Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista mexicana de ciencias agrícolas
versão impressa ISSN 2007-0934
Rev. Mex. Cienc. Agríc vol.7 no.5 Texcoco Jun./Ago. 2016
Articles
Periodic application of low concentrations of paclobutrazol and salicylic acid in potatoes in greenhouse
1Sitio Experimental Metepec-INIFAP. Toluca-Zitácuaro. Carretera Vial Adolfo López Mateos, Col. San José Barbabosa, km 4.5. Zinacantepec, Estado. México. C. P. 51350. Tel: (722) 278.43 31). (rmtzzm@yahoo.mx; lopez.humberto@inifap.gob.mx; irammarin1989@gmail.com).
The objective of this research was to determine the use of low concentrations of salicylic acid and paclobutrazol in Solanum tuberosum (Crystal and clone 981824) in the greenhouse for increased performance and potato starch. The experiments were performed in 2012 in Zinacantepec, Mexico. Treatments consisted of spraying with AS: 10-8, 10-10, 10-12 y 10-14 M, paclobutrazol: 1, 0.75, 0.5, 0.25 mg L-1 and the control, arranged in a randomized complete blocks, determined height, chlorophyll, IAF, fresh weight, number of tubers and starch content. In clone 981824, the control plants and AS (10-8 and10-10) had higher, while for Cristal were AS, witness and paclobutrazol 0.25 mg L-1. Chlorophyll and IAF there were no significant differences. In clone 981824, the witness had higher fresh weight (148 g per plant) instead Cristal showed no differences between treatments. The total number of minitubers was higher with paclobutrazol 0.5 mg L-1 in both genotypes (Crystal: 12.93 and 981824: 23.3). All paclobutrazol treatments and AS 10-8, 10-10 and 10-14 had higher starch content compared with the control in the clone 981824, while in Cristal were paclobutrazol 0.75 and 1.0 mg L-1 to 241 and 231 mg g-1, 10-8 and 10-12 of AS 165 and 173 mg g-1. In conclusion paclobutrazol reduces plant height and both this and the AS increase the starch content of the tuber at the indicated concentrations.
Keywords: Solanum tuberosum; greenhouse; minitubers
El objetivo de esta investigación fue determinar el uso de bajas concentraciones de ácido salicílico y paclobutrazol en Solanum tuberosum (Cristal y clon 981824) en invernadero para el incremento de rendimiento y almidón en papa. Los experimentos se realizaron en 2012 en Zinacantepec, México. Los tratamientos consistieron en aspersión con AS: 10-8, 10-10, 10-12 y 10-14 M, paclobutrazol: 1, 0.75, 0.5, 0.25 mg L-1 y el testigo, arreglados en bloques completos al azar, se determinó altura, clorofila, IAF, peso fresco, número de tubérculos y contenido de almidón. En el clon 981824, las plantas testigo y las de AS (10-8 y10-10) presentaron mayor altura, mientras para Cristal fueron las de AS, testigo y paclobutrazol 0.25 mg L-1. En clorofila e IAF no hubo diferencias significativas. En el clon 981824, el testigo presento mayor peso fresco (148 g por planta), en cambio Cristal no presentó diferencias entre tratamientos. El número total de minitubérculos fue superior con paclobutrazol 0.5 mg L-1 en ambos genotipos (Cristal: 12.93 y 981824: 23.3). Todos los tratamientos de paclobutrazol y de AS 10-8, 10-10 y 10-14 presentaron mayor contenido de almidón con respecto al testigo en el clon 981824, mientras que en Cristal fueron: paclobutrazol 0.75 y 1.0 mg L-1 con 241 y 231 mg g-1 y 10-8 y 10-12 de AS con 165 y 173 mg g-1. En conclusión el paclobutrazol disminuye la altura de las plantas y tanto este como el AS incrementan el contenido de almidón de tubérculo a las concentraciones indicadas.
Palabras clave: Solanum tuberosum; invernadero; minitubérculos
Introduction
The use of growth regulators in agriculture has been made for various purposes; as growth promoters (gibberellins, cytokinins and auxins) and inhibitors or growth retardants, use of the latter is made among other purposes for fruit set and control the height of crops without demerit in production Jankiewicz (2003). Potato have been evaluated in vitro, for maintenance of germplasm, as inducers of tuberisation and tolerance to high and low temperatures, greenhouse using them is aimed at limiting the growth and development of the plant to facilitate the work of culture and increase the number of tubers per plant and per square meter, i.e. to promote the process of tuberisation (Flores, 2009).
