Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista mexicana de ciencias agrícolas
versão impressa ISSN 2007-0934
Rev. Mex. Cienc. Agríc vol.7 no.7 Texcoco Set./Nov. 2016
Articles
Rating wild strains of Azospirillum sp. and Gluconacetobacter sp. as promoters of plant growth
1Universidad Tecnológica de Izúcar de Matamoros-Programa Educativo de Agrobiotecnología. Prolongación Reforma 168, Barrio de Santiago Mihuacán C. P. 74420, Izúcar de Matamoros, Puebla. Tel: 01(243) 436 38 95. (disolga_2610@hotmail.com; humberto950@hotmail.com; jadolfoft18@hotmail.com).
The current patterns of plant nutrition involve extensive use of chemical fertilizers that bring with it numerous side effects among which pollution of groundwater and progressive soil erosion, reflecting the urgent need for alternatives that allow replace efficiently those schemes. The proposal for this work alternative, developed in the facilities of the Technological University of Izucar de Matamoros in 2013, is to promote the use of bio-fertilizers prepared from indigenous bacteria in the region, for which, were performed assays in vitro and inplanta demonstrating its effect as plant growth promoters through mechanisms such as biological nitrogen fixation (FBN) and the production of growth regulators. Two strains were isolated using media lacking culture nitrogen, both characterized in part, the first of which belongs to the genus Azospirillum called M1 isolated from roots of Jatropha, the second genus Gluconacetobacter called G1 isolated from roots of sugar cane. In tests on cuttings of dragon fruit, corn plants and sugarcane both in vitro and in planta it was observed that both strains have the ability to promote the formation of lateral roots and that the number of these is higher compared to witnesses not it inoculated likewise, it was observed that the strain M1 has a similar effect on the G1 rhizogenesis strain and co-inoculation of both strains in these plant models.
Keywords: bacteria; bio-fertilizers; native
Los esquemas actuales de nutrición vegetal implican el uso extensivo de fertilizantes químicos que acarrean consigo numerosos efectos secundarios entre los cuales destacan la contaminación de los mantos freáticos y la progresiva erosión del suelo, lo cual refleja la imperiosa necesidad de alternativas que permita reemplazar de forma eficiente aquellos esquemas. La alternativa propuesta por este trabajo, desarrollado en las instalaciones de la Universidad Tecnológica de Izúcar de Matamoros en 2013, es impulsar el uso de biofertilizantes elaborados a base de bacterias autóctonas de la región, para lo cual, se realizaron ensayos in vitro e in planta que demuestran su efecto como promotores de crecimiento vegetal por medio de mecanismos como la fijación biológica de nitrógeno (FBN) y la producción de reguladores de crecimiento. Se aislaron dos cepas empleando medios de cultivo carentes de nitrógeno, ambas caracterizadas de forma parcial, la primera de ellas pertenece al género Azospirillum denominada M1 aislada de raíces de Jatropha, la segunda del género Gluconacetobacter denominada G1 aislada de raíces de caña de azúcar. En los ensayos realizados en esquejes de pitahaya, plantas de maíz y caña de azúcar tanto in vitro como in planta se observó que ambas cepas poseen la capacidad de promover la formación de raíces laterales y que el número de estas es mayor en comparación con testigos no inoculados, del mismo modo, se observó que la cepa M1 posee un efecto semejante en rizogénesis al de la cepa G1 y que la coinoculación de ambas cepas en estos modelos vegetales.
Palabras clave: autóctonas; bacterias; biofertilizantes
Introduction
For optimal performance in any crop agronomic interest, significant amounts of chemical fertilizers are required; however, use a negative impact on its profitability, and increase the problem of salinity due to the physicochemical characteristics of the soil. An alternative to the use of chemical fertilizers are promoters of plant growth or microorganisms (PGPR) called "bioestimulantes", "biofertilizers" "inoculants" (Cárdenas et al, 2010).
