Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista mexicana de ciencias agrícolas
versão impressa ISSN 2007-0934
Rev. Mex. Cienc. Agríc vol.7 no.7 Texcoco Set./Nov. 2016
Articles
Characterization of storage proteins and mineral content melon seed (Cucumis melo L.)
1Tecnológico Nacional de México-Instituto Tecnológico de Roque. Carretera Celaya-Juventino Rosas, km 8. C. P. 38110. Celaya, Guanajuato, México. (garamirez@itroque.edu.mx; alberh1@hotmail.com; cesar.aguirre.m@hotmail.com; jor_covarru-jrg@hotmail.com; gaiturriaga@itroque.edu.mx).
The chinese melon seed has been used traditionally to make horchata; however, it has lost the habit of consuming it. To expand the knowledge of this plant resource reservation soluble proteins were extracted from the seed. The total protein content was 27.34% for cantaloupe and 24.9% for honeydew (smooth). The globulins constitute the majority faction and prolamins, the minority. In the electrophoretic pattern shows globulin fraction abundant and well-defined bands. The precipitated proteins with hydrochloric acid or cold showed an electrophoretic pattern similar in both varieties. Proteins were extracted at pH values best between 5 and 12, but not values of 2 to 4. The treatment with trypsin and chymotrypsin showed accumulation of bands on the front of the gel, demonstrating the presence of digested peptides. The inhibitory activity against trypsin is greater at the albumins fraction with 741 UI mL-1 and globulin fraction with 587 UI mL-1. The mineral content was 0.5% for phosphorus and magnesium 0.28 for cantaloupe; for honeydew was 0.36% for potassium and 0.28% for phosphorus. The oil content was 35.8% in seed cantaloupe and 23.2% in the honeydew, which is known to 67.5% are unsaturated fatty acids; oil was amber color, pleasant odor. The use of melon seed is a plant resource to be promoted for its high content of protein and oil, as it is valuable for human consumption. It can also be used in the formulation of livestock feed or cultured insect larvae in the laboratory.
Keywords: digestibility; marginal resource; oil; protein reserve
La semilla de melón chino se ha usado de manera tradicional para elaborar horchata; sin embargo, se ha perdido la costumbre de consumirla. A fin de ampliar el conocimiento sobre este recurso vegetal se extrajeron las proteínas de reserva solubles de la semilla. El contenido total de proteína fue de 27.34% para el melón chino y de 24.9% para el melón verde (liso). Las globulinas constituyen la fracción mayoritaria y las prolaminas, la minoritaria. En el patrón electroforético, la fracción globulina muestra bandas abundantes y bien definidas. Las proteínas precipitadas con ácido clorhídrico o frío mostraron un patrón electroforético semejante en ambas variedades. Las proteínas se extrajeron mejor a valores de pH entre 5 y 12, pero no a valores de 2 a 4. El tratamiento con tripsina y quimotripsina mostró acumulación de bandas en el frente del gel, lo cual demuestra la presencia de péptidos digeridos. La actividad inhibitoria contra tripsina es mayor en la fracción albúminas con 741 UI mL-1 y fracción globulina con 587 UI mL-1. El contenido mineral fue de 0.5% para fósforo y de 0.28 para magnesio en melón chino; para el verde fue de 0.36% para potasio y de 0.28% para fósforo. El contenido de aceite fue de 35.8% en semilla de melón chino y de 23.2% en el verde, del cual se sabe que 67.5% son ácidos grasos insaturados; el aceite fue de color ámbar, de olor agradable. El uso de la semilla de melón es un recurso vegetal que debe promoverse por su alto contenido de proteína y aceite, ya que es valioso para la alimentación humana. También puede usarse en la formulación de alimento para ganado o de larvas de insecto cultivadas en laboratorio.