The tuberisation process is influenced by photoperiod, temperature, conditions governing, low levels of endogenous growth regulators Mauk and Langille (1978), increased carbohydrate at the apex of stolons in the concentration of gibberellins Martínez et al. (2001) and abscisic acid increased Xu et al. (1998). Gibberellins acting as inhibitor tuberisation Krauss (1985) and in induction conditions tuberisation low contribution of altered nitrogen radius of abscisic acid hormones: gibberellins, increased the abscisic acid and decreased the amount of gibberellins, which it coincided with the start of the tuberisation Krauss and Marschner (1981). Endogenous levels of gibberellins in potato plants are high in non-inductive conditions (high temperatures and long photoperiod) and decrease under conditions of induction tuberisation (Vreugdenhil and Sergeeva, 1999).
Several growth inhibitors have been used as antagonistic agents gibberellins in non-inductive conditions tuberisation (high temperature and long photoperiods), such as: tetcyclasis, cycocel, paclobutrazol, uniconazole, alar, ancymidol and daminozide, some of the which increase the formation of tubers in conditions in vitro and greenhouse (López et al., 1998; Rodríguez et al., 2006; Tekalign et al., 2005; Flores et al., 2011).
The paclobutrazol inhibits the biosynthesis of gibberellins, by oxidation of ent-kaurene Dicks (1980) and blocks oxidative reactions between kaurenoic acid and kaurene. The restoration of growth of the plant with application of gibberellin found that this effect is due to paclobutrazol (Balamani and Poovaiah, 1985). It has been shown that pretreatments paclobutrazol in sweetpotato increases its antioxidant system (Kuan-Hung, 2006). In potatoes, in vitro conditions paclobutrazol concentrations of 0.001 mg L-1 produced microtubers weight and diameter greater than the control Kianmeher et al. (2012), likewise, applications kinetin 2 .5 mg L-1 had no significant influence on the formation of tubers, however applications along with paclobutrazol (0.001 mg L-1) significantly increased tuber. While the stimulus paclobutrazol tuber initiation and inhibited growth (Simko, 1993).
On the other hand, it lowers greenhouse plant height, growth rate and reduces crop partition assimilated to leaves, stems, roots and stolons, tubers increasing it to (Flores et al., 2011); (Balamani and Poovaiah, 1985) also causes the increase of chlorophyll a and b, root diameter increases and becomes thicker layer palisade mesophyll cells and spongy tissue (Tsegaw et al., 2005); at high temperature, improves productivity and reduces tuber stem growth (Tekalign et al., 2004).
Applications 150 and 450 mg L-1 paclobutrazol increased the number of minitubers in soilless (Lim et al., 2004), while Bandara and Tanino (1995), obtained on greenhouse variety Norland almost twice tubers with a single application of 450 mg paclobutrazol L-1. However, Flores (2011) mention that with 150 mg L-1 paclobutrazol or 20 mg L-1 uniconazole, the plant height is reduced without affecting the number of tubers and biomass. However, there are also studies where there may be an adverse effect by applying paclobutrazol, mentioned which can reduce the biomass of the entire plant (Tekalign et al., 2005) and even the performance of minitubers (Hughes and Keith, 2003).
Furthermore, salicylic acid (AS) has pleiotropic effects, is directly involved in plant growth, thermogenesis, floral induction and assimilation ions (Hayat et al., 2007). It mentioned as a growth promoter to promote increased height and stem diameter in chrysanthemum and habanero (Guzmán et al., 2012; Villanueva et al., 2009), it has been shown to increase biomass productivity and fruits in jalapeno (Sánchez et al., 2011), likewise, studies show their participation in the induction of stress tolerance low temperatures, shocks high temperature and salinity in potato in vitro conditions and (Mora and López, 2006; Scoot et al., 1999; Sajid and Aftab, 2012) and induces tolerance to some diseases such as late blight of potato, purple top syndrome potato (Halim et al., 2007; López et al., 2013) and Rizoctonia solani (Hadi and Baladi, 2010), the latter author mentions that applications of 2 mM of AS increased the number of tubers in plants infected with the fungus. Because of all the properties shown the AS is considered a true plant hormone involved in plant defense and immune reactions to abiotic stress, it contributes to the regulation of growth and development of plants (Rivas and Plasencia, 2011).
So far it is not known that the AS intervene antagonistically with the gibberellins; however, several genes are under the transcriptional regulation of this, including genes involved in the synthesis of jasmonic acid and ethylene (Galis and Matsuoka, 2007). López and Scott (1996), induced in potato tuber crops in vitro by using 7.5 x 10-4 acetylsalicylic acid (ASA). It has also been achieved growth arrest using ASA and CCC in in vitro potato plants (López et al., 1998). While in the field, ASA applications increased performance and improved quality of tubers for industry (Nickell, 1991).
