Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista mexicana de ciencias agrícolas
versão impressa ISSN 2007-0934
Rev. Mex. Cienc. Agríc vol.7 no.6 Texcoco Ago./Set. 2016
Articles
Synergistic effect of Trichoderma asperelleum T8A and captan 50 ® against Colletotrichum gloeosporioides (Penz.)
1Universidad Tecnológica de Izúcar de Matamoros, Izúcar de Matamoros, Puebla, México. (mitsuki_abi@hotmail.com; reyes9503@hotmail.com).
2Departamento de Ciencias del Agua y Medio Ambiente, Instituto Tecnológico de Sonora, Cd. Obregón, Sonora, México. (yepezglz@gmail.com).
3Catedrático CONACYT-Instituto Tecnológico de Sonora, Cd. Obregón, Sonora, México.
4Campo Experimental Norman E. Borlaug, Cd. Obregón, Sonora, México. (fipc04@gmail.com).
The objective of this study was to quantify the interaction Captan 50® - Trichoderma asperelleum T8a as synergistic alternative to control Colletotrichum gloeosporioides ATCC MYA 456, causal agent of anthracnose in mango. The macroscopic, microscopic and molecular characterization confirmed the taxonomy of these organisms as T. asperelleum (T8a) and C. gloeosporioides (ATCC MYA 456), the latter showed the ability to cause anthracnose to 100% of fruits inoculated. The bio-control agent T. asperellum T8a, showed an in vitro inhibition of 91% in the growth of phyto pathogen through parasitic fungi mechanisms. Thus, trials of minimum inhibitory concentration of Captan 50®vs. microorganisms under study were performed, noting that concentrations equal to or greater than 0.5 g L-1 fungicide completely inhibited pathogen growth, however, lower concentrations (0.1 and 0.25 g L-1) inhibited the growth of this only 36 %. Furthermore, T. asperellum T8a growth was inhibited 6% at doses of 0.5 g L-1 of Captan 50®, not observing effects on this strain at lower doses of the fungicide. Thus, the concentration of 0.1 g L-1Captan50® was co-applied in vitro with T. asperellum T8a, observing a synergistic inhibition of C. gloeosporioides ATCC MYA 456, it reached 99%. The data indicate the potential of this integrated alternative to reduce the application of Captan 50®, enhance control of C. gloeosporioides, and reduce economic/environmental problems by the use of this fungicide in field.
Keywords: biological control agent; chemical control; anthracnose; minimum inhibitory concentration
El objetivo del presente estudio fue cuantificar la interacción Captan 50® - Trichoderma asperellum T8a como alternativa sinérgica para el control de Colletotrichum gloeosporioides ATCC MYA 456, agente causal de la antracnosis en el mango. La caracterización macroscópica, microscópica y molecular confirmaron la taxonomía de estos microorganismos como T. asperellum (T8a) y C. gloeosporioides (ATCC MYA 456), este último mostró la capacidad de causar antracnosis 100% de los frutos inoculados. El agente de control biológico, T. asperellum T8a, mostró una inhibición in vitro de 91% en el crecimiento del fitopatógeno, mediante mecanismos micoparasíticos. Así, ensayos de concentración mínima inhibitoria de Captan 50® vs. los microorganismos en estudio fueron realizados, observando que concentraciones iguales o mayores a 0.5 g L-1 del fungicida inhibieron completamente el crecimiento del fitopatógeno, sin embargo, concentraciones menores (0.1 y 0.25 g L-1) inhibieron el crecimiento de éste sólo en 36%. Por otro lado, el crecimiento de T. asperellum T8a fue inhibido 6% en dosis de 0.5 g L-1 de Captan 50®, no observando efectos sobre esta cepa a dosis menores del fungicida. De esta manera, la concentración de 0.1 g L-1 de Captan 50® fue co-aplicada in vitro con T. asperellum T8a, observando una inhibición sinérgica de C. gloeosporioides ATCC MYA 456, alcanzó 99%. Los datos indican el potencial de la presente alternativa integrada para reducir la aplicación de Captan 50®, potenciar el control de C. gloeosporioides, y reducir los problemas económicos/ambientales por el uso de este fungicida en campo.
Palabras claves: agente de control biológico; antracnosis; control químico; concentración mínima inhibitoria
Introduction
Mango (Mangifera indica L.) has been cultivated and naturalized so widely that their distribution can be considered pantropical -even when its exact origin is unknown (Saints-Villalobos et al., 2011). In addition, this fruit is considered one of the most popular in tropical areas and developing countries (FAO, 2013).
World production of mango is approximately 31 × 106 t yr-1. India contributes 43% of this production (largest producer), and Mexico ranks fifth in this sector with a production of more than 4%. The outlook for our country changes when considering fresh mango exports in the international market because Mexico is the second largest exporter worldwide with a share of 21%, representing about 2 × 105 t yr-1 (FAO, 2013), resulting in approximately $ 86 million USD.
National and global production of mango has a chain value similar, where have been identified steps that destabilize (critical points), due to the lack of scientific knowledge or sustainable technologies around them, these are: flowering event, generation/use of agro industrial waste and control of anthracnose, the latter being the main problem in pre and post-harvest (de los Santos-Villalobos et al., 2011).
