Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista mexicana de ciencias agrícolas
versión impresa ISSN 2007-0934
Rev. Mex. Cienc. Agríc vol.7 no.7 Texcoco sep./nov. 2016
Research note
Analysis seeds Encyclia adenocaula (La Llave & Lex.) Schltr (Orchidaceae) for ex situ conservation
1Universidad Autónoma del Estado de México Campus "El Cerrillo"-Ciencias Agropecuarias y Recursos Naturales-Facultad de Ciencias Agrícolas. El Cerrillo Piedras Blancas C. P. 50200. Toluca, Estado de México.
2Universidad Veracruzana-Laboratorio de Micropropagación Vegetal-Facultad de Ciencias Biológicas y Agropecuarias Campus Córdoba.
Seeds Trompillo (Encyclia adenocaula (La Llave & Lex.) Schltr. Orchidaceae) from mature capsules of a population, were analyzed for: size, structure, weight, number of seeds per gram and viability by tetrazolium test with without pretreatment of Ca(OCl)2 and Tween, asymbiotic test germination conditions in vitro to compare the viability of them. Germination was evaluated in germination percentage and days in three semi-solid culture media (MS + CV; MS + banana extract; MS + coconut water). The seed size was 0.56±0.08 mm long by 0.09±0.01mm wide, covering an area of 3.25±0.5 mm2, with an approximate weight of 1.6±0.08μg, registering 63 500 seeds per gram. The percentage of viability by tetrazolium test showed statistically significant differences (p≤0.05) between treatments, excelling treatments Tetrazolium 1 % with and without Tween with 50.3 and 48.2% respectively, pretreatment with Ca(OCl)2 affected directly the viability of the seeds. In asymbiotic germination were significant statistical differences (p≤ 0.05) between treatments, highlighting MS + CV with 56.9% germination at 30 days, followed by medium MS + coconut water with 31.4% germination to 55.7 days, finally medium MS + banana extract with 23% to 59.1 days.
Keywords: coconut water; banana extract; germination; mature capsules; trompillo
Semillas de Trompillo (Encyclia adenocaula (La Llave & Lex.) Schltr. Orchidaceae), procedentes de cápsulas maduras de una población, fueron analizadas respecto a: tamaño, estructura, peso, número de semillas por gramo y la viabilidad mediante prueba de Tetrazolio con y sin pretratamiento de Ca(OCl)2 y Twenn , la prueba de germinación asimbiótica en condiciones in vitro para comparar la viabilidad de las mismas. La germinación, se evaluó en porcentaje y días a germinación en tres medios semisólidos de cultivo (MS+CV; MS+extracto de plátano; MS + agua de coco). El tamaño de la semilla fue de 0.56±0.08 mm de largo por 0.09±0.01mm de ancho, cubriendo un área de 3.25±0.5 mm2, con un peso aproximado de 1.6±0.08(μg, registrando 63 500 semillas por gramo. El porcentaje de viabilidad mediante la prueba de Tetrazolio presentó diferencias estadísticas significativas (p≤ 0.05) entre los tratamientos, sobresaliendo los tratamientos de Tetrazolio al 1% con y sin Twenn con 50.3 y 48.2% respectivamente, el pretratamiento con Ca(OCl)2 afectó directamente la viabilidad de las semillas. En la germinación asimbiótica se presentaron diferencias estadísticas significativas (p≤ 0.05) entre los tratamientos, destacando MS + CV con 56.9% de germinación a los 30 días, seguidos de medio MS + agua de coco con 31.4% de germinación a los 55.7 días, por último el medio MS + extracto de plátano con 23% a los 59.1 días.
Palabras clave: agua de coco; cápsulas maduras; extracto de plátano; germinación; trompillo
Trompillo, angels or encyclia rabbit (Encyclia adenocaula (La Llave & Lex.) Schltr) is an endemic orchid of Mexico distributed in the states of Nayarit, Jalisco, Michoacán, Mexico, Guerrero and Oaxaca. In the state of Mexico it is in the municipalities of Temascaltepec, Tejupilco and Valley de Bravo (Ruiz et al, 2008; Szeszko, 2011; Téllez, 2011), is one of the 188 species listed in NOM-059-SEMARNAT- 2010 (SEMARNAT, 2010) in the "endangered". Because of its showy flowers (inflorescences 60 cm to a meter long, with 10 to 35 flowers) and its pleasant fragrance noon, the species has been very collected involuntarily causing considerable decrease in natural populations (Ruiz et al, 2008; Szeszko, 2011), adding, climate change and forest disasters is suffering its ecosystem. It is important to conserve the species both in situ and ex situ conditions, a key strategy of the latter, is the implementation of genebanks (Lascurain et al., 2009; Sánchez and Jiménez, 2010).