Materials and methods
The experiments were conducted from June to November 2012 in greenhouses INIFAP in Zinacantepec, Mexico at 19° 17’ 21” north latitude and 99° 42’ 49” west longitude at 2 640 meters. The average temperature inside the greenhouse was 14.9 °C with a maximum of 36 and minimum of -1.5 °C respectively.
The pots volume of 1 L were used, with a horticultural grade perlite 1 to 4 mm in diameter and peat 1:1 v/v. First experiment were used clone 981824 and then two experiments with the variety Cristal was established in vitro plants free of virus. The experimental design was randomized complete block with four replications and 24 pots per repetition of which only ten plants were taken for data collection: for the experiment with clone 981824 only were taken: height, fresh weight, number and diameter tubers and starch content in tubers. In experiments with the variety Cristal was also taken, tubers dry weight of starch in tubers, SPAD readings and leaf area index (IAF). The amount of starch in minitubers was determined by the method described by Peña and Ortega (1991), chlorophyll content was evaluated by the method of Lichtenthaler and Wellburn (1983) and correlated with the SPAD units obtained with the meter Konica Minolta Chlorophyll plus 502 SPAD- according to (Rodríguez et al., 1998).
The SPAD readings were taken in the first fully expanded leaf, making three readings per plant at three different times, 22 days after the first application. In addition, IAF readings were made the day after taking SPAD readings, and were made with the Ceptometro Accupar, model LP-80 PAR / IAF.
The plants were irrigated by drip 8 L h-1 with four outputs distributor. They were scheduled, two irrigations per day at a cost of 66 ml per pot in each irrigation to a maximum expenditure of 133 ml per pot, with the solution of 160 N; 60 P; 250 K, 250 Ca; 75 Mg; 3 Fe; 0.5 Cu; 0.5 Mn and 0.5 B in mg L-1 and pH was adjusted to 6 + -0.1 and 2 mS cm-1 of CE.
Treatments with different concentrations of salicylic acid and paclobutrazol were applied twice a week after the 22 days of transplantation (DDT) to 85 DDT spray of 3.0 to 4.5 ml per plant solution 75 to 110 ml per replicate, four concentrations of AS (10-8, 10-10, 10-12 and 10-14 M) and four concentrations of paclobutrazol (1, 0.75, 0.5, 0.25 mg L-1), the witness was applied only with water.
Results and discussion
Overall height with respect to the variable significant differences between treatments in the three experiments, showed clone 981824 taller plants were those for the witness, the concentrations of 10-8 and 10-10 of AS, and lower height corresponded to treatments paclobutrazol, while the Cristal variety in the average of both experiments shows that plants taller were treatments with AS, the witness and those corresponding to the lower paclobutrazol concentration (0.25 mg L-1) and equal to each other statistically, plants presenting lower height paclobutrazol treatments of 1, 0.75 and 0.5 mg L-1 (Figure 1). Regarding AS, Galis and Matsuoka (2007) mention that there are no reports that it is antagonistic gibberellin; also it mentioned that favors height and stem diameter increase in chrysanthemum and habanero (Guzmán et al., 2012; Villanueva et al., 2009), which is not consistent with the results obtained in this work, since treatments with SA were in the range equal to the witness Cristal and clone 981824 plants had lower height than this.

Under Cristal. Var. Cristal and 981824 clone. Tukey α= 0.05. Treatments with the same letter do not differ statistically.
Figure 1 Average plant height and mean comparison with regular applications of Paclobutrazol and AS.
While paclobutrazol known as synthesis inhibitor gibberellin as has been achieved restoring growth of the plant with application of gibberellins, which proves that the effect of growth inhibition is due to paclobutrazol, which inhibits biosynthesis (Balamani and Poovaiah, 1985). And while there are various jobs that match what is reported here growth arrest (Flores et al. 2011); Balamani and Poovaiah, 1985) there are no reports regarding the continuous application of low levels of this inhibitor.
However, the results could indicate that the continued application of it if possible accumulation causes of this inhibitor on the ground, causing growth arrest. Also, a single application of paclobutrazol of 1 mg L-1 at 15 days after transplantation in potato produces no growth arrest.