Anthracnose, caused by Colletotrichum gloeosporioides, is the disease of most impact on productivity and quality of mango, which is distributed in all producing areas of the world (Litz, 2000). The taxonomic classification of the causative agent is class: Sordariomycetes, subclass: Hypocreomycetidae, order: Hypocreomycetidae incertaesedis, family: Glomerellaceae, genre: Glomerella, species: cingulata, being C. gloeosporioides its anamorph state (NCBI, 2014).
This ubiquitous pathogen produces hyphae hyaline unicellular, ovoid and elongated, with slightly curved conidia (10 to 15 microns in length and 5 to 7 microns wide). The acervuli produced in infected tissue can be placed in the form subepidermal, epidermal or subcuticular with upright short conidiophores (da Silva and Michereff Bruce, 2013). This disease is present worldwide and in all phenological stages of the crop, causing considerable losses in production and causing the fall of more than 90% of the fruit when the disease is present in intensities over 80%, even when damage levels reach 40%, fruit drop is greater than 50% (Espinosa et al., 2004). For this reason, the management of anthracnose in mango requires constant control due to weather conditions (temperature and humidity) promote infection and disease development, negatively impacting the productivity of this crop
For decades, agricultural practice mostly used for the control of this disease has been the application of synthetic fungicides, which include benzimidazole, dithiocarbamate, chloroalquitio and inhibitors of the synthesis of ergosterol: difenoconazole, myclobutanil, and prochloraz (Peres et al., 2004; Arias and Carrizales, 2007). However, the use of these fungicides is not an economically and environmentally sustainable practice because the overuse of these has led to serious problems of environmental pollution, generating toxic residues on fruits and pathogen resistance (Wu et al., 2009). Thus, for years, social pressure to reduce the use of these synthetic fungicides in mango production and its presence in the environment has increased significantly, leading to the need to develop sustainable alternatives against anthracnose that allow to decrease the use and problems caused by these synthetic fungicides.
In recent years, the application of control agents of microbial origin has increased significantly as an alternative to combat diseases in agricultural crops, through various mechanisms, such as antagonisms (parasitism and antibiosis), and competition for space and nutrients, i.e. the successful control of C. gloeosporioides in mango has been achieved through the use Burkholderia cepacia XXVI (siderophore production), Trichoderma viride Tv1 (unknown mechanism of action), Bacillus subtilis Pla10 (production of antibiotics), among others (Ragazzo-Sanchez et al., 2011; de Santos-Villalobos et al., 2012; Lakshmi et al., 2013). However, the level of success of this alternative in field is variable due to their biological nature, where its efficiency is altered by environmental stimuli (biotic and abiotic) to which they are exposed. The aim of this work was the development and in vitro evaluation of an efficient and environmentally friendly alternative to control the causal agent of anthracnose in mango, C. gloeosporioides. This alternative is based on the combined and synergistic use of a control agent of microbial origin (T. asperellum T8a) and a synthetic fungicide (Captan 50®), in order to minimize losses in mango production caused by this disease, thus as environmental problems from the use of synthetic fungicides used conventionally.
Materials and methods
Phyto pathogenic fungi and control agents of microbial and synthetic origin used. The phyto pathogen used as causal agent of anthracnose in mango was the strain ATCC MYA 456 from Colletotrichum gloeosporioides, obtained from the American Type Culture Collection (ATCC). The strain Trichoderma asperellum T8a (isolated from mango tree) was used as biological control agent against C. gloeosporioides ATCC MYA 456. The strain T8a was provided by the edaphic and endophyte collection of microorganisms (hive) from the Biotech laboratory of Microbial Source, at Instituto Tecnologico de Sonora. Also, Captan 50® was used as synthetic fungicide of conventional use againt the phyto pathongen fungi under study.
Macroscopic, microscopic and molecular characterization of microorganisms under study. The microorganisms used were identified through their macroscopic and microscopic characteristics, such as hyaline hyphae; mycelium color and conidia shape (Gunnel and Gubler, 1992, de los Santos-Villalobos et al., 2013.). Furthermore, these microorganisms were molecularly identified by amplification of 5.8S rRNA gene in its genomic DNA, by the polymerase chain reaction (PCR) (White et al., 1990). Previously, the microorganisms under study were grown in broth Potato Dextrose (PDB) to obtain mycelium (incubation for 3 days at 28 °C), which was used for extraction of genomic DNA (Rader and Broda, 1985). The amplicons (~ 300 bp) were purified using the GFX PCR DNA kit and Gel Band Purification (Illustra) and sequenced in both directions by Sanger platform. Electropherograms obtained were analyzed using the Sequence Scanner v1.0 (Applied Biosystems). The sequences obtained were compared with those deposited in the gene bank from NCBI.
Pathogenicity tests of C. gloeosporioides ATCC MYA 456 in vivo. Pathogenicity tests of C. gloeosporioides ATCC MYA 456 were carried out in green mangoes (five fruits per treatment), by inoculating 1 x 106 spores in five distant points of each fruit. Subsequently, this were incubated in a humid chamber at 28 ± 1 °C, relative humidity of 85 ± 5%, 12 h photoperiod (540 Lux) - 12h (20 Lux) for 15 days. The number of fruits that showed the characteristic symptoms of anthracnose were recorded, isolating the pathogen under study from lesions observed in these fruits (de los Santos-Villalobos et al., 2013).