Palabras clave: aceite; digestibilidad; proteína de reserva; recurso marginal
Introduction
The melon (Cucumis melo L.) is an annual herbaceous crop, creeping, thick stems and leaf petioles, belonging to the Cucurbitaceae family. The melon is widely cultivated for its edible fruit and vegetables is one of the most important, both global and national, for their production and planted area. In 2012 there were 32 million tons worldwide. The melon genome contains 27 427 genes allegedly encoding proteins and is proposed as a model for studying fruit ripening, sex determination and physiology of phloem (García-Mas et al., 2012).
The protein consumed by mankind comes mostly from vegetables, legumes being the main group of these, with soy as the most important crop, followed by beans. The grains of edible legumes are nutritionally important, as the main source of protein (20 to 40% or more of the daily intake) low-cost diet of the mexican population (Chagas and Santoro, 1997; Pérez et al, 2002).
The legume family and in particular the case of soybeans, is a clear example of seeds rich in protein used in food, and in the case of the family cucurbitaceae can be taken as an example to the seed or pumpkin seed (Cucurbita pepo), as the seed contains 31% protein, 36% fat and 15% carbohydrates, widely consumed in Mexico, but wasted in other countries (Martínez et al, 2011). The melon seed, besides having medicinal qualities (Ravishankar and Vishnu, 2012), is a source of protein (27%) and oil (35%), which would improve nutrition and help rescue the culinary roots (Leyva and Pérez, 2015).
Protein concentrates, such as phaseolins, have been added to meat products, biscuits and bread, successfully. The stability of the food emulsions is affected by the source of proteins, the extraction method, the pH and the addition of other compounds such as polysaccharides. A energy drinks for athletes are added branched chain amino acids (valine, leucine, isoleucine) by the assumption that these decrease the synthesis of serotonin in the brain, a neurotransmitter associated with fatigue, and that intake of branched chain amino acids may delay fatigue and enhance athletic performance. Melon proteins provide 167% of daily intake needs isoleucine, 113% of leucine, 51% of lysine, 37% of methionine and 169% of phenylalanine (Hu and Ao, 2007; Makri and Doxastakis, 2006).
Furthermore, proteins melon seed antifungal properties found (Ferreira et al, 2007). A better understanding of proteins reserve allows its use for new applications such as the production of vaccines (Maruyama et al, 2014) or special uses, such as diet to prevent or treat diseases (Tandang et al., 2011). For example, protein intake, lysine relationship: arginine is recommended to be less than 2 as this is important for the control of hyperlipidemia and arteriosclerosis (Martínez and Sánchez, 2004). The melon seed is a resource used in human food in a traditional way but has fallen into disuse and we believe that their study will allow food and reconsider as a valuable resource for its composition.
Materials and methods
The seed (approximately 50 g) was finely ground and degreased with a mixture of chloroform-methanol 2:1. The defatted was used for protein extraction (Syros et al, 2003). Storage proteins were extracted sequentially incubating 1 h each time, first with distilled water to remove albumins; the end of the hour incubation with stirring, 25 min was centrifuged at 13 000 x g in microfuge (Eppendorf mark) and the supernatant was taken; the precipitate was added 0.5 M sodium chloride in 50 mM Tris pH 8, for extracting globulins, incubating one hour at room temperature with stirring, centrifuged as mentioned and the supernatant was taken; the pellet was added 2-propanol 55% (v/v), incubated one hour stirring and centrifuged again to remove prolamins, saving the supernatant.
A tablet was added 0.1 M borate buffer with 0.5% SDS, pH 8; glutelins for extraction, was incubated one hour with stirring at room temperature (Syros et al., 2008). For the separation of protein denaturing gels 10% they were employed with tricine buffer (Shagger and von Jagow, 1987). For protein extraction to different pH values (2, 4, 6, 7, 8, 10, 12); 1 g of defatted flour was weighed and added 5 mL of distilled water and brought to the appropriate pH by adding small amounts of NaOH or HCl (1N or 0.1 N), the volume was completed to 10 mL, it was incubated with stirring for one hour and centrifuged one hour at 14 000 xg in centrifuge (Wolf and Sathe 1998; Meng and Ma, 2002; Tavano and Neves, 2008). The moisture content of the seed was determined by weighing 20 grains and incubating at 80 C for 24 h (Raya et al., 2014). Cryoprecipitation: 0.1 g of defatted it weighed and added 0.5 mL of water, stirred for one hour and centrifuged for 30 min at 15 700 x g in microfuge; the supernatant was removed.