Chlorophyll concentration. The linear regression model to determine the relationship between chlorophyll and Spad readings presented an R2 of 0.89, α= 0.01, so there is significant relationship between chlorophyll concentration and SPAD readings: total chlorophyll = 0,121882 + (2.86474) (SPAD), by substituting in the formula the values of SPAD, the following results (Table 1) were: in the Cristal variety of plants with the highest values of chlorophyll corresponded to the treatments of 0.5, 0.75 of paclobutrazol and 10-8 of AS, 22 days after the first application and statistically equal to the control, while for the second sampling 55 DDA, only the concentration of 0.5 mg L-1 showed the highest value; however, it was equal to the witness, paclobutrazol remaining treatments and two concentrations of SA (10-12 and 10-14), and the DDT 80 no significant differences between treatments. For this variable, the results were inconclusive and although some authors mention that the paclobutrazol increases the amount of chlorophyll Tsegaw et al. (2005) in this work do not get upset. The SA they not for two treatments (10-8 and 10-10), in the second reading had the lowest values for chlorophyll in both experiments.
Table 1 Contents of Chlorophyll (in potato plants at 22, 52 and 80 DAA in the Cristal variety with salicylic acid and paclobutrazol.
| Tratamiento | Clorofila (µg*ml-1) | |||||
| Tukey | MEDIA | Tukey | MEDIA | Tukey | MEDIA | |
| 22 DDA | 52 DDA | 80 DDA | ||||
| Testigo | ab | 151.22 | ab | 151.3 | a | 145.76 |
| Paclo. 0.25 | ab | 152.69 | ab | 154.33 | a | 151.79 |
| Paclo. 0.5 | a | 155.4 | a | 153 | a | 151.55 |
| Paclo. 0.75 | a | 154.33 | ab | 155.51 | a | 152.9 |
| Paclo. 1.00 | ab | 150.6 | ab | 152.59 | a | 152.9 |
| A. S. 10-8 | a | 154.61 | b | 146.58 | a | 149.82 |
| A. S. 10-10 | b | 144.99 | b | 146.44 | a | 145.77 |
| A. S. 10-12 | ab | 149.96 | ab | 153.25 | a | 149.4 |
| A. S. 10-14 | ab | 149.57 | ab | 154.43 | a | 151.39 |
| CV | 5.946 | 5.393 | 4.849 | |||
Tukey α= 0.05. Treatments with the same letter do not differ statistically.
Regarding the IAF were taken two readings, the first 75 DDA and second before harvest, no significant difference between treatments was found; however, it occurs in both readings trend lower IAF (Figure 2) in the treatment of 1 mg L-1 paclobutrazol (Figure 2) higher leaf area index was presented in treatment plants of 0.75 mg L-1, with no significant statistical difference; however, this index showed normal values above 3 units, which is enough to capture more than 99% of solar radiation.
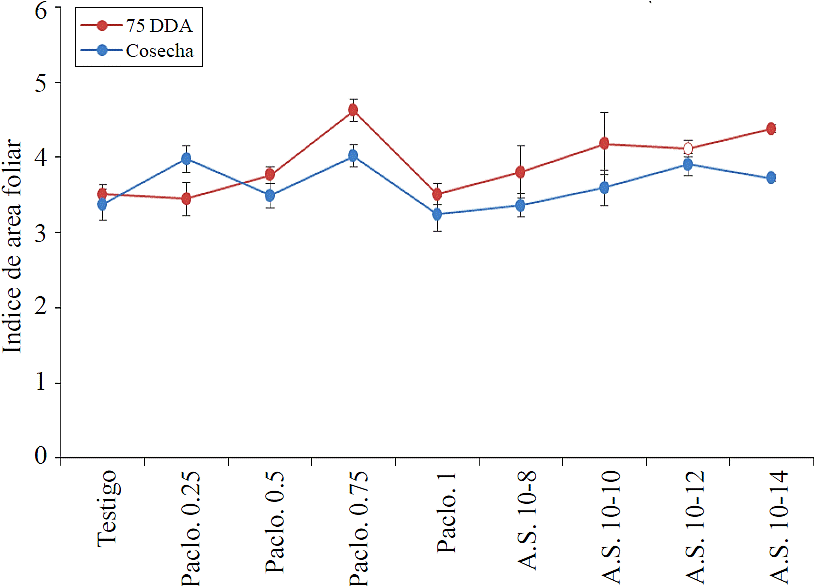
Paclo= paclobutrazol; AS= salicylic acid.
Figure 2 Leaf area index in the range Cristal 75 days after the first application of treatments.
These results contrast with mentioned by Flores et al. (2011), who detected a decrease relative to the control IAF up 54% using 250 mg L-1 of paclobutrazol in one application.
The fresh weight of tubers if was affected by treatments in clone 981824, but not in the variety Cristal, which showed no significant difference between treatments, while the clone 981824 the control treatment with 137.48 g was statistically equal to plants paclobutrazol treatments 0.25 mg L-1 and salicylic acid 10-8 higher, 18 to 31.5%, the other treatments with paclobutrazol and SA, which decreased the fresh weight of tubers (Table 2).