The most commonly used for this purpose microorganisms are bacteria belonging to the genera, Rhizobium, Azospirillum, Azotobacter, Gluconacetobacter and Pseudomonas, as well as mycorrhizal fungi of the genus Glomus which generally come from other regions which limits their actions thereby affecting the process production (Hirsch et al., 2001; Sevilla et al., 2001). Thus, studies on adaptation and biofertilizer efficiency of new strains shall be conducted in the regions where they are to be used. Inoculation with Azospirillum brasilense is highly beneficial in grasses such as corn, sugar cane, grasses and sorghum, as it provides 30 to 50% nitrogen of these crops (Martínez et al., 2003; Vivienne et al., 2004). Besides fixing nitrogen, this bacterium is able to produce plant growth hormones such as indole acetic acid (AIA), generating significant root growth, including increased absorption and chemical fertilizers own applied (Aguilar et al., 2008).
Gluconacetobacter diazotrophicus, microorganism whose discovery opened a new chapter in nitrogen fixation in non-leguminous plants, potential expanded when Fuentes et al. (1999) showed that releases up to 50% of fixed nitrogen and produces various auxins, cytokinins and mainlyAIA (Lee et al, 2004; Madhaiyan et al, 2006). The Acetobacteraceae, belongs to the family, which in turn is inserted into the a-proteobacteria subclass. However, it is clear that there is still much research in the field of biofertilization, regarding the effect which can produce adequate incorporation of G. diazotrophicus the plant system, given the benefits and potential of this organism. The objective of this research is to isolate and characterize microorganisms of the genders Azospirillum and Gluconacetobacter as promoters of plant growth, promising for the production of bio-fertilizers which would enable the traditional patterns of chemical fertilization and that in turn generate a positive impact on the ground where they are applied.
Materials and methods
Getting isolates of Azospirillum and Gluconacetobacter. Azospirillum was isolated in culture medium PY, supplemented with 20 ug mL-1 nalidixic acid and 10 ug mL-1 oftetracycline (Caballero-Mellado et al. (1992). In the case of Gluconacetobacter the culture medium was used LGI semisolid taking root cuttings 2 cm length of Jatropha and sugar cane, washed and surface sterilized with 70% ethanol for 5 min, rinsed in sterile distilled water and transferred to the media above culture remaining in incubation for 10 days at 30 °C (Reis et al, 1994).
Characterization of isolates. Culture media were screened positive growth and the methodology described by Dóbereiner et al. (1995) was followed for identifying Azospirillum and by Collins and Lyne (1980) for Gluconacetobacter. In both cases the ability to grow in different carbon sources according to the methodology proposed by Barbosa (2006) was also determined. Additionally an amplification protocol developed by polymerase chain reaction ofthe DNAr16S gene using the primers F 5-AGAGTTTGATCCTGGCTCAG-3' and R 5-TACCTTGTTTTACGACTT-3' which generate an amplified described by Singh et al. (2013) of1499 pb in Azospirillum sp. and AC 5'-CTGTTTCCCGCAAGGGAC-3: and D1 5'-GCGCCCCATT GCTGGGTT-3' generating an amplified described by Sievers et al. (1998) of 445 pb in Gluconacetobacter sp., additionally were used primers 1 440 5'-GTTGGCTTAGAAGCAGCC-3' and AD 5'-TGCGGCAAAAGCCGGAT-3' generating a product described by Kirchhof et al. (1998) of 411 pb of gene ADNr 23S of Gluconacetobacter diazotrophicus and AzD 5' GTGAGTAACACGTGGGAACCTG 3' and AzR 5' GCTTCCACTTAGACCTCATGCT 3' that generate an amplified of 780 pb of gene DNAr 16S of Azospirillum brasilense.
The reaction mixture consisted of5 µL ofgenomic DNA, 2.5 µL of enzyme Buffer 10X, 3 µL of MgCl2 50 mM, 1.25 µL of mixture of dNTPs, 1.25 µL of each primer, 0.2 µL of Taq Polymerase and water free DNAse to a 25 µL volume. The reaction was performed in a BioRad® thermocycler Mycicler of and programmed as follows: Initial denaturation 94 °C 5 min, followed by 30 cycles with a denaturation step at 95 °C for 1 min, 71 °C alignment, 66 °C, 61 °C and 60 °C for 1 min for F-R, AC-D1, 1440-AD and AzD-AzR respectively, extension 73 °C for 1 min for AC-D1, 1440-AD and AzD-AzR, FR 2 minutes with a final extension at 73 °C for 10 min all cases. All amplicons were run on agarose gels stained with 0.7% ethidium bromide and visualized under a system UV documentation of gel GelDocXR + from BioRad®.