In vitro confrontation assays. Confrontation tests were conducted in order to quantify the control ability of T. asperellum T8a against C. gloeosporioides ATCC MYA 456. These assays were developed co-inoculating 1 x 105 spores of strain ATCC MYA 456 with 1 x 105 spores of strain T8a (at a distance of 6 cm from the pathogen), in petri dishes containing potato dextrose agar (PDA) as culture medium, incubating them at 28 °C for 8 days (de los Santos-Villalobos et al., 2013). The inhibition percentage of C. gloeosporioides ATCC MYA 456 was calculated using the equation.
Where: % I = inhibition percentage (%), A1 = area of the Petri dish (in mm2) covered by C. gloeosporioides inoculated control without the control agent, A2 = area of the Petri dish (in mm2) covered by C. gloeosporioides co-inoculated with T. asperelleum T8a (de los Santos-Villalobos et al., 2013).
In vitro minimum inhibitory concentration assays. The impact of different doses of Captan 50® on C. gloeosporioides ATCC MYA 456 and T. asperelleum T8a was evaluated through minimum inhibitory concentration assay. For this, 1 x 105 spores of each microorganism under study were inoculated in Petri dishes containing PDA as culture medium, added with a concentration gradient of Captan 50® (g L-1): 0, 0.1, 0.25, 0.5, 1.0, 1.5, 3.0, 6.0, 9.0, 12.0, 15.0. Petri dishes were incubated at 28 ° C for 8 days. Growth rates were quantified by the equation:
Where: TC = growth rate (mm2 h-1); C1 = initial growth (mm2); C2 = final growth (mm2); T1 = initial time (h); T2 = final time (h) (de los Santos-Villalobos et al., 2013).
Synergism assays T. asperelleum T8a - Captan 50® against C. gloeosporioides ATCC MYA 456. Triple confrontation assays were conducted in order to quantify the control ability from synergism T. asperelleum T8a - captan 50® against C. gloeosporioides ATCC MYA 456. The assays were developed by co-inoculating 1 x 105 spores of strain ATCC MYA 456 and T8a (at a distance of 6 cm) in Petri dishes containing PDA as culture medium supplemented with 0.1 g L-1 capture 50®. Inoculated Petri dishes were incubated at 28 °C for 8 days (de los Santos-Villalobos et al., 2013). The inhibition percentage of C. gloeosporioides ATCC MYA 456 was calculated using equation 1.
Statistical analysis. All experiments were replicated three times independently. The data obtained were analyzed by analysis of variance test (ANOVA) one-way and Tukey-Kramer method (p= 0.05) using the JMP-SAS v 8.0.2 software.
Results and discussion
Macro and microscopic characteristics of the organisms studied. Anthracnose, caused by the plant pathogenic fungus C. gloeosporioides, is the main disease affecting mango production worldwide, leading to losses of 90% of production when environmental conditions are favorable for the development of this causal agent (Arauz, 2000). In the present study was evaluated the control of this plant pathogen through the synergism between a biological control agent and a synthetic fungicide (T. asperelleum T8a and captan 50®, respectively).
Strain ATCC MYA 456 of C. gloeosporioides showed at macroscopic level, hyaline mycelium that quickly became cottony dark gray, showing slow growth in Petri dishes containing PDA as culture medium, with full coverage after 7 days, incubation at 28 °C (Figure 1a); microscopically showed mycelium and septate conidia, the latter ovoid (Figure 1b), these characteristics have been previously reported for this species by Montero -Tavera et al., (2010). The control agent of microbial origin, T. asperellum T8a, macroscopically showed mycelium with green/white concentric rings in the presence of periods of 12 h light/dark grown in Petri dishes containing PDA as culture medium, and incubated at 28 oC (Figure 1c), and at microscopic level showed septate hyphae and the presence of typical phialides for this genre, housing conidia (Figure 1d) (Druzhinina et al., 2011). Molecular characterization of these microorganisms was performed by amplification and sequencing the 5.8S rRNA gene, showed 100% coverage and 99% identity compared with the nucleotide sequences of the species C. gloeosporioides and T. asperellum previously reported in the database from NCBI, confirming the taxonomy and identity of the microorganisms under study.
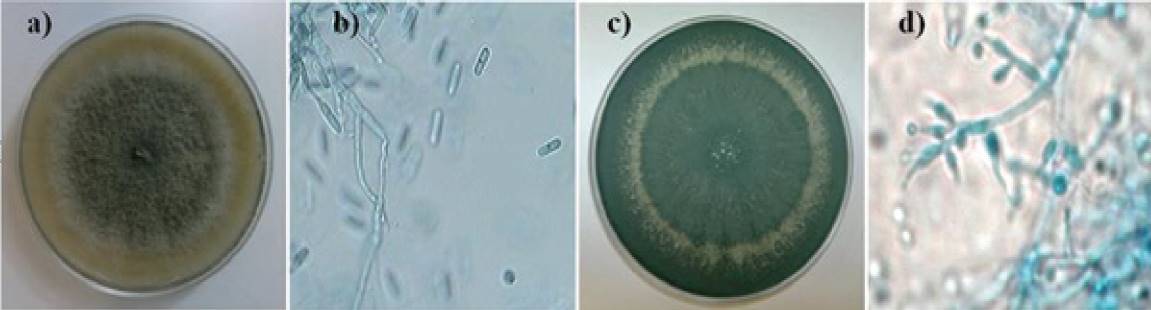
Figure 1. Microorganisms used in this study. Macroscopic and microscopic characteristics of C. gloeosporioides ATCC MYA 456 (a and b), and T. asperellum T8a (c and d), inoculated in Petri dishes containing PDA as culture medium, and incubated at 28 oC for 7 days.