In Mexico the vast majority of genebanks are intended to conserve species of agrifood interest, although some banks responsible for collecting wild, priority species, with viability problems in some category of risk, etc. (Lascurain et al, 2009). The efforts to preserve the Orchidaceae family have been exhaustive, by implementing plant germplasm banks under in vitro conditions by method of minimal growth or slow growth. However, as in gardens or living collections germplasm maintenance requires adequate facilities and specialized maintenance more expensive maintenance costs, conservation of germplasm from seed is a viable strategy to preserve the greatest genetic variability possible with lower costs and very tight spaces.
Determine the quality of stored seeds is an important factor in determining the success or failure of conservation in genebanks point. In the analysis of seeds should study physical and biological a lot to assign a value features, these tests should be performed in two stages: first, immediately after extraction and clean seed; and second, before being planted or used for any other destination and the main analyzes are, weight and size of seeds, viability, purity, moisture, etc (Raó et al., 2007).
Ossenbach et al. (2007), they conducted feasibility tests on orchid seeds of 22 accessions of 10 different species and determined that pretreatment with Ca(OCl)2 as a desiccant significantly affects the viability of the seeds and tetrazolium test it is a very valid to determine the viability of seeds of orchids method, however, they determined that the accuracy of these studies is affected by the ability of the analyst who interprets them.
There are several studies that evaluate the germination of orchid seeds, however, its main objective is to evaluate germination rates and germination rate in different media in order to set the protocol in vitro of each of the species propagation, only Salazar-Mercado (2012), tested for germination tetrazolium comparing to demonstrate feasibility of Colombian tropical seeds.
The aim of this study was to analyze the physical and physiological quality of Encyclia adenocaula as model and priority species in the State of Mexico, for their conservation in a genebank seed.
The study was conducted at the laboratory of tissue culture and greenhouse 5 which houses the collection of orchids of the Faculty of Agricultural Sciences at the Autonomous University of the State of Mexico. The mature seeds capsules were used in a population of E. adenocaula pollinated in 2012 and collected in May 2013 from plants of the community called "El Peñon" in the town of Temascaltepec, Mexico, located UTM 14Q 381736 and 2106024 with 1789 masl; in the laboratory he proceeded to remove the remnants of flowers and placed in paper envelope until dehiscence at room temperature, once you open the capsules proceeded to completely open along the longitudinal lines on a glass surface. Once extracted all the seeds, we proceeded to remove foreign particles or debris capsule with forceps for further storage in amber bottle under refrigeration (10 °C) until use.
In the analysis of E. adenocaula seeds were taken into account aspects such as size, weight and structure of the seed and the viability of it. For size and structure of the seed sample that was placed on three slides using a light microscope (Leica Microsystems Ltd. Leica Application Suite V4) and the software package Image Tool 3.00 (UTHSCSA) took 300 seeds they were measured species data taking as long and wide seed and coverage areas structures seeds. To determine the weight, 0.0001 g of seed were taken in triplicate and counted, by rule of three weight and number of seeds per gram was obtained.