Quantification of AIA. Isolates were grown in liquid Luria-Bertani (LB) supplemented with tryptone casein as a source of tryptophan, with incubation at 30 °C and 150 rpm constant shaking. In addition to quantifying the AIA produced by each strain, factors that could affect the production of the secondary metabolite were determined, the variables to be evaluated are the concentration of tryptone in LB (2, 4 and 8 g L-1), the pH (4, 5, 6 and 7) and water activity (0.01M, 0.1M and 1M NaCl and 20% and 30% sucrose) quantized latter in minimal medium with sucrose as carbon source and supplemented with 0.5 g/L tryptophan. The 1 mL aliquots of culture were collected at 24, 48, 72 and 96 hours incubation and centrifuged at 9 000 rpm for 5 min, the supernatant mixed with 3 ml of reagent Salkowski prepared according Glickman and Dessaux (1994) and it was measured in a UV-VIS spectrophotometer Jenway® at 535 nm with reference to a standard curve of AIA comercial sigma-aldrich®.
Quantification of ammonia nitrogen. To quantify the fixed nitrogen 50 µL of each bacterial suspension was inoculated into 5 mL of soil extract broth 10% and 5ml minimal medium incubating at 30 °C for 72 h at120 rpm. 700 µL of culture was taken and added 1.5 mL of KCl 2M, the tubes were vortexed for 5 minutes and then allowed to stand for an additional 1 h. Subsequently was added 150 mL of an alcoholic solution of phenol 10%, 150 µL of 0.5% sodium nitroprusside and 700 µL of oxidant solution. It stirs to mix and then rested for 1 hour blue coloration was observed, making reading spectrophotometer at 633nm. Concentrations were calculated on a calibration curve obtained with serial dilutions of a solution of 100 ppm of ammonium chloride (García et al, 2010).
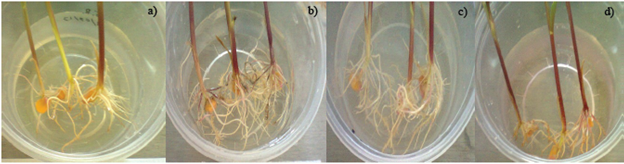
Effect of inoculation in corn, sugar cane and pitahaya. The effect of inoculating as determined the strains in vitro assays in maize seeds germinated on agar water plates for 48 h inoculated with 1X107 UFC of M1 and G1 grown Dóbereiner et al. (1995) in LB liquid medium supplemented with 8 g L-1 tryptone, incubated for 72 h at 30 °C and 150 rpm constant stirring, once germinated seeds were placed in nutrient solution and simple inoculations of each bacterial model were performed and co-inoculation, the inoculated seeds were allowed to incubate for 10 days and its effect was assessed by measuring the root mass and number of roots. Further field tests were conducted in sugarcane variety ATE MEX 9640 which was inoculated 10 days after germination with bacterial suspensions containing 1X1011 UFC/mL (Table 1), treatments were distributed in a randomized complete block design with 5 replications, the were measured variables stem thickness, height, weight and number of sprouts at 12 months after planting (Dibut et al, 2005). Finally the same microbial models in pitahayas propagated ex vitro through cuttings which were inoculated 20 days after transplantation with 50 mL of a solution containing 1X108 UFC mL-1 using as reference control were assessed - uninoculated and control + with Radix rooting 1500®, in this case the root mass and number of roots at 60 days post inoculation was measured.
Table 1 Treatments evaluated in sugar cane.