To the residue (tablet) was again added 1 mL of distilled H2O and extracted for 12 h at 4 °C (overnight), the next day centrifuged again under the above conditions, and the supernatants of both extractions were pooled in a and 1.5 mL tube were taken to refrigeration (4 °C) for 12 h 45 min was centrifuged at15 700 x g. The supernatant was hydrochloric acid added to achieve a concentration of (HCl) at 0.025 M. It was refrigerated for an hour and 30 minutes was centrifuged at 15 700 x g to obtain the protein precipitate (Montoya et al., 2008). The protein was quantified in triplicate by the method Bradford (1976).
Mineral quantification was performed using a scanning electron microscope, Jeol mark coupled to a dispersive X-ray probe tablets of melon seed meal. At least three analyzes were made per sample. This quantification technique gives results comparable to others and has been used for biological samples (Liu et al., 2007; Raya and Mancilla, 2009).
In vitro digestibility: samples were taken in triplicate 100 µl of protein extract and placed separately adjusted with HCl to pH 2; 30 min preincubated at 39 °C in a water bath and 10µl was added pepsin enzyme (10 mg/mL) (from porcine gastric mucosa pepsin, Sigma); An aliquot of 25 µl was taken and the rest was added trypsin-chymotrypsin (10 mg mL-1) (Trypsin type IXS from porcine pancreas, Sigma; chymotrypsinogen A type II from bovine pancreas, Sigma) and 120 min was incubated at 39 °C ; taking 20 µL of each of the aliquots obtained in vitro digestibility, samples were loaded on the gel. After the electrophoresis the gel was stained with Coomassie blue (Montoya et al., 2008).
Oil extraction: The 1 g of flour was weighed; it was placed in a pre-weighed filter and this was put into a glass; was added hexane in a ratio of4:1 volume/weight, the glass was covered to prevent the solvent to evaporate and allowed to incubate for18 h. At the end of this time the vessel was taken to Soxhlet Tecator brand model 1043; the glass on the team was heated for two hours and distilled for two hours and evaporated the solvent. Once extracted oil and filter glass and weighed by difference, the oil yield was obtained. inhibitory activity against trypsin enzymes: was determined by measuring the absorbance increase at 253 nm in a spectrophotometric reader 722-2000 VELAB model using as synthetic substrate and the N-a-Benzoil-L-Arginina p-nitroanilida (BApNA) (Raya et al, 2014).
This activity is defined as the number of inhibited enzyme units, and is calculated by the formula:
Where: IU/mL= milliliter inhibition units; Abs Enz= absorbance enzyme; Abs Inh= Absorbance inhibitor; Vi= volume in mL inhibitor.
The 60 µL of each protein extract were used, was used to control bovine enzyme trypsin (200 µg mL-1) and as substrate was used the N-benzoil-arginina-p-nitroanilida (BApNA).
Determination of ash: two grams of sample were weighed into a tared crucible and dry; the crucible and its contents were fired first on a low flame, avoiding possible excessive soot formation, until charred and then transferred to a muffle for 4 h.
Results and discussion
The oil content seed melon is reported as 35.36%, similar to that found in this study for the cantaloupe content, but the honeydew presented a lower content (23.28%), but important, than other oilseeds (Hu and Ao, 2007; Raya et al., 2012). A cantaloupe can contain about 14 grams of seed per fruit on average; green or smooth can yield about 21 grams. The composition of the seed oil is a melon 53.9% of linoleic, 12.1% of oleic, 23.9% of palmitic and 5.7% of stearic. A 67.5% are unsaturated fatty acids (Hu and Ao, 2007). Comparatively, olive oil contains 71.9% of oleic (18:1) and 7.5% of linoleic (18:2) (Dubois et al, 2007).