Pathogenicity assays of C. gloeosporioides ATCC MYA 456. The main problem in anthracnose control is the great genetic diversity and in consequence pathogenic, strains of C. gloeosporioides in field, for example, in 1992 were reported around 600 variants of this species (Bailey and Jeger, 1992). Moreover, Bonde et al., (1991) distinguished various pathogenic forms based on their specific hosts and cultural characteristics. Thus, studies on the degree of specific pathogenicity of each strain of the phytopathogen are necessary to design efficient control strategies in field. Thus, testing pathogenicity of C. gloeosporioides ATCC 456 were developed in vivo, noting that this strain showed the ability to cause anthracnose at 100% of the inoculated mangos, disease characterized by the appearance of black spots that coalesce to form irregular lesions (Figure 2). This reinforces the evidence reported on species of the genus Colletotrichum, considered the most successful within the group of plant pathogenic fungi for the severity of the disease causing its host plant. Moreover, its ability to cause latent or quiescent infections places this genus within the major postharvest pathogens (Jeffries et al., 1990).
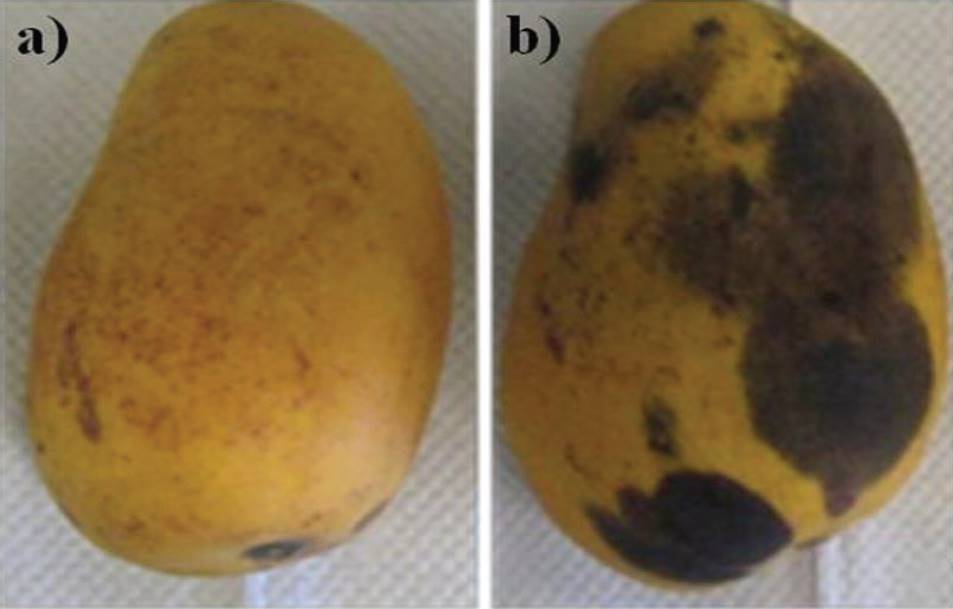
Figure 2. Pathogenicity assay of C. gloeosporioides ATCC MYA 456 in vivo. a) uninoculated fruit; b) fruit inoculated with 1 x 106 spores of the phyto-pathogen studied, and incubated in a humid chamberb incubated (28 ± 1 ° C, relative humidity 85 ± 5%, photoperiod of 12 h (540 Lux) - 12 h (20 Lux) For 15 days).
Confrontation assays between T. asperelleum T8a and C. gloeosporioides ATCC MYA 456. In recent years, control of anthracnose has focused primarily on the use of physical and chemical methods (pruning, ultraviolet light and modified atmospheres) and chemicals (synthetic fungicides, copper and cholesterol inhibitors) (Stevens et al., 1997; Ker, 2001; Karabulut and Baykal, 2004; Arias and Carrizales, 2007). However, these alternatives have economic, environmental and efficiency problems in controlling the causative agent. Therefore, generation or optimization of alternative sustainable control of this disease is crucial to increase the competitiveness of mango. In this paper, strain T. asperelleum T8a was evaluated as microbial control agent origin against C. gloeosporioides ATCC MYA 456, and then the impact of the incorporation of Captan 50® to such interaction was studied.
Confrontation assays showed the ability T. asperellum T8a to inhibit growth of C. gloeosporioides ATCC MYA 456, in 91%, this through mycoparasitic mechanisms, observing an overgrowth in the phytopathogenic fungus by strain T8a (Figure 3), demonstrating the ability of T8a strain as a promising alternative of control of microbial origin against the causative agent of anthracnose (de los Santos-Villalobos et al., 2013). The mycoparasitic behavior observed of strain T8a has been demonstrated for Trichoderma (teleomorph state: Hypocrea), which has been studied by possessing biotrophic forms of life and saprofite, whose mycoparasitic ability (through biosynthesis of lytic enzymes) has been focused for the protection of plants against various fungal diseases, that is, its application as a biological control agent (Atanasova et al., 2013).