*G1= Gluconacetobacter sp., M1= Azospirillum sp., G1-M1= coinoculación Gluconacetobacter sp. y Azospirillum sp., FQ= fertilización química al 100% (220-80-80) con nitrato de potasio, fosfato diamónico y cloruro de potasio. El testigo y los tratamientos 1, 2 y 3 tuvieron fertilización química al 30%.
Statistical analysis. All assays were performed in triplicate and data were subjected to statistical analysis of variance and mean comparison Tukey at a significance level of p≤ 0.05 using the SAS package (Statistical Analysis System).
Results and discussion
The strains were obtained in each of the culture media and colony morphology suggestive phenotypic characteristics of Azospirillum genus which was called the M1 and Gluconacetobacter called G1 described by Hynes et al. (2008); Cavalcante et al. (1988), isolated from root samples of jatropha and sugar cane respectively (Table 2). Additionally it observed microscopically that the M1 strain has mobility spirally ovoid, amid Nfb with congo red observed scarlet red colonies with rounded and slightly mucoid edges according to Pérez and Casas (2005), for the G1 strain observed orange mucoid colonies of irregular shape with acid production in the middle LGI and coffee-brown potato dextrose agar colony, able to tolerate sucrose concentrations up to 30% and production of acetic acid (Fuentes et al, 2001).
Table 2 Biochemical characterization and use of carbon sources M1 and G1 strains.

*Cat= catalasa; Ox= oxidasa; Mov= movilidad; Sac= sacarosa; Glc= glucosa; Lac= lactosa; Cit= citrato; Man= manitol; Mal= maltosa; Et= etanol; Gli= glicerol; Ino= inocitol; Ac= acetato sódico, (+) crecimiento abundante, (-) sin crecimiento, (+/-) crecimiento ligero.
The results of biochemical and morphological characterization for M1 and G1 strains match the descriptions of manual classification of bacteria Bergey for the genders Azospirillum and Gluconacetobacter respectively (Holt et al., 1994).
The PCR protocol of rDNA 16S gene M1 and G1 strains amplified products 1.5 Kb and 450 pb respectively (Figure 1), according to what reported by Singh et al. (2013) and Sievers et al. (1998). The gene amplification DNAr 23S for G1 strain generated a 410 pb product estimated reported for species of the genus Gluconacetobacter (Kirchhof et al, 1998).
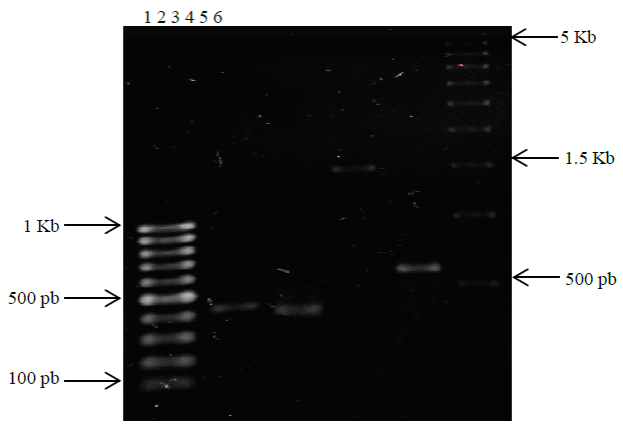
Figure 1 The PCR amplification products of DNAr 16S of G1 and M1 and DNAr23S genes of G1. Lane 1 MPM 100 pb, lane 2 DNAr 16S of G1, lane 3 DNAr 23S of G1, lane 4 DNAr 16S M1 primers F-R, lanes 5 DNAr 16S M1 primers AzD-AzR, lane 6 MPM 500 pb.