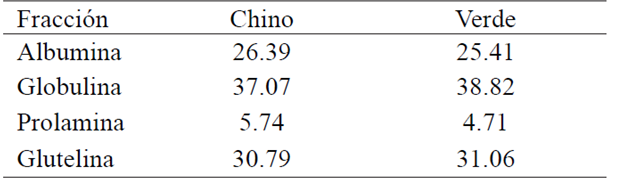
The total protein content, gave 27.34% for cantaloupe (Hu and Ao, 2007). For honeydew a value of 29.9% (Table 1), similar to that found in this study reported amount. The distribution of the protein fractions to the defatted (Table 2) shows that the fraction is greater presence globulin 37% while that found in lower content is the prolamine fraction with 6% compared to the protein. The seed chokecherry more water-soluble protein (albumin fraction) was extracted with other solvents, but also managed to extract a good deal with saline and borates. This is consistent with those reported by other authors, as dicots presented as major proteins of albumins and globulins reservation. Other authors have reported differences in the protein content and the relative abundance of different fractions, which could be due to the varieties used or culture conditions (Mandal and Mandal, 2000; Tavano and Neves, 2008; Raya, et al, 2012).
Table 1 Content of oil, protein, ash and moisture seed cantaloupe and honeydew.

El contenido de carbohidratos se calculó por diferencia con el resto de los componentes.
The distribution of the protein fractions to flour honeydew (Table 2) shows that the most abundant fraction is 38% globulin while found in lesser amount is the prolamine fraction with 4.7%; globulins fraction in a concentration of 5.83 mg/mL was obtained. In creole beans, albumins are a little more than half of the extracted soluble protein (Raya et al, 2014), although legumes contain high amounts of globulins (Tavano and Neves, 2008).
The fraction most abundant protein honeydew is the globulin fraction is in the third lane (Figure 1A, B), followed by the glutelin fraction in the fifth lane and where one can see that is low molecular weight. In the second lane is albumen which contains protein of high molecular weight low, being most abundant low molecular weight. The less abundant is the prolamin fraction with low molecular weight proteins (Figure 1).

Figure 1 Electrophoretic pattern of protein fractions melon seed. A. Chinese melon. Lane 1 molecular weight markers (MPM); 2 albumin fraction; 3 globulin fraction; 4 prolamin fraction; and 5 glutelin fraction. B. green melon. Lane 1 MPM; 2 albumin fraction; 3 globulin fraction; 4 prolamin fraction; and 5 glutelin fraction.
In Figure 1B is appreciated electrophoretic seed protein pattern of honeydew, very similar to the cantaloupe (1A). This would indicate that at least at this level there is little diversity and a variety probably gave rise to the other.
In Figure 2 soluble proteins precipitated both varieties, where again a very similar pattern is observed between the two varieties shown. In some bands enriched green slightly below the height of the molecular weight marker (66 kDa, lane 1) are observed. Protein extraction melon seed pHs different. According to the data obtained for the concentration of protein extracts, treatment resulted in a higher concentration was water extraction at pH 8, 6.29 mg mL-1.

Figure 2 Electrophoretic patterns of precipitated protein with hydrochloric acid or low temperature. Lane 1 MPM (66 kDa); lane 2 cantaloupe protein precipitated with hydrochloric acid; lane 3, protein precipitated with hydrochloric acid honeydew. Lane 4, cantaloupe cryoprecipitated protein; lane 5, protein cryoprecipitated honeydew.