Table 2 Effect on the fresh weight of tubers produced in greenhouses, variety Cristal and clone 981824 per share of paclobutrazol and salicylic acid.
| Tratamiento | Exp. Cristal | Exp. 981824 | ||
| Tukey | Media | Tukey | Media | |
| P. Frs. | P. Frs. | |||
| Testigo | a | 104.16 | a | 137.48 |
| Paclo. 0.25 | a | 99.32 | ab | 118.25 |
| Paclo. 0.50 | a | 109.25 | bcd | 108.65 |
| Paclo. 0.75 | a | 113.54 | bcd | 104.61 |
| Paclo. 1.00 | a | 98.83 | d | 94.17 |
| A. S. 10-8 | a | 101.12 | ab | 120.88 |
| A. S. 10-10 | a | 102.59 | bc | 111.78 |
| A. S. 10-12 | a | 108.8 | cd | 101.3 |
| A. S. 10-14 | a | 107.6 | bcd | 105.33 |
| CV | 15.778 | 34.447 | ||
Tukey α= 0.05.
In general can be observed in clone 981824 in some treatments was presented reduced fresh weight of tuber with paclobutrazol. Reports in the literature differ and therefore no results found supporters here, as reports where biomass is not affected or is less according to the concentration used (Flores et al., 2011; Balamani and Poovaiah, 1985).
As for the number of minitubers in both genotypes were no differences between treatments were observed in tubers over 15 mm in diameter; however, considering the total tubers if there is significant difference between treatments in both genotypes; for variety Cristal treatment with more minitubers it was that of paclobutrazol 0.5 mg L-1 with 12.9 tubers per plant, and was statistically equal treatment of 0.25 and 0.75 mg L-1 of paclobutrazol and concentrations AS of 10-12 and 10-14, while the witness present 11.25 minitubers per plant, and treatment with the lowest number of them was AS to 10 -10 M, while in clone 981824 results were inconclusive because the treatment with the highest number of tubers was with 0.25 mg L-1 and was equal to both the witness and the other treatments except for AS of 10-8 M with present only 16.9 tubers per plant (Table 3). There are contradictory statements regarding paclobutrazol, if it favors tuber Tekalign et al. (2005) mention that may cause decreased performance, Flores et al. (2011) confirmed to mention that some of the concentrations studied from 200 to 250 mg L-1 decrease biomass tubercle, while at concentrations of 150 mg L-1 no significant difference with the control without paclobutrazol in this sense.
Table 3 Effect on the number of tubers produced in greenhouses, variety Cristal and clone 981824 for effect of paclobutrazol and salicylic acid.
| Tratamiento | Exp. Cristal | Exp. 981824 | ||||||
| Tukey | Media | Tukey | Media | Tukey | Media | Tukey | Media | |
| TT | T>15 mm | TT | T>15 mm | |||||
| Testigo | bc | 11.2581 | a | 8.4086 | ab | 20.4 | a | 12.85 |
| Paclo. 0.25 | abc | 11.6316 | a | 9.0526 | a | 23.3 | a | 14.5 |
| Paclo. 0.50 | a | 12.9302 | a | 9.5698 | ab | 22.35 | a | 13.4 |
| Paclo. 0.75 | ab | 12.0625 | a | 9.2396 | ab | 21.3 | a | 12.45 |
| Paclo. 1.00 | bc | 10.9684 | a | 8.9789 | ab | 18.3 | a | 12.5 |
| A. S. 10-8 | bc | 10.4471 | a | 8.5294 | b | 16.9 | a | 12.25 |
| A. S. 10-10 | c | 10.2895 | a | 8.6184 | ab | 19 | a | 13.05 |
| A. S. 10-12 | abc | 11.383 | a | 9.234 | ab | 21.3 | a | 14.55 |
| A. S. 10-14 | abc | 11.6787 | a | 9.0217 | ab | 19.35 | a | 12.45 |
| CV | 31.299 | 30.819 | 29.182 | 24.558 | ||||
Tukey α= 0.05. TT= total tubers; T> 15 mm= tubers over 15 mm diameter.
Also Tekalign et al. (2005) mention that can enhance productivity of tubers in high temperature conditions. In both AS, there are no references to the moment the effect of low concentrations as about 10-12 minituber production; however, Benavides et al. (2005) mentions that applications AS of 10-4 and 10-6 increased the number of tubers per plant but not production or the average weight per tuber. In relation to the different response in both genotypes, it could present because the Cristal variety produces fewer tubers and is intermediate, while clone 981824 is late and has a potential for increased production. This confirms that genetic variability between genotypes can give a different response to different growing conditions.