The seed viability was determined by two techniques: 1) Tetrazolium test (TDZ) is a rapid test and germination. In the first methodology followed Bohm (1996), cited in Ossenbach et al, 2007. A 0.0001 g was established design completely random with three replications, where four treatments, which consisted of immersing filter paper packages were evaluated seeds: T1) 24 h imbibition in distilled water, was then replaced by solution of Ca(OCl)2 for two hours and subsequently 24 h in solution TDZ; T2) 24
hours in distilled water imbibition and 24 h in solution TDZ; T3) 24 h imbibition in distilled water, then was replaced with solution of Ca(OCl)2 for two hours and subsequently 24 h in solution TDZ plus two drops of Tween 80 and T4) 24 hours of soaking in distilled water and 24 h Tetrazolium solution plus two drops of Tween 80.An analysis of variance and Tukey 5% was made using the statistical program SAS System for Windows 9.0; and 2) the germination test was used a completely randomized design with ten repetitions using the technique sowing of in vitro. The culture medium was Murashige and Skoog (MS) (1962) basal medium supplemented with glycine (2 mg L-1), myo-inositol (100 mg L-1), thiamine HCl (0.4 mg L-1), pyridoxine (0.5 mg L-1), nicotinic acid (2 mg L-1), sucrose 30 mg L-1 added as treatments: 1 g L-1 charcoal (MS+CA); 10% ofbanana extract (MS+P); 10% coconut water (MS+AC); pH was adjusted to 5.7±1 with 6 g L-1 agar. The cultures were incubated at 24 ± 2 °C, 18/6 h photoperiod at a light intensity of 27 μmol m-2s-1.
Germination was considered phenological stage called initial protocorm, this because it is easy to detect visually stage. Using a stereoscopic microscope, a camera and software package Image Tool 3.00 (UTHSCSA) images of a field repetition were obtained. An analysis of variance and Tukey test at 5% was performed with the help of The SAS System for Windows 9.0 statistical software.
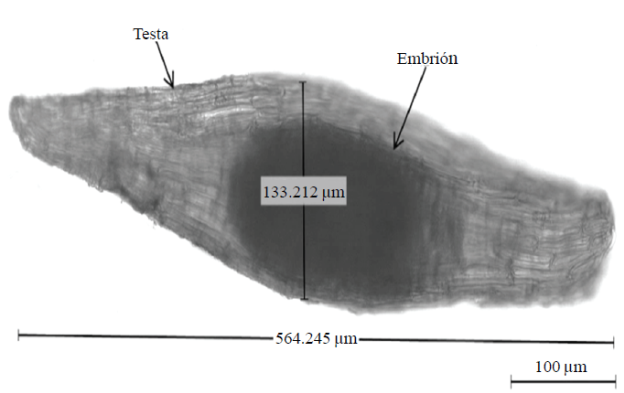
After studies it was determined E. adenocaula presents filiform and long elongated seeds, with a length of 0.56±0.08 mm and a width of 0.09±0.01 mm, covering an area of 3.25±0.5 mm2, these data were taken from whole seeds (embryo and testa) (Figure 1), results consistent with those reported for other species of orchids since 1992 by Arditti as well as Hagsater et al. (2005); Verdugo et al. (2007); Tellez (2011) and Aguilar and López (2013), reporting seed sizes from 0.25 to 1.2 mm long and 0.09 to 0.27 mm wide.
The seed presented the two basic structures of seeds corresponding to the Orchidaceae family; an embryo lacking endosperm (reserve nutrients) with an approximate area of 0.01±0.01 mm2. The approximate weight of a seed was 1.6±0.08 g, equivalent to approximately 63 500 seeds per gram. The viability of seeds to determine whether a seed is alive and has the ability to germinate (Ruiz, 2009). According to feasibility testing, testing TDZ, called rapid test, statistically significant differences (p≤ 0.05) between treatments were recorded, showing no significant difference T2 (50.3%) and T4 (48.2%) treatments; i.e. that pretreatment with Ca(OCl)2 directly affected the viability of the seeds, where this solution is used the percentages were lower corresponding to T1 and T3 with 24.1% and 48.2% respectively, results that agree with Ossenbach et al. (2007) for five species of orchids including Encyclia ochracea obtained where 95% viability without pretreatment with Ca(OCl)2 and 85% with pretreatment.
Lallana and Garcia (2013) report a viability of 90 and 85% in Trichocentrumjonesianum seeds, Salazar-Mercado (2012), in Cattlella mendelii seeds with 93% viability and Salazar and Cancino (2012) reported 87.2 and 80.6% seeds feasibility of P. vespa y S. klotzcheana respectively with the same method. According to statistical analysis there was no any significant effect on the addition of drops of Tween 80 with respect to the loss of viability, however, with the addition of this product was achieved better observation on the coloration of the embryo.