The high protein solubility is due to the low hydrophobicity of the surface; high solubility at neutral pH and acid enables use in beverages and liquid food (Meng and Ma, 2002). The chokecherry seed protein is insoluble to very acidic pH (Raya et al., 2012), red bean protein ("red bean") has a minimum solubility at pH 5; something similar to what was observed with melon, which is poorly soluble at pH 4 (lane 4, Figure 3), (Chagas and Santoro, 1997). The cicer arietinum protein has a minimum solubility between pH 4.5 to 6 and practically insoluble at pH 5 (Tavano and Neves, 2008).
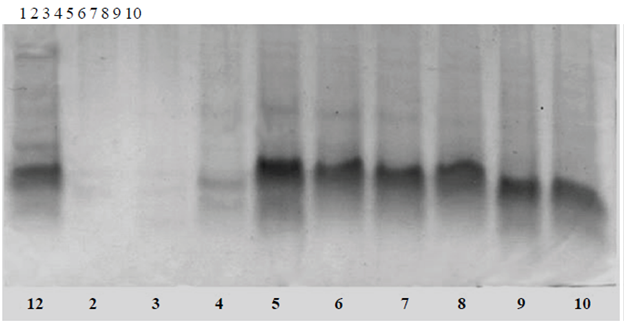
Figure 3 Removing protein at different pH valúes. Protein extraction at pH 8 seems to be the best; at acidic pH, 2.3 and 4 hardly extracted protein, probably due to the low solubility of these pH. Most values are extracted basic or slightly acidic pH (pH5.6).
In Figure 4 the digestion of protein extracted from seed and treated with digestive enzymes shown; observations indicate that a protein is easily digested. This would lead for use on special diets where protein is required with these characteristics. For example, baby food, sports (Ardura et al., 2000).

Figure 4 Soluble proteins melon treated with digestive enzymes. Shown in lane 1 single with pepsin digested protein. In lane 2, protein and chymotrypsin pepsin digested. In lanes 3 and 4 the peptides are observed in the bottom of the gel treatments with pepsin, trypsin and chymotrypsin.
The extract showed higher inhibitory activity against protease protein extract was obtained with water, which corresponds to the albumin fraction; extracts corresponding to globulins and prolamins have similar activity between them (Figure 5), globulins extract with reducing agent dithiothreitol (DTT) shows a large decrease in enzyme activity and glutelin extract has little inhibitory activity. In chokecherry seed and that of C. arietinum, albumin fraction has the highest activity, followed by the glutelin (Tavano and Neves, 2008; Raya et al, 2012). However, observations in the laboratory indicate that these seeds are easily colonized and consumed by storage pests, its high protein and lipid certainly content can propose them as an ingredient for diets such organisms, something that would be worth exploring (Wang et al, 2014).
The seed cantaloupe contains large amount of phosphorus (P) in relation to the other elements containing followed magnesium (Mg) (Figure 6), so can be considered to seed as a food with good supply of phosphorus and magnesium. Compared to seed honeydew, cantaloupe contains that of silicon (Si) and chlorine (Cl) (Figure 7).
The honeydew melon seed is rich in potassium (K), followed by phosphorus (P), magnesium (Mg) and sulfur (S); the melon contains between 0.3% and 0.5% phosphorus (Figure 7), a content somewhat lower than reported for seed Nicotiana glauca (Hoeking, 1980). Both varieties showed a potassium content between 0.2 and 0.35%; Nicotiana seed reported 1 030 mg/100 g and chokecherry 700 mg/100 g. The sulfur approaches that reported for Nicotiana (190 mg/100 g), the same as the magnesium 350 mg/100 g; for seed chokecherry 120 mg/100 g were found (Hoeking, 1980; Raya et al, 2012).