Starch content. It is observed in clone 981824, in general, a tendency to accumulate in larger tuber starch content in treatment compared with the control, as seen in (Figure 3). All treatments with paclobutrazol starch showed higher values than the control, while with AS of four concentrations tested three of them were slightly higher than the control.
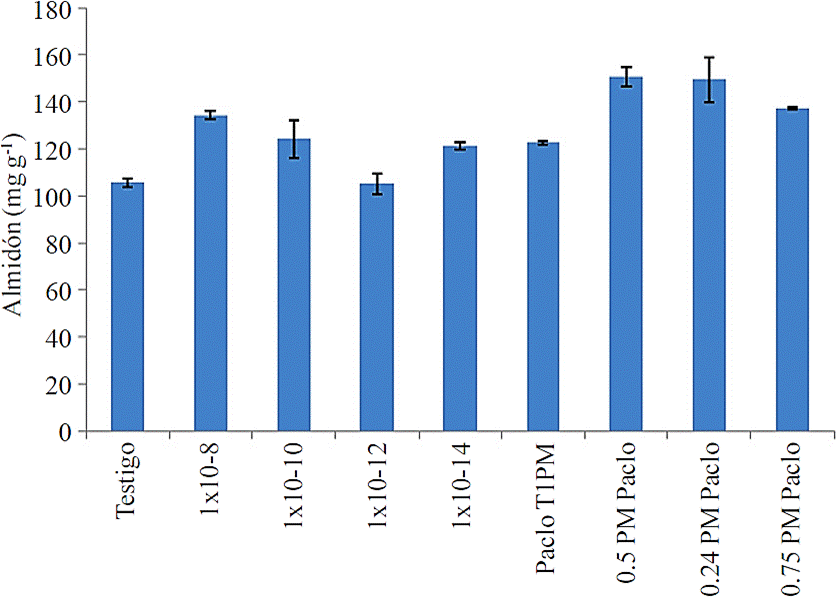
Figure 3 Concentration of starch in the tuber with salicylic acid and paclobutrazol in potato clone 981824.
Also, in the Cristal variety the results were similar to experiment with clone 981824, since according to the analysis of variance and differentiation test Tukey (Table 4), there are differences between treatments α= 0.0001 detected that two of treatments paclobutrazol 1 and 0.75 mg L-1 the highest concentrations of starch, with 241.92 and 231.24 mg g-1 fresh weight, respectively, while the concentrations of 0.5 and 0.25 mg L-1 starch content in tubers was similar to that detected in tubers of control treatment, these results agree with those mentioned by Balamani and Poovaiah (1985) who claim that the paclobutrazol decreased the contribution of assimilates to stems, leaves, roots and stolons, increasing it to the tubers and Tsegaw et al. (2005) mention that increases paclobutrazol starch synthesis in cells of the marrow stem cells and cortical stem and root. Salicylic acid treatments 10-8 and 10-12 were also superior to the control in the tuber starch content, with 173.96 and 165.26 mg g-1 fresh weight, respectively. While treatments 10-10 and 10-14 were statistically equal to the control. These results are consistent with results obtained by Nickell (1991), who with ASA applications under field conditions increased yield and improved quality of tubers for industry.
Table 4 Mean Difference starch content in tubers of the variety Cristal in response to periodic applications of paclobutrazol and salicylic acid.
| Tukey | Media mg g-1 p. fresco | Tratamiento |
| a | 241.92 | PACL 0.75 |
| a | 231.24 | PACL 1.0 |
| b | 173.96 | S. A. 10-12 |
| c b | 165.26 | S. A. 10-8 |
| c b d | 153.64 | S. A. 10-10 |
| e d | 140.28 | Testigo |
| e d | 125.74 | PACL 0.5 |
| e | 116.65 | PACL 0.25 |
| e | 116.17 | S. A. 10-14 |
Tratamientos con la misma letra no son significativas. Tukey α= 0.05.
Conclusions
Low and regular spray applications of paclobutrazol potato inhibit plant growth, increase chlorophyll content and starch accumulation favor, with a tendency to increase the number of tubers at low concentrations.
Low and regular applications of salicylic acid tend to favor the accumulation of starch in the tuber without affecting plant growth and production of minitubers.