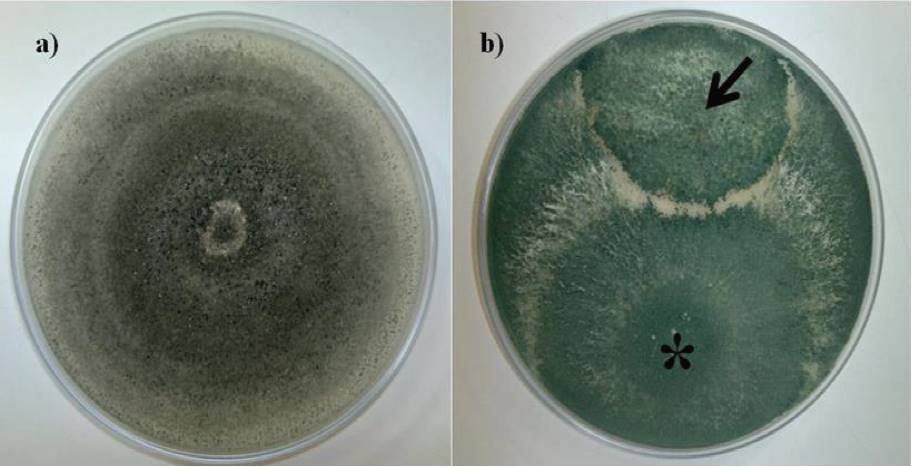
Figure 3. Comparison tests between T. asperellum T8a and C. gloeosporioides ATCC MYA 456, through mycoparasic mechanisms (indicated by an arrow).
Minimum inhibitory concentration assays of microorganisms studied by captan 50®. Captan 50® is a very powerful contact fungicide containing N-trichloromethylthio, and belongs to the group of phthalimides. This fungicide interacts with cellular thiols producing thiophosgene, which is a toxic compound that interferes with breathing process of fungal cells, inhibiting spore germination and hindering their growth and development (Bravoag, 2013).
Anthracnose control in mango through captan 50® has been carried out using five applications of 2 kg ha-1 each, for a total of 10 kg ha-1; howefver, this fungicide is very persistent in the environment (Alpuche, 1990), leading to residual effects on the fruits. In addition, these applications of captan 50® favor the generation of resistance from plant pathogenic fungi, thus the repression of the population and diversity of those fungi susceptible to this fungicide, limiting their ecological role.
Minimum inhibitory concentration assays of captan 50® on C. gloeosporioides ATCC MYA 456 and T. asperelleum T8a were developed in order to know: i) the potential inhibitory effect of residual fungicide on T. asperelleum T8a when it is inoculated, which would limit its effectiveness; and ii) the minimum concentration of captan 50® that in co-inoculation with T. asperelleum T8a does not interfere with its development but contribute to increased control of C. gloeosporioides ATCC MYA 456.
These essays showed that concentrations equal to or greater than 0.5 g L-1 captan 50® added to Petri dishes containing PDA as culture medium, entirely inhibited the growth of C. gloeosporioides ATCC MYA 456; however, lower concentrations (0.1 and 0.25 g L-1) inhibited the growth of the pathogen by 36% (Figure 4). Furthermore, the growth of T. asperellum T8a was inhibited 93% when inoculated at the highest concentration tested (15 g L-1), and only 16% when 0.5 g L-1 of captan 50® was used, in comparison to 100% inhibition in growth of the phytopathogenic at the same dose. These results probe greater resistance from control agent of microbial origin to the inhibitory effects of captan 50® compared to the phytopathogenic fungus under study, similar observations on the resistance of strains of the genus Trichoderma to synthetic fungicides have been reported, i.e. Trichoderma asperelloides and Trichoderma harzianum strains developed in increased concentrations of synthetic fungicides such as captan, 2350 ppm; thiabendazole, 20 ppm; and a mixture of capture-carboxin, 1500 ppm (Chaparro et al., 2011).
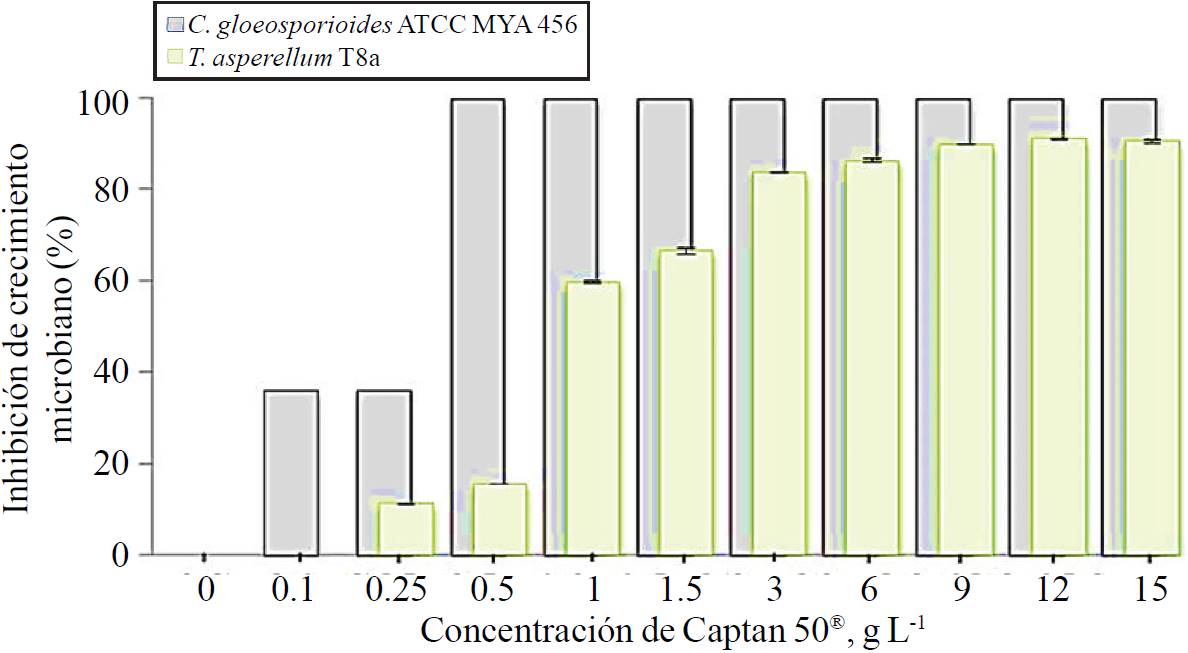
Figure 4. Minimum inhibitory concentration of Captan 50® on C. gloeosporioides ATCC MYA 456 (gray bars) and T. asperellum T8a (green bar) in petri dishes containing PDA as culture medium and incubated at 28 oC for 7 days.