Quantification of AIA. In quantifying this growth regulator it found that the maximum production of AIA occurs at concentrations of 0.01 M of NaCl both M1 to G1 at 96 hours of incubation (Figure 2) with values of 19.19 and 27.28 mg L-1 respectively, just as it is observed that the concentration of AIA decreases significantly for both strains under osmotic stress it is more severe (Spaepen et al., 2007). Finally it was found that G1 continues to present good growth and producing this compound, at lower concentrations, even at 20 and 30% sucrose (6.24 and 7.46 mg L-1 of AIA of incubation (Figure 2) with values of 19.19 and 27.28 mg L-1 respectively, just as it is observed that the concentration of AIA decreases significantly for both strains under osmotic stress it is more severe (Spaepen et al., 2007). Finally it was found that G1 continues to present good growth and producing this compound, at lower concentrations, even at 20 and 30% sucrose (6.24 and 7.46 mg L-1 of AIA respectively) which speaks of its ability to tolerate high concentrations of carbohydrate contrary to M1 whose production is reduced to 0.8 and 1.1 mg L-1 at the same concentrations of sucrose, probably due to a decrease in their growth under these conditions.
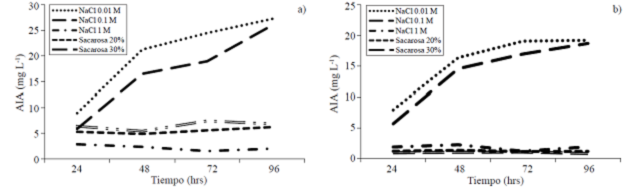
Figure 2 Production of indole acetic acid with osmotic stress induced by different concentrations of NaCl and sucrose in minimal medium a) strain G1; and b) strain M1.
It was found that the optimum pH for the production of AIA is 7 for both M1 to G1 (Table 3) and both strains are affected production of this metabolite to lower pH values, such as reported by Sahasrabudhe (2011) , it should be noted that G1 has greater tolerance and thus increased production of AIA M1 at lower pH values. It is also noted that the concentration of tryptone directly affects the production of this compound, so that high concentrations of this protein increase considerably the amount of tryptophan and production of AIA in both strains, indicating the possibility of that the synthesis of the secondary metabolite follow dependent pathways of tryptophan (Aguilar et al, 2008). As shown in Table 2, G1 is able to produce significantly more growth regulator compared to M1 and co-inoculation no statistically significant increase in each pH value when compared with G1, these results are consistent with reported by Lara et al. (2012) and Cheang et al. (2005) in relation to the amount of AIA strains produced by Azospirillum and Gluconacetobacter.
Table 3 Production of AIA at different pH values and concentrations tryptone
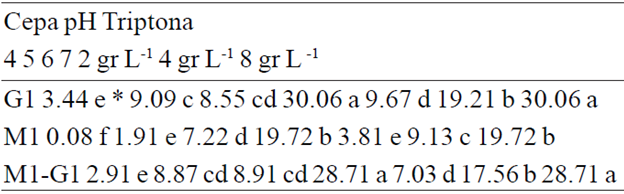
*Concentración de AIA en mg/L, valores con la misma letra son estadísticamente iguales para p≤ 0.05.
Quantification of ammonia nitrogen. In determining ammonium notes that both M1 and G1 are capable of fixing nitrogen (Table 4), shows that extract broth soil strain M1 produces significantly greater amounts (14.74 umol L-1) to those produced by G1 (5.17 umol L-1) and generated by co-inoculation of both strains (11.21 umol L-1), higher concentrations of ammonium for both strains are also observed in soil extract broth compared to minimal medium. The ammonium concentrations found in this study are consistent with those previously reported for strains of Azospirillum sp. (García et al., 2010).
Table 4 Quantification of ammoniacal nitrogen in different culture media.
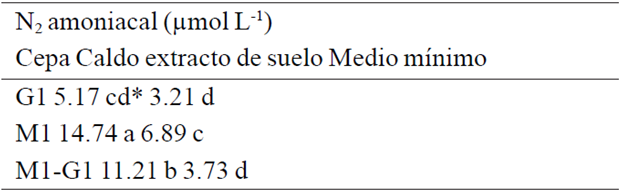
*Valores con la misma letra son estadísticamente iguales para p≤ 0.05.
Effect of M1 and G1 in corn, sugar cane and pitahaya. In assessing the inoculation of M1 and G1 in different plant models it shows that both strains have positive effects on cuttings pitahaya both root mass and number of roots as they present statistically equal values at+control for both variables (Table 5), the positive effects of inoculation of strains of the genus Azospirillum in various crops have been attributed mainly to the improvement in the development of the root (Fallik and Okon, 1996; Burdman et al, 1997; Dóbbelaere et al, 2002).