The percentage viability assessment by the technique of in vitro germination, showed statistically significant differences (p≤ 0.05) between treatments as better treatment resulting MS+CA with 56.9% germination at 30 days, followed by medium MS+AC with 31.4% germination to 55.7 days, finally the MS + P medium with 23% to 59.1 days. Results consistent with those reported in Ruiz et al. (2008) for the same species 62.4% germination at 27 days, although the difference in percentages can be given mainly by the origin of the material established in experiments. However, it is noteworthy that germination in the work can be considered from the imbibition of seeds as they consider various authors to the presence of first leaves, this must be due perhaps to the objective of the work.
However, in the same treatment the seeds germinated asynchronously; i.e. in the same condition after two months of establishing the different stages of germination and growth in vitro of the species were observed: imbibition, presenting swelling and embryo took a pale green caused by water absorption (Figure 2A); green seed, the seeds showed a pronounced green coloration and the embryo begins to increase in size within 15 days after sowing (Figure 2B); germination, at 30 days the embryo breaks testa (Figure 2C), initial protocorm; 36 days cell complex very faint green formed, the leaf primordium (Figure 2D) appearing; protocorm late, the protocorm continued growth, being born at the apex a foliar primordium 40 days after the establishment (Figure 2E); leaf development; the leaf primordium continued to grow, protocorm body began to decrease and to differentiate the leaves to about 47 days (Figure 2F); root development, was presented to the 57 days or so, the root primordia developed near the base of the leaves white (Figure 2G); full seedling, roots and leaves perfectly (Figure 2H) distinguished; They were seen as stages coincided with that reported with Flores et al. (2008), Salazar and Cancino (2012), Aguilar and López (2013) in some species of orchid.
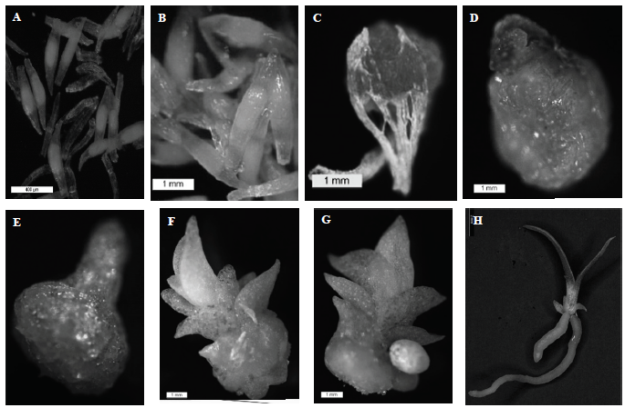
Figure 2 Germination and in vitro development of Encyclia adenocaula. A. imbibition to ±7 days after sowing, scale bar 400 μm B. Green seeds, ± 15 days. Scale bar 1 mm. C. Germination, ± 30 days, 1 mm, scale bar. D. initial protocorm, ± 36 days, 1 mm, scale bar. E. protocorm late, ± 40 days, scale bar 1 mm. F. Development of leaves, ± 47 days, scale bar 1 mm. G. root development, ± 57 days, 1 mm, scale bar. H. Full seedling, scale bar 2 mm. Photomicrographs.
Conclusions
This work allowed to determine some physical characteristics of seeds Encyclia adenocaula as: the structure, size and number of seeds per gram (0.56±0.08 mm long by 0.09±0.01 mm wide and 63 500 seeds per gram).
As for the physiological quality was checked for E. adenocaula that viability by testing Tetrazolium 1%, it is easy and quick (within three days reached 50.3%) compared with the results obtained by in vitro germination on MS medium supplemented with activated coal at 30 days (56.9% reached). It was observed that pretreatment with Ca(OCl)2 (desiccant) seed viability decreases.
The in vitro germination of E. adenocaula was presented asynchronously achieving determine the germination process and development in vitro of the species from its imbibition until the formation of seedling.
Conserve resources in a seed bank eliminates intrinsic problems of ex situ conservation, such as loss of genetic viability due to founder effect, analyzing the quality of the seeds we can store them with the assurance that at any given time achieve its regeneration smoothly.