Literatura citada
Balamani, V. and Poovaiah, W. 1985. Retardation of shoot growth and promotion of tuber growth of potato plants by Paclobutrazol. Am. Potato J. 62:363-369. [ Links ]
Bandara, S. and Tanino, K. 1995. Paclobutrazol enhances minituber production in Norland potatoes. J. Plant Growth Regulation. 14(3):151-155. [ Links ]
Benavides, A.; Morales, S; Ramírez, H.; Fuentes, O. y Mendoza, R. 2005. Aplicación de inductores de tolerancia en plantas de papa. http://abenmen.com/a/papa_inductores.pdf. [ Links ]
Dicks, W. 1980. Recent developments in the use of plant growth retardants. In: Clifford, D. R. and Lenton, J. R. (Ed.) recent developments in the use of plant growth retardants. Monograf. 4. British Plant Growth Regul. Group, Wantage, UK. 1-14 pp. [ Links ]
Flores, R.; Sánchez, F.; Rodríguez, E.; Mora, R.; Colinas, T. y Lozoya, H. 2011. Paclobutrazol, Uniconazol y Cycocel en la producción de tubérculo-semilla de papa en cultivo hidropónico. Rev. Chapingo. 2:173-182. [ Links ]
Galis, I. and Matsuoka, K. 2007. Transcriptomic analysis of salicylic acid-responsive genes in tobacco BY-2 cells. In: Hayat, S. and Ahmad, A. (Eds.). Salicylic acid - a plant hormone. Springer, Dordrecht. The Netherlands. 371-396 pp. [ Links ]
Guzman, A.; Borges, L.; Pinzón, L.; Ruiz, E. y Zúñiga, J. 2012. Efecto del ácido salicílico y la nutrición mineral sobre la calidad de plántulas de chile habanero. Agron. Mesoam. 23(2):247-257. [ Links ]
Hadi, M. and Baladi, G. 2010. The effect of salicylic acid on the reduction of Rizocthonia solani damage in tubers of Marfona potato cultivar. American- Eurasian J. Agric. and Environ. Sci. 7(4):492-496. [ Links ]
Halim, V.; Eschen, L.; Altmann, S.; Birschwilks, M.; Scheel, D. and Rosahl, S. 2007. Salicylic acid is important for basal defense of Solanum tuberosum against Phytophthora infestans. Molecular Plant-Microbe Interactions. 20(11):1346-1352. [ Links ]
Hayat, B. and Ahmad, A. 2007. Salicylic acid: Biosynthesis, metabolism and physiological role in plants (Chapter 1). In: Hayat, S. and Ahmad, A. (Eds.). Salicylic acid-a plant hormone. Springer, Dordrecht. The Netherlands. 1-14 pp. [ Links ]
Hughes, R. and Keith, N.F. 2003. Effect of Paclobutrazol treatments on growth and tuber yields in greenhouse - grown Shepody seed potatoes. Acta Hortic. (619):271-277. [ Links ]
Jankiewicz, S. L. 2003. Retardadores y otros inhibidores sintéticos del crecimiento y sustancias que modifican el desarrollo en plantas. In: Jankiewicz, S. L. (Ed.). Reguladores del crecimiento, desarrollo y resistencia en plantas. Propiedades y acción. Universidad Autónoma de Chapingo. Ed. Mundiprensa. 241-256 pp. [ Links ]
Kianmeher, B.; Otroshy, M.; Parsa, M.; Mohallati, N. M. and Moradi, K. 2012. Effect of plant growth regulation during in vitro phase on potato minituber production. Int. J. Agric. Sci. 4(15):1060-1067. [ Links ]
Kuan, L.; Chao, T.; Shih, H.; Long, C. and Hsiao, L. 2006. Paclobutrazol pre-treatment enhanced flooding tolerance of sweet potato. J. Plant Physiol. 163:750-760. [ Links ]
Krauss, A. 1985. Interaction of nitrogen nutrition, phytohormones in tuberization potato physiology. Academic, Pres, London. 209-231. [ Links ]
Krauss, A. and Marschner, H. 1991. Influence of nitrogen nutrition, daylength and temperature on contents of gibberellic and absisic acid in potato plants. [ Links ]
Lichtenthaler, K. and Wellburnt, R. 1983. Determinations of total carotenoids and chlorophylls a y b of leaf extracts in different solvents. V. Biochem. Soc. Trans. 11:591-592. [ Links ]
Lim, T.; Yoon, C.; Choi, S. and Dithal, P. 2004. Application of gibberellic acid and Paclobutrazol for efficient production of potato (Solanum tuberosum L.) minitubers and their dormancy breaking under soilless culture system. J. Korean Soc. Hortic. Sci. 45(4):189-193. [ Links ]
López, H. and Scott, I. 1996. Induction of in vitro tuberization of potato microplants by acetylsalicylic acid. J. Plant Physiol. 151:74-78. [ Links ]
