-silver nitrate. Acta Physiol. Plantarum. 26(3):299-305. [ Links ]
González, V. M. E. 2003. Estudio del proceso de callogénesis en genotipos promisorios de cafeto (Coffea canephora P.). Rev. Colomb. Biotecnol. 5(1):16-22. [ Links ]
Kumar, V.; Ramakrisha, A. and Ravishankar, G. A. 2007. Influence of different ethylene inhibitors on somatic embryogenesis and secondary embryogenesis from Coffea canephora P ex Fr. Plant Cell Tissue and Organ Culture. 43(6):602-607. [ Links ]
Kumar, V.; Parvatam, G. and Ravishankar, G.A. 2009. AgNO3-a potential regulator of ethylene activity and plant growth modulator. Elec. J. Biotechnol. 12(2):1-15. [ Links ]
López-Gómez, P.; Iraeheta-Donjuan, L.; Castellanos-Juárez, M.; Méndez-López, I.; Sandoval-Esquivez, A.; Aguirre-Medina, J. F.; Ojeda-Zacarías, M. C. y Gutiérrez-Díez, A. 2010. Influencia del explante y medio de cultivo en la embriogénesis somática en hojas de café. Rev. Fitotee. Mex. 33:205-213. [ Links ]
López-Gómez, P.; Iracheta-Donjuan, L.; Castellanos-Juárez, M.; Méndez-López, I.; Aguirre-Medina, J.F.; Gutiérrez-Díez, A.; Ojeda-Zacarías, M.C. y Pérez-Pérez, B. R. 2011. Variación en la tolerancia a desinfectantes de genotipos élite de Coffea spp., cultivados in vitro. Rev. Mex. Ciene. Agrie. 2:645-657. [ Links ]
Montes, S.; Aldaz, J. P.; Cevallos, M.; Cabrera, J. C. y López, M. 2000. Uso del bioregulador Peetimorf en la propagación acelerada del Anthurium cúbense. Cultivos Tropicales. 21(3):29-32. [ Links ]
Murashige, T. and Skoog, F. 1962. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 15:473-497. [ Links ]
Pierson, E. S.; Van Lammern, A. A. M.; Sehel, H. N. and Staritsky, G. 1983. In vitro development of embryoids from punched leaf dishes of Coffea canephora. Protoplasma. 115:208-216. [ Links ]
SAS Institute Inc., 2002, Cary, NC, USA. [ Links ]
Wang, K. L. C.; Hai, L. and Eeker, J. R. 2002. Ethylene biosynthesis and signaling networks. Plant Cell. s131-s151 supplement. [ Links ]
Van Boxtel, J. and Berthouly, M. 1996. High frequency somatic embryogenesis from coffee leaves. Plant Cell Tiss. Org. Cult. 44:7-17. [ Links ]
Yasuda, T.; Fujii, Y.; and Yamaguehi, T. 1985. Embryogenic callus induction from Coffea arabica leafexplants by benzyladenine. Plant Cell Physiol. 26:595- 597. [ Links ]
Received: January 2016; Accepted: March 2016











 texto en
texto en 