In the seed of bean (Phaseolus vulgaris L.) the content of molybdenum (Mo) differs by an order of magnitude between rich and poor soils in this element; the amount of minerals present in the seed is, in some cases, which will make available for plant development (Tyler and Zohlen, 1998). In grain sorghum (Sorghum halepense), concentrations of phosphorus (P), potassium (K) and magnesium (Mg) decreases significantly decrease when supplementation of these elements (Tyler and Zohlen, 1998). The content of cobalt (Co) and the sulfur is greater than in the seed of cantaloupe. The Co is required for biological nitrogen fixation, but absorption in melon could simply be that present in the soil; the cobalt present in the seed can prevent chlorosis of the future plant (Tyler and Zohlen, 1998). There is a large genetic component as to the accumulation of mineral elements by plants (Hodson et al, 2005). The National Institute of Nutrition in Mexico, recommends a daily intake for adults, 350 mg of Mg, phosphate 800 mg and potassium 900-2 of 700 mg daily.
The melon seed is a suitable source of potassium, phosphorus (phosphate) and magnesium, sulfur and even in the ease of seed honeydew or smooth. The proteins were extracted sequentially, using water, saline, alcohol (2-propanol) and alkali (pH 9.5 borate buffer), which has allowed us to determine that more and better protein extracted with saline. Moreover, the oil extracted is amber and pleasant smell. The inhibitory activity against trypsin was measured and virtually zero, indicating that this protein can be eaten raw without worrying about this. Measuring inhibitory activity was performed by a spectrophotometric method using a synthetic substrate and. The mineral content of the seed is similar to other (beans, corn, chokecherry) but highlights the presence of molybdenum.
The protein is also extracted at different pH, which allows us to know that does not dissolve well in acidic solution, but if it can dissolve in neutral pH or alkaline. This would allow use in foods with this feature. The melon seed contains 35% oil, which makes it susceptible to profit for the extraction of this, either for human use or biodiesel (Dubois et al., 2007). This could be particularly suitable, human use in areas where caloric intake is deficient; i.e. marginalized and rural areas (Leyva and Pérez, 2015). To be collected and used melon seed could certainly be equated with pumpkin in terms of volume handled. They exist in the flour to prepare horchata market, which apparently contain rice flour and lots of sugar; it is more healthy and nutritious definitely a flour-based melon seed; She was tested to combine it with amaranth flour to improve its amino acid composition, achieving good acceptance among testers. This was rated as very good in flavor, acidity and appearance.
Conclusions
The electrophoretic profile of the protein fractions of the materials used as well as the electrophoretic profile of cryoprecipitated protein materials used similar types of proteins. The cryoprecipitation of proteins yielded well-defined bands, enabling proposed technique as a method of fractionation. The cantaloupe and honeydew have a similar amount of protein; 2, 3, 4 pH not extracted protein. The melon seed protein to the diet could be integrated either as a protein extract or consuming the seeds, as apparently, it is easily digestible for not being rich in protease inhibitors. The oil content was higher in the cantaloupe that in the honeydew; this makes possible its use for food purposes or even for obtaining biodiesel. The melon seed is a suitable source of minerals, especially potassium, phosphorus (phosphate) and magnesium.