In the present study the concentration of captan 50® selected for further trials was 0.1 g L-1, this in order to enhance the control of the phytopathogen through the synergy of the fungicide and the mycoparasitic strain T8a, due to 0.1 g L-1 captan 50® inhibits the growth of C. gloeosporioides ATCC MYA 456 in 36%, without showing inhibitory effects on T. asperellum T8a.
Synergism assays T. asperelleum T8a - captan 50® against C. gloeosporioides ATCC MYA 456. The confrontation assay between the co-inoculation of control agents (0.1 g L-1 capture 50® + T. asperelleum T8a) vs. C. gloeosporioides ATCC MYA 456 showed a synergistic effect of this interaction for control of the causal agent of anthracnose; i.e., the selected dose captan 50® (0.1 g L-1) inhibited 36% growth of C. gloeosporioides ATCC MYA 456 and T. asperellum T8a in 91% after 8 days of incubation at 28 °C, using PDA as culture medium. However, the interaction of control agents (0.1 g L-1 captan 50® + T. asperellum T8a) increased growth inhibition of the phytopathogen to 99%, under the same culture conditions (Figure 5).
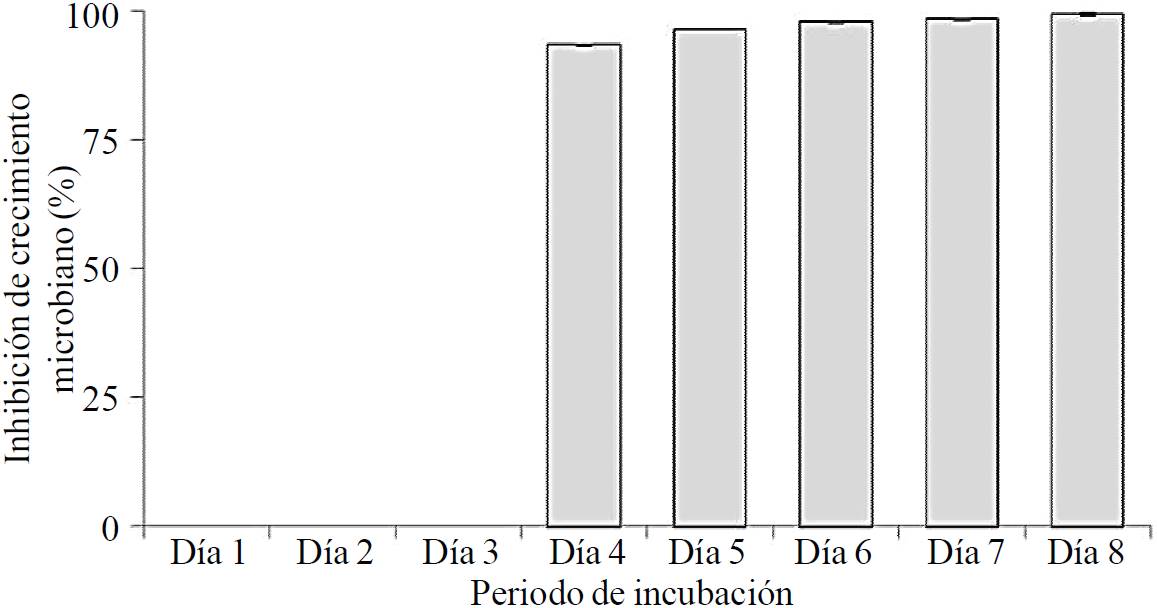
Figure 5. Growth inhibition of C. gloeosporioides ATCC MYA 456 through the synergy between T. asperellum T8a and 0.1 g L-1 Captan 50®. The assays were carried out in Petri dishes containing PDA as culture medium, added with 0.1 g L-1 Captan 50®, co-inoculating the microorganisms under study at a distance of 6 cm, incubated at 28 oC for 8 days.