Table 5 Effect of inoculation of M1 and G1 on pitahaya and sugar cane.

Valores con la misma letra son estadísticamente iguales para p< 0.05. *masa radicular medida en gramos, **Altura medida en centímetros, ***peso medido en kilogramos.
Also, sugar cane for the variables height, weight and number of outbreaks observed that inoculation of G1 presented statistically equal to the control values + in accordance with those reported by Sevilla et al. (1998) who observed a significant increase in plant biomass sugar cane inoculated with promoters of plant growth compared to uninoculated plants, likewise found in this investigation that the effect of inoculation is larger compared to negative controls used for each model plant (Muñoz and Caballero, 2003). It can also be seen that co-inoculation has no greater effect than the simple inoculation of M1 and G1 in the variables measured for each plant model (Table 5). The production of indole acetic acid, and the high sensitivity of the roots to the hormone are fundamental, in response to inoculation of Azospirillum where is often observe more radical development, which results in higher surface absorption of nutrients, and so, further development of the aerial part of the plant (Cholula, 2005).
The inoculation of maize seeds shows that M1 has higher effects compared to G1 in root volume (Figure 3), we can see that the co-inoculation of both bacterial models results in a similar to that shown by M1 effect and the inoculated treatments have greater root volume than the uninoculated (Ilyas et al., 2012). These results demonstrate the potential of microbial models isolated as plant growth promoters.
Conclusions
According to the results observed in each of the tests it demonstrated that isolates are promoting plant growth partially characterized as Azospirillum sp. (G1) and Gluconacetobacter sp. (M1). Both microbial models are capable of synthesizing AIA with increased production by G1, on the other hand, M1 shows increased production capacity of ammonia nitrogen. It was found that both the concentration of Tryptone, such as pH and osmotic stress are factors that directly affect production in AIA. Finally we can say that there is no greater effect on plant generated by co-inoculation of both strains compared with schemes simple inoculation in three plant models used and finally that both G1 and M1 inoculated at a rate of 200 mL/36 m2 with concentration of 1X1011 UFC mL-1 achieve reductions of up to 70% in the schemes of chemical fertilizer in sugar cane obtaining variables such performance control +, so these microbial models can be considered promising in the development of bio-fertilizers in this region.
Literatura citada
Aguilar, P. J.; Xiqui, V. M.; García, G. S. and Baca, B. E. 2008. Producción del ácido indol 3- acético en Azospirillum brasilense. Rev. Latinoam. Microbiol. 50(1-2):29-37. [ Links ]
Barbosa, E. A.; Perin, L. y Reis, V. M. 2006. Uso de fuentes de carbono por G. diazotrophicus isolados de plantas de cana-de-afúcar. Pesq. Agropec Bras. 41:827-833. [ Links ]
Burdman, S.; Kigel, J. and Okon, Y. 1997. Effects of Azospirillum brasilense on nodulation and growth ofcommon bean (Phaseolus vulgaris L.). Soil Biol. Biochem. 29:923-929 [ Links ]
Caballero, M. J.; Carcaño, M. G. and Mascarúa, E.A. 1992. Field inoculation of wheat (Triticum aestivum) witthAzospirillum brasilense under temperate climate. Symbiosis 13(1-3):243-253. [ Links ]