Literatura citada
Ardura, F. J.; de Hoyos, L. C.; de Llano, J. M. A.; Aldana, G. J. y Revilla, R. M. A. 2000. Actimetría y cronobiología en el cólico infantil. Efecto de dos dietas especiales. Boletín Pediatría 40:8-18. [ Links ]
Bradford, M. M. 1976. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein dye binding. Analytical Biochem. 72:248-252. [ Links ]
Chagas, E. P. and Santoro, l. G. Y. 1997. Globulin and albumin protein in dehulled seeds of three Phaseolus vulgaris cultivars. Plant Foods for Human Nutrition 51:17-26. [ Links ]
Dubois V.; Breton, M. S.; Linder, F. J. and Parmentier, M. 2007. Fatty acid profiles of 80 vegetables oils with regard to their nutritional potential. Eur. J. Lipid Sei. Teehnol. 109:710-732. [ Links ]
Ferreira, S. F. F.; Oliveira, M. A.; Fernandes, K. V. S; Carvalho, A. O.; Perales, J. and Gomes, V. M. 2007. A new peptide of melón seeds which shows sequence homology with vicilin: partial characterization and antifungal activity. Scientia Hortic. 111:399-405. [ Links ]
García-Mas, J.; Benjak, A.; Sanseverino, W.; Bourgeois, M.; Mir, G.; González, V.M.; Hénaff, E.; Cámara, F.; Cozzuto, L.; Lowy, E.; Alioto, T.; Capella-Gutiérrez,; Blanca, J.; Cañizares, J.; Ziarsolo, P.; Gonzalez-Ibeas, D.; Rodriguez-Moreno, L.; Droege, M.; Du, L.; Alvarez-Tejado, M.; Lorente-Galdos, B.; Melé, M.; Yang, L.; Weng, Y.; Navarro, A.; Marques-Bonet, T.; Aranda, M.A.; Nuez, F.; Pieó, B.; Gabaldón, T.; Roma, G.; Guigó, R.; Casaeuberta, J.M; Arús, P. and Puigdoménech, P. 2012. The genome of melón (Cucumis melo L.). Procedings Natural Academie Science. 109:11872-11877. [ Links ]
Hoeking, P. J. 1980. The composition of phloem exudate and xilem sap from tree tobacco (Nicotiana glauca Grah). Annals Bot. 45:633-643. [ Links ]
Hodson, M. J.; White, P. J.; Mead, A. and Broadley, M. R. 2005. Phylogenetie variation in the silicon composition of plants. Annals Bot. 96:1027-1046. [ Links ]
Hu, M. H. and Ao, Y. 2007. Characteristics of some nutritional composition of melon (Cucumis melo hybrid 'Chunli') seeds. Inter. J. Food Sci. Technol. 42:1397-1401. [ Links ]
Leyva, T. D. A. y Pérez, V. A. 2015. Perdida de las raíces culinarias por la transformación en la cultura alimentaria. Rev. Mex. Cienc. Agric. 6:867-889. [ Links ]
Liu, D.; Kottke, I. and Adam, D. 2007. Localization of cadmium in the root cells of Allium cepa by energy dispersive X-ray analysis. Biologia Plantarum. 51:363-366. [ Links ]
Majkowska, G. J. 2009. Mineral content of melon fruit (Cucumis melo L.). J. Elemental. 14:717-727. [ Links ]
Makri, E. A. and Doxastakis, G. I. 2006. Study of emulsions stabilized with Phaseolus vulgaris and Phaseolus coccineus with addition of Arabic gum, locust bean gum, and xanthan gum. Food Hydroeolloids. 20:1141-1152. [ Links ]
Mandal, R. K. and Mandal, S. 2000. Seed storage protein and approaches improvement of their nutritional quality by genetic engineering. Current Sei. 79: 576-589. [ Links ]
Martínez, A. Y. O.; Martínez, Y. J.; Córdova, L. M.; Valdivié, N. y Estarrón, E. M. 2011. Fitoesteroles y escualeno como hipocolesterolémicos en cinco variedades de semillas de Cucurbita máxima y Cucurbita moschata (calabaza). Rev. Cubana Plantas Medicinales. 16:72-81. [ Links ]
Martínez, A. O. y Sánchez, de M. F. 2004.Arginina, óxido nítrico y función endotelial. Ars Pharm 45:303-317. [ Links ]
Maruyama, N.; Fujiwara, K. K.; Yokoyama, C. C.; Hasegawa, K. H.; Takagi, K.; Nishizawa, Y.; Uki, T.; Kawarabayashi, M.; Shouji, M.; Ishimoto, P. and Terakawa, T. 2014. Stable accumulation of seed storage proteins containing vaccine peptides in transgenic soybean seeds. J. Bios. Bioeng. 118: 441-447. [ Links ]