These results suggest the following mechanism of action of the combination of control agents used: the concentration of 0.1 g L-1 capture 50® does not affect the growth of T. asperelleum T8a, but it does affect the development of C. gloeosporioides ATCC MYA 456, whereby, when both microbial strains are co-inoculated in Petri dishes containing PDA supplemented with 0.1 g L-1 captan 50®, it is inhibited or retards the growth of the phytopathogen without affecting the development of T. asperellum T8a, first providing an advantage in the competition for space and nutrients of T8a strain, and then when T. asperelleum T8a intercepts the mycelium of the phytopathogen with stunted growth by the dose of captan 50®, mycoparasitism starts. In this way, it is demonstrated the ability of strain T8a to survive in environments with remaining molecules of captan 50®, which enhances its biocontrol ability under the specific compatibility with the synthetic fungicide, representing a promising tool for sustainable control of fungal diseases in crops.
Similar results have been reported by Shaiesta et al., (2013), showing the ability of a strain of Trichoderma harzianum to tolerate high doses of captan (3 g L-1), carbendazim (1g L-1), bitertanol (1g L-1), hexaconazole (1g L -1) and mancozeb (3 g L-1), quantifying growth inhibition in 36.6%, 90.8%, 40%, 16.1% and 11.7%, respectively. Furthermore, Ordentlich et al., (1990) reported an increase in productivity of potato cv. Draga and cv. Desiree 46% and 80%, respectively, due to the reduction of the infection caused by Verticillium dahliae through an integrated control applying Trichoderma harzianum and captan.
Conclusion
The integrated use of T. asperellum T8a and a low dose and captan 50 ® (0.1 g L-1) led to in vitro growth inhibition, in an increased manner and synergistically of, C. gloeosporioides ATCC MYA 454, pathogenic strain responsible for anthracnose in mango. Which represents a promising alternative for sustainable control of anthracnose in mango orchards, thus control of various diseases in crops of interest. The application of this alternative has potential advantages compared to the excessive application of synthetic fungicides, i) T8a strain was isolated from the rhizosphere of mango trees from orchards in production, suggesting a successful plant x microorganism and microorganisms x microorganism interaction when applied in field; ii) T. asperelleum T8 maintained its ability to inhibit the growth of the phytopathogen in vitro in presence of synthetic fungicide, which shows that its mycoparasitic capacity is not compromised even when there are remnants of captan 50® molecules in the agro-system, as a result of excessive and constant application to mango cultivation; and iii) use of this technology will allow reduced application of captan 50® due to the low dose used, which in combination with T8a strain will boost the control of C. gloeosporioides, reducing the economic and environmental problems by the use of captan 50®.
Literatura citada
Alpuche, L. 1990. Los fungicidas: aspectos generales. In: Albert, L. (Coord.). Los plaguicidas. El Ambiente y la Salud. Centro de Ecodesarrollo. 215-217 pp. [ Links ]
Arauz, L. F. 2000. Mango anthracnose: economic impact and current options for integrated management. Plant Dis. 84(6):600-611. [ Links ]
Arias, B. y Carrizales, L. 2007. Control químico de la antracnosis del mango (Mangifera indica L.) en pre y postcosecha en el municipio Cedeño, estado Monagas, Venezuela. Bioagro. 19(1):19-25. [ Links ]
Atanasova, L.; Druzhinina, I. S. and Jaklitsch, W. M. 2013. Two hundred Trichoderma species recognized on the basis of molecular phylogeny. In: Mukherjee, P. K.; Horwitz, B. A.; Singh, U. S.; Mukherjee, M. and Schmoll, M. (Eds.). Trichoderma: biology and applications. CABI, Wallingford. 10-42 pp. [ Links ]
Bailey, J. A.; O'Connell, R. J.; Pring, R. J. and Nash, C. 1992. Infection strategies of Colletotrichum species. In: Bailey, J. A. and Jeger, M. J. (Eds.). Colletotrichum: biology, pathology and control,. Wallingford UK: CAB International. 88-120 pp. [ Links ]
Bonde, M. R.; Peterson, G. C. and Maas, G. L. 1991. Isozyme comparisons for identification of Colletotrichum spp. pathogenic to strawberry. Phytopathology. 81(12):1523-1528. [ Links ]
Bravoag, 2013. Captan 50. http://Salicitudes/fichas_tecnicas/_ft_captan50.pdf. [ Links ]
Bruce da Silva, C. F. and Michereff, S. J. 2013. Biology of Colletotrichum spp. and epidemiology of the anthracnose in tropical fruit trees. Rev. Caatinga. 26(4):130-138. [ Links ]
Chaparro, A.; Carvajal, L. and Orduz, S. 2011. Fungicide tolerance of Trichoderma asperelloides and T. harzianum strains. Agric. Sci. 2:301-307. [ Links ]