Caballero, M. J.; López, R. L. y Bustillos, C. R. 1999. Presence of 16S rRNA genes in multiple replicons in Azospirillum brasilense. FEMS Microbiol. Lett. 178(2):283-288. [ Links ]
Cárdenas, D. M.; Garrido, M. F.; Bonilla, R. R. y Baldani V. L. 2010. Aislamiento e identificación de cepas de Azospirillum sp. en pasto guinea (Panicum maximum Jacq.) del Valle del Cesar. Pastos y Forrajes. 33(3):67-76. [ Links ]
Cavalcante, V.A. and Dóbereiner, J.A. 1988. New acid-tolerant nitrogen fixing bacterium associated with sugarcane. Plant Soil. 108:23-31. [ Links ]
Cholula, L. P. 2005. Estudio de la producción de poli β-hidroxibutirato (PHB) en Azospirillum brasilense Sp7. Centro de Biotecnología Genómica IPN Tamaulipas, México. 42-82 pp. [ Links ]
Manzano, J. and Heydrich, M. 2005. Physiological characterization of sugarcane's endophytic microbial community. Rev. Colomb. Biotecnol. 7(1):66-75. [ Links ]
Collins, C. H. and Lyne, P. M. 1980. Microbiological methods. Butterworth and Co. (Publishers) Ltd., London. 272-273 pp. [ Links ]
Dibut, B.; Ortega, M.; Martínez, R.; Fey, L. y Ríos, Y. 2005. Nuevos asilados de Gluconacetobacter diazotrophicus en cultivos de importancia económica para Cuba. Cultivos Tropicales. 26(2):5-10. [ Links ]
Dóbbelaere, S.; Croonenborghs, A.; Thys, A.; Ptacek, D.; Okon, Y. and Vanderleyden, J. 2002. Effect of inoculation with wild type Azospirillum brasilense and A. irakense strains on development and nitrogen uptake of spring wheat and grain maize. Biol. Fertil. Soils 36:284-297. [ Links ]
Dóbereiner, J. 1995. Isolation and identification of aerobic nitrogen-fixing bacteria from soil and plants. In: methods in applied soil microbiology and biochemistry. Editado por Kassem Alef y Paolo Nannipieri. Academic Press Limited. London, Great Britain. 134-139 pp. [ Links ]
Fallik, E. and Okon, Y. 1996. Inoculations of Azospirillum brasilense: biomass production, survival and growth promotion of Setaria italica and Zea mays. Soil Biol. Biochem. 28:123-126. [ Links ]
Fuentes, R. L. E.; Caballero, M. J.; Sepúlveda, J. and Martínez, R. E. 1999. Colonization of sugarcane by Acetobacter diazotrophicus, and indolacetic is inhibited by high N-fertilization. FEMS. Microbiol. Ecol. 29:117-128. [ Links ]
Fuentes, R. L. E.; Bustillos, R.; Tapia, A.; Jiménez, S. T.; Wang, E. T., Martínez, R. E. and Caballero, M. J. 2001. Novel nitrogen-fixing acetic acid bacteria, Gluconacetobacter johannae sp. nov., and Gluconacetobacter azotocaptans sp. nov., associated with coffee plants. Int. J. Syst. Evol. Microbiol. 51:1305-1314. [ Links ]
García, F.; Muñoz, H.; Carreño, C. and Mendoza, G. 2010. Characterization of native strains of Azospirillum spp. and its effect on growth of Oryza sativa L. "rice" in Lambayeque. Scientia Agropecuaria. 1:107-116. [ Links ]
Glickman, E. and Dessaux, Y. 1994. A critical examination of the specificity of the Salkowsky reagent for indolic compounds produced by phytopathogenic bacteria. Am. Soc. Microbiol. 61(2):793-796. [ Links ]
Hirsch, A. M.; Lum, M. R. and Downie, J. A. 2001. What makes the rhizobia-legume symbiosis so special? Plant Physiol. 127:1484-1492. [ Links ]
Holt, J.; Bergey, D. and Krieg, N. 1994. Bergey's manual of determinative bacteriology. In: Sneath, Staley, J. and Williams, S. 9th (Ed.). Lippincott Willliams and Wilkins, Baltimore, USA. 56 p. [ Links ]
Hynes, R. K.; Leung, G. C.; Hirkala, Y. and Nelson, L. M. 2008. Isolation, selection, and characterization of beneficial rhizobacteria from pea, lentil, and chickpea grown in western Canada. Can. J. Microbiol. 54:248-258. [ Links ]