Montoya, C.A.; Leterme P.; Victoria, N. F.; Toro O.; Souffrant W.B.; Beebe, S. and Lailles, J. P. 2008. Susceptibility of phaseolin to in vitro proteolysis is highly variable across common bean varieties (Phaseolusvulgaris). J. Agrie. Food Chem. 56:2183-2191. [ Links ]
Pérez, H. P.; Esquivel, E. G.; Rosales, S. R. y Acosta, G. J. A. 2002. Caracterización física, culinaria y nutricional de frijol del altiplano subhúmedo de México. Arch. Latinoam. Nutric. 52:172-180. [ Links ]
Ravishankar, K. and Vishnu, P. P. S. 2012. In vitro antioxidant activity of ethanolic seed extracts of Macrotyloma uniflorum and Cucumis melo for therapeutic potential. Int. J. Res. Pharmacy Chem. 2:2231-2781. [ Links ]
Raya, P. J. C.; Aguirre, M. C. L; Tapia, A. R; Ramírez, P. J. C. y Covarrubias, P. J. 2012. Composición elemental y caracterización de las proteínas de reserva de la semilla de capulín (Prunus serotina). Polibotanica 34:203-215. [ Links ]
Raya, P. J. C.; Gutiérrez, B. G. M; Ramírez, P. J. G.; Covarrubias, P. J. y Aguirre, M. C. L. 2014. Caracterización de proteínas y contenido mineral de dos variedades nativas de frijol de México. Agron. Mesoam. 25:1-11 [ Links ]
Raya, P. J. C. y Aguirre, M. C. L. 2009. Composición elemental de algunas especies de plantas silvestres mexicanas. Rev. Chapingo Ser. Cienc. Forest. Amb. 15:95-99. [ Links ]
Ribeiro, S. F. F.; Agizzio, A. P.; Machado, O. L. T.; Neves, F.; Oliveira, M. A. A. G. C.; Fernández, K. V. S.; Carvalho, A. O.; Perales, J. and Gomes, V. M. 2007. Anew peptide of melon seeds which shows sequence homology with vicilin: partial characterization and antifungal activity. Scientia Hortic. 111:399-405. [ Links ]
Sehägger, H. and von Jagow, G. 1987. Trieine-sodium dodecyl sulfate polyacrylamide gel electrophoresis for the separation of protein in the range from 1-100 kDa. Anal. Biochem. 166:368-379. [ Links ]
Shewry, P. R. 2007. Improving the protein content and composition of cereal grain. J. Cereal Sci. 46:239-250. [ Links ]
Shewry, P. R. and Halford, N. G. 2001. Cereal seed storage proteins: structures, properties and role in grain utilization. J. Exp. Bot. 53: 947-958. [ Links ]
Shewry, P. R.; Napier, J.A. and Tatham, A. S. 1995. Seed storage proteins: structures and biosynthesis. The Plant Cell. 7:945-956. [ Links ]
Syros, T.; Yupsanis, T. and Economou, A. 2003. Fractionation and electrophoretic patterns of storage protein of Ebanus creatica. Apreliminary survey as a tool in taxonomy. Biologia Plantarum. 46:435-443. [ Links ]
Tandang, S.; Tecson, M. R. G.; Mikami, M. E. M; Utsumi, B. and Maruyama, N. S. 2011. Molecular design of seed storage proteins for enhanced food physicochemical properties. Ann. Review Food Sci. Technol. 2:59-73. [ Links ]
Tavano, O. L. and Neves, V. A. 2008. Isolation, solubility and in vitro hydrolysis of chickpea vicilin-like protein. LWT 41:1244-1251. [ Links ]
Tyler, G. and Zohlen, A. 1998. Plant seeds as mineral nutrient resource for seedlings-A comparison of plants from calcareus and silicate soils. Ann. Bot. 81:455-459. [ Links ]
Wang, Y. Ch.; Zhang, S. K. and Ren, J. S. 2014. Effects of dietary additives in artificial diets on survival and larval development of Cnaphalocrocis medinalis (Lepidoptera: crambidae). Florida Entomologist. 97:1041-1045. [ Links ]
Received: June 2016; Accepted: August 2016











 texto em
texto em