López, H. A.; Jimenez, M. and Scott, I. 1998. Storage of potato microplants in vitro in the presence of acetylsalicylic acid. Plant Cell. Tissue Organ Culture. 54:145-152. [ Links ]
López, H. A.; Mora, M.; Martínez, R. and Sánchez, S. 2013. Short and long term effects of salicylic acid and protection to phytoplasmaassociated stress in potato plants. In: Hayat et al. (Eds.). Salicylic acid. Springer Science + Business media Dordrecht. [ Links ]
Simko, I. 1993. Effects of kinetin, paclobutrazol and their interactions on the microtuberization of potato stem segments cultured in vitro in the light. Plant Growth Regulation. 12:23-27. [ Links ]
Martínez, F.; García, N. L.; Bou, J. and Prat, S. 2001. The interaction of gibberellins and photoperiod in the control of potato tuberization. J. Plant Growth Regulation. 20(4):377-386. [ Links ]
Mauk, C. and Langille, A. 1978. Cis-Zeatin riboside in the potato plant: its identification and changes in endogenous levels as influenced by temperature and photoperiod. Plant Physiol. 62:438-442. [ Links ]
Mora, M. E. y López, H. A. 2006. Tolerancia a baja temperature inducida por ácido salicílico y peroxido de hidrógeno en microplantas de papa. Rev. Fitotec. Mex. 29. (2):81-85. [ Links ]
Nickell, L. 1991. Aspirin and sulfonilamide improve processed potato quality. Proceedings 18th annual meeting. Plant Growth Regulators Society of America. 115-120. [ Links ]
Peña, B. and Ortega, L. 1991. Nonstructural carbohydrate partitioning in Phaseolus vulgaris after vegetative growth. J. Sci. Food Agric. 55:563-577. [ Links ]
Rodríguez, M. N.; Alcántar, G.; Aguilar, A.; Etchevers, D. y Santizo, A. 1998. Estimación de la concentración de nitrógeno y clorofila en tomate mediante un medidor portátil de clorofila. Rev. Terra. 16(2):135-141. [ Links ]
Rodríguez, M.; Bou, J. and Prat, S. 2006. Seasonal control of tuberization in potato: conserved elements with the flowering response. Ann. Rev. Plant Biol. 57:151-180. [ Links ]
Rivas, M. and Plasencia, J. 2011. Salicylic acid beyond defense: its role in plant growth and development. J. Exp. Bot. 62(10):3321-3338. [ Links ]
Sajid, Z. and Aftab, F. 2012. Salicylic acid in amelioration of salt tolerance in potato (Solanum tuberosum L.) under in vitro conditions. Pak. J. Bot. 44:37- 42. [ Links ]
Sánchez, E.; Barrera, R.; Muñoz, E.; Ojeda, L. D. y Álvaro, A. 2011. Efecto del ácido salicílico sobre biomasa, actividad fotosintética, contenido nutricional y productividad del chile jalapeño. Rev. Chapingo Ser. Hortic. 17(1):63-68. [ Links ]
Scott, I.; López, H. and Foyer, C. 1999. Salicylic acid and hydrogen peroxide in abiotic stress signaling in plants. Phyton Plant Physiol. 39(3):13 -17. [ Links ]
Tekalign, T. and Hammes, S. 2004. Response of potato grown under noninductive condition to Paclobutrazol: shoot growth, chlorophyll content, net photosynthesis, assimilate partitioning, tuber yield, quality, and dormancy. Plant Growth Regulation . 43:227-236. [ Links ]
Tekalign, T. and Hammes, S. 2005. Growth and biomass production in potato grown in the hot tropics as influenced by Paclobutrazol. Plant Growth Regulation. 45(1):37-46. [ Links ]
Tsegaw, T.; Hammes, S. and Robbertse, J. 2005. Paclobutrazol-induced leaf, stem, and root anatomical modification in potato. Hortscience. 40(5):1343-1346. [ Links ]
Villanueva, E.; Alcántar, G.; Sánchez, P.; Soria, M. y Larqué, A. 2009. Efecto del ácido salicílico y dimetil sulfoxido en la floración de [chrysantemum morifolium (ramat) kitamura] en Yucatán. Rev. Chapingo Ser. Hortic. 15(2):23-31. [ Links ]
Vreugdenhill, D. 2004. Comparing potato tuberization and sprouting opposite phenomena? Amer. J. Potato Res. 81:275-280. [ Links ]
Vreugdenhil, D. and Sergeeva, L. 1999. Gibberellins and tuberization in potato. Potato Res. 42:471- 481. [ Links ]
Xu, X.; Van Lammeren, M. A.; Vermeer, E. and Vreugdenhil, D. 1998. The role of gibberellin, abscisic acid, and sucrose in the regulation of potato tuber formation in vitro. Plant Physiol. 117:575-584. [ Links ]
Received: February 2016; Accepted: May 2016











 texto em
texto em 