Ilyas, N.; Bano, A.; Iqbal, S. and Iqbal, N. 2012. Physiological, biochemical and molecular characterization of Azospirillum spp. isolated from maize under water stress. Pak. J. Bot. 44:71-80. [ Links ]
Kirchhof, G.; Baldani, J. I.; Reis, V. M. and Hartmann, A. 1998. Molecular assay to identify Acetobacter diazotrophicus and detect its occurrence in plant tissues. Can. J. Microbiol. 44:12-19. [ Links ]
Lara, C.; Oviedo, L. and Alemán, A. 2012. Strain native with potential in the indol acetic acid production to improve the agriculture. Biotecnología en el sector Agropecuario y Agroindustrial. 9(1):17-23. [ Links ]
Lee, S.; Flores, E. M.; Contreras, Z. M.; García, F. L.; Escamilla, J. E. and Kennedy, C. 2004. Indole-3-acetic acid biosynthesis is deficient in Gluconacetobacter diazotrophicus strains with mutations in cytochrome c biogenesis genes. J. Bacteriol. 186:5384-5391. [ Links ]
Madhaiyan, M.; Poonguzhali, S.; Hari, K.; Saravanan, U. S. and Sa, T. 2006. Influence of pesticides on the growth rate and plant-growth promoting traits of Gluconacetobacter diazotrophicus. Pesticide Biochem. Physiol. 84:143-154. [ Links ]
Martínez, M. L. J.; Soto, U. L.; Baca, B. E. and Sánchez, J. A. 2003. Indole-3-butyric acid (IBA) production in culture medium by wild strain Azospirillum brasilense. FEMS Microbiol. Lett. 228(2):167-173. [ Links ]
Muñoz, R. J. and Caballero, M. J. 2003. Population dynamics of Gluconacetobacter diazotrophicus in sugarcane cultivars and its effect on plant growth. Microbial Ecol. 46:454-464. [ Links ]
Pérez, J. y Casas, M. 2005. Estudio de la interacción planta-Azospirillum en el cultivo de caña de azúcar (Saccharum sp.). INICA. Boyeros, Cuba. Cultivos tropicales. 26(4):13-19. [ Links ]
Reis, V. M.; Olivares, F. L. and Dobereiner, J. 1994. Improved methodology for isolation of Acetobacter diazotrophicus and confirmation of its endophytic hábitat. World J. Microbiol. Biotech. 10:401-405. [ Links ]
Sahasrabudhe, M. 2011. Screening of rhizobia for indole acetic acid production. Ann. Biological Res. 2(4):460-468. [ Links ]
Sevilla, M.; De Oliveira, A.; Baldani, I. and Kennedy, C. 1998. Contributions of the bacterial endophyte Acetobacter diazotrophicus to sugarcane nutrition. A preliminary study. Symbiosis 25:181-191. [ Links ]
Sevilla, M.; Burris, R. H.; Gunapala, N. and Kennedy, C. 2001. Comparison of benefit to sugarcane plant growth and 15N2 incorporation following inoculation of sterile plants with Acetobacter diazotrophicus wild-type and Nif mutant strains. Mol. Plan-Microbe Int. 14:358-366. [ Links ]
Sievers, M.; Schlegel, H. G.; Caballero, M. J.; Dóbereiner, J. and Ludwig, W. 1998. Phylogenetic identification of two major nitrogen-fixing bacteria associated with sugarcane. System. Appl. Microbiol. 21:505-508. [ Links ]
Singh, D.; Sharma, A. and Kaur, G. 2013. Biochemical and molecular characterization of the bacterial endophytes from native sugarcane varieties of Himalayan region. Biotech. 3:205-212. [ Links ]
Spaepen, S., Vanderleyden, J. and Remans, R. 2007. Indole-3-acetic acid in microbial and microorganisms-plant signaling. FEMS Microbiol. Rev. 31:425-448. [ Links ]
Vivienne, N.; Matiru, F. D. and Dakora, S. 2004. Potential use of rhizobial bacteria as promoters of plant growth for increased yield in landraces ofAfrican cereal crops. Afr. J. Biotechnol. 3(1):1-7. [ Links ]
Received: April 2016; Accepted: July 2016











 texto em
texto em