de los Santos-Villalobos, S.; Barrera-Galicia, G. C.; Miranda-Salcedo, M. A. and Peña-Cabrieles, J. J. 2012. Burkholderia cepacia XXVI siderophore with biocontrol capacity against Colletotrichum gloeosporioides. World J. Microbiol. Biotechnol. 28(8):2615-2623. [ Links ]
de los Santos-Villalobos, S.; de-Folter, S.; Délano-Frier, J. P.; Gómez-Lim, M. A.; Guzmán-Ortiz, D. A.; Sánchez-García, P. and Peña-Cabriales, J. J. 2011. Critical aspects on the integral management of mango: flowering, anthracnose and industrial waste. Rev. Mex. Cienc. Agríc. 2(2):221-234. [ Links ]
de los Santos-Villalobos, S.; Guzmán-Ortiz, D. A.; Gómez-Lim, M. A.; Délano-Frier, J. P.; de-Folter, S. and Peña-Cabriales, J. J. 2013. Potential use of Trichoderma asperellum (Samuels, Liechfeldt et Nirenberg) T8a as a biological control agent against anthracnose in mango (Mangifera indica L.). Biological Control. 64(1):37-44. [ Links ]
Druzhinina, I. S.; Seidl-Seiboth, V.; Herrera-Estrella, A.; Horwitz, B. A.; Kenerley, C. M.; Monte, E.; Mukherjee, P. K.; Zeilinger, S.; Grigoriev, I. V. and Kubicek, C. P. 2011. Trichoderma: the genomics of opportunistic success. Nat. Rev. Microbiol. 9(10):749-759. [ Links ]
Espinosa, J.; Arias, J.; Rico, H.; Miranda, M. y Chávez, X. 2004. Dinámica del daño y control de la antracnosis Colletotrichum gloeosporioides (Penz.) en mango en Michoacán. INIFAP 5:1-28. [ Links ]
FAO. 2013. FAOSTAT: http://faostat.fao.org/. [ Links ]
Gunnell, P. S. and Gubler, W. D. 1992. Taxonomy and morphology of Colletotrichum species pathogenic on strawberry. Mycologia 84:157-165. [ Links ]
Jeffries, P.; Dodd, J. C.; Jeger, M.J. and Plumbley, R. A. 1990. The biology and control of Colletotrichum species on tropical fruit crops. Plant Pathol. 39(3):343-366. [ Links ]
Karabulut, O. A. and Baykal, N. 2004. Integrated control of postharvest diseases of peaches with a yeast antagonist, hot water and modified atmosphere packaging. Crop Protection 23(5):431-435. [ Links ]
Ker, C. K. 2001. Sensitivity of mango anthracnose pathogen, Colletotrichum gloeosporioides, to the fungicide prochloraz in Taiwan. Proc Natl. Sci. Counc. Repub. China B. 25(3):174-179. [ Links ]
Lakshmi, B. K. M.; Kumari, D. A.; Kumar, A. K.; Babu, J. D. and Reddy, P. N. 2013. Mitigating postharvest losses caused by anthracnose disease in mango by using bio agents, botanicals and ISR chemicals. Acta Hortic. 1012:661-670. [ Links ]
Litz, R. E. 2000. World mango breeding problems and perspectives: a biotechnology overview. In: Proceedings Simposium Mango. Control de la floración y mejoramiento genético. INIFAP. 1-7. [ Links ]
Montero- Tavera, V.; Morales- García, J. L.; González- Chavira, M. M. y Anaya- López, J. L.; Corona-Torres, T. y Gálvez- Mariscal, A. 2010. Diversidad genética, patogénica y morfológica del hongo Colletotrichum gloeosporioides (Penz.) de Michoacán, México. Rev. Mex. Cienc. Agríc. 1(2):159-174. [ Links ]
National Center for Biotechnology Information (NCBI). 2014. http://www.ncbi.nlm.nih.gov/taxonomy/browser/wwwtax.cgi?id=474922. [ Links ]
Ordentlich, A.; Nachmias, A. and Chet, I. 1990. Integrated control of Verticillium dahliae in potato by Trichoderma harzianum and captan. Crop Protection. 9(5):363-6. [ Links ]
Peres, N. A.; Souza, N. L.; Peever, T. L. and Timmer, L. W. 2004. Benomyl sensitivity of isolates of Colletotrichum gloesporioides from Citrus. Plant Dis. 88(2):125-130. [ Links ]
Rader, U. and Broda, P. 1985. Rapid preparation of DNA from filamentous fungi. Lett Appl Microbiol. 1(1):17-20. [ Links ]
Ragazzo-Sánchez, J.; Robles-Cabrera, A.; Lomelí- González, L.; Luna- Solano, G. y Calderón- Santoyo, M. 2011. Selección de cepas de Bacillus spp. productoras de antibióticos aisladas de frutos tropicales. Rev. Chapingo Ser. Hortic. 17(1):5-11. [ Links ]
Shaiesta, S.; Sahera, N. and Shaheen, K. 2013. Efficacy of Fungicides against Trichoderma spp. causing green mold disease of oyster mushroom (Pleurotus sajor-caju). Res. J. Microbiol. 8(1):13-24. [ Links ]
Stevens, C.; Khan, V. A. and Lu, J. Y. 1997. Integration of ultraviolet (UV-C) ligth with yeast treatment for control of postharvest storage rots of fruits and vegetables. Biol. Control. 10(2):98-103. [ Links ]
White, T. J.; Bruns, T.; Lee, S. and Taylor, J. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis, M. A.; Gelfand, D. H.; Sninsky, J. J.; White, T. J. (Eds.). PCR protocols: a guide to methods and applications. Academic Press, San Diego. 315-322 pp. [ Links ]
Wu, C. H.; Bernard, S. M.; Andersen, G. L. and Chen, W. 2009. Developing microbe-plant interactions for applications in plant-growth promotion and disease control, production of useful compounds, remediation and carbon sequestration. Microb Biotechnol. 2(4):428-440. [ Links ]
Received: January 2016; Accepted: March 2016











 texto em
texto em 