Literatura citada
Aguilar, M. M. A. y López, E. A. L. 2013. Germinación in situ de Laelia speciosa (Kunth) Schltr., una herramienta para su conservación ex situ . In: Pulido-Flores, G. and Monk, S. (Eds.). Estudios científicos en el estado de Hidalgo y zonas aledañas. Volumen II, Lincoln, NE: Zea Books. [ Links ]
Arditti, J. 1992. Fundamentals of Orchid Biology. John Wiley & Sons, Inc. USA. 18-24 pp. [ Links ]
Flores, E. G.; Legaría, S. J. P.; Gil, V. I. y Colinas, L. M. T. 2008. Propagación in vitro de Oncidium stramineum Lindl. Una orquídea amenazada y endémica de México. Rev. Chapingo Ser. Hortic. 14(3):347-353. [ Links ]
Lallana, V. H. y García, L. F. 2013. Efecto de pretratamientos en la prueba de viabilidad de semillas de Trichocentrum jonesianum (Orchidaceae). Argentina. Investigación Agraria. 15(2):129-132. [ Links ]
Lascuráin, M.; List, R.; Barraza, L.; Díaz, E. P.; Gual, F. S.; Maunder, M.; Dorantes, J. y Luna, V. E. 2009. Conservación de especies ex situ, en capital natural de México. Estado de conservación y tendencias de cambio. Conabio, México. 517-544 pp. [ Links ]
Murashige, T. and Skoog, F.1962.A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiolgia Plantarum. 15(3):473-497. [ Links ]
Ossenbach, C.; Arce, J. y Warner, J. 2007. Almacenamiento de semillas de diferentes especies de orquídeas para su conservación en un banco de germoplasma: II. Deshidratación, almacenamiento y pruebas de viabilidad de las semillas. Tierra Tropical. 3(1):47-59. [ Links ]
Raó, N. K.; J. Hanson, M. E.; Dulloo, K.; Ghosh, D.; Novell y Larinde M. 2007. Manual para el manejo de semillas en bancos de germoplasma. Manuales para bancos de germoplasma No. 8. Bioversity International, Roma, Italia. 165 p. [ Links ]
Ruíz, B. C.; Laguna, C. A.; Iglesias, A. L. G.; Damon, A.; Marín, H. T. N. J.; Azpíroz, R. H. S. and Moreno, M. J. L. 2008. In vitro germination of Encyclia adenocaula (La Llave & Lex.) Schltr (Orchidaceae). Rev. Int. Bot. 77:203-215. [ Links ]
Ruiz, M. A. 2009. El análisis de tetrazolio en el control de calidad de semillas. Caso de estudio: cebadilla chaqueña. Estación Experimental Agropecuaria Anguil "Ing. Agr. Guillermo Covas". Argentina. Publicación técnica 17. 20 p. [ Links ]
Salazar-Mercado, S. A. 2012. Germinación asimbiótica de semillas y desarrollo in vitro de plántulas de Cattleya mendelii Dombrain (Orchidaceae). Acta Agronómica. 61(1):69-68. [ Links ]
Salazar, S. A. M and Cancino, G. O. 2012. Evaluation of the effects of two organics supplements on in vitro germination of native orchids in the province of Pamplona, Colombia. Rev. Colomb. Biotecnol. 14(1):53-59. [ Links ]
Sánchez, Ch. N. y Jiménez, V. M. 2010. Técnicas de conservación in vitro para el establecimiento de bancos de germoplasma en cultivos tropicales. Agron. Mesoam. 21(1):193-205. [ Links ]
SEMARNAT. 2010. Norma Oficial Mexicana NOM-059-SEMARNAT-2010, Protección Ambiental-Especies nativas de México de Flora y Fauna Silvestres-Categorías de riesgo y especificaciones para su inclusión, exclusión o cambio- lista de Especies en riesgo. Diario Oficial de la Federación 30 diciembre. México. [ Links ]
Szeszko, D. R. F. 2011. La orquideoflora mexiquense. Biblioteca mexiquense del bicentenario, colección mayor del estado de México: patrimonio del pueblo. Consejo Editorial de la Administración Pública Estatal. 362 p. [ Links ]
Téllez, V. M.A.A. 2011. Diagnóstico de la familia Orchidaceae en México. Universidad Autónoma Chapingo (UACH). Texcoco, Edo de México. 179 p. [ Links ]
Received: May 2016; Accepted: June 2016











 texto en
texto en