Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista mexicana de ciencias agrícolas
versión impresa ISSN 2007-0934
Rev. Mex. Cienc. Agríc vol.7 no.4 Texcoco may./jun. 2016
Articles
Acclimation of Agave americana var. Oaxacensis obtained in vitro
1 División de Estudios de Postgrado e Investigación-Instituto Tecnológico del Valle de Oaxaca. Ex Hacienda de Nazareno, Xoxocotlán Oaxaca. C. P. 71230. México. Tel: 01 (951) 5170444. (elvia_year@hotmail.com; jenriquezdelvalle@yahoo.com; vicvel5@hotmail.com; geraro65@gmail.com).
Because of the importance of Agave americana variety oaxacensis as raw material for the craftsmanship of mezcal. The growth of 260 micro-propagated plants was evaluated during 245 days having on average: 4.6 leaves, 10.1 cm in the longest leaf (AHML), 8.5 cm in diameter crown (DC) and 20.1 g cm-2 chlorophyll leaf blade (CL). The plants were established according to a completely randomized design with factorial arrangement (4 x 5), using pots of 300 cm3 with four different substrates: sand (1), sand-vermiculite (1:1), sand-peat (1:1) sand and peat-vermiculite (1:1:1), using daily irrigation with five different compositions 1) water and; 2) some aliquot: 25, 50, 75 and 100% of the universal solution Steiner. Each treatment, had ten repetitions of a pot with a silver each. The first 49 days remained in a greenhouse with high relative humidity and solar radiation decreased 50%. The last 196 days under full sunlight greenhouse, ventilation and lower relative humidity. The analysis of variance for the main factors was performed after the first five weeks and at the end of the experiment was performed for all treatments, in addition to the comparison of means (Tukey, p> 0.05. The largest increase in AHML (1.63 cm), number of leaves (NH) (0.81), DC (1.17 cm) and CL (6.50 g cm-2) what caused the sand-peat without fertilizer substrate. The micro- propagated plants are replacing sheets between 35 and 70 days acclimatization.
Keywords: Agave americana var. Oaxacensis L.; chlorophyll; micropropagation; substrate; Steiner solution
Debido a la importancia de Agave americana variedad oaxacensis como materia prima para la elaboración artesanal de mezcal. Se evaluó durante 245 días el crecimiento de 260 plantas micropropagadas que tenían en promedio: 4.6 hojas, 10.1 cm de altura en la hoja más larga (AHML), 8.5 cm de diámetro de corona (DC) y 20.1 μg cm-2 de clorofila en lámina foliar (CL). Las plantas se establecieron de acuerdo con un diseño completamente al azar con arreglo factorial (4 x 5), utilizando macetas de 300 cm3 con cuatro diferentes sustratos: arena (1), arena-vermiculita (1:1), arena- turba (1:1) y arena-vermiculita-turba (1:1:1), aplicando diariamente riego con cinco diferentes composiciones 1) agua y 2) alguna alícuota: 25, 50, 75 y 100% de la solución universal Steiner. Cada tratamiento, tuvo diez repeticiones de una maceta con una plata cada una. Los primeros 49 días permanecieron en invernadero con humedad relativa alta y radiación solar disminuida al 50%. Los últimos 196 días en invernadero bajo radiación solar plena, ventilación y humedad relativa menor. Se realizó el análisis de varianza para los factores principales después de las primeras cinco semanas y, al final del experimento se realizó para todos los tratamientos, además de la comparación de medias (Tukey, p> 0.05. El mayor incremento en AHML (1.63 cm), número de hojas (NH) (0.81), DC (1.17 cm) y CL (6.50 μg cm-2) lo provocó el sustrato arena-turba sin fertilización. Las plantas micropropagadas presentan sustitución de hojas entre los 35 y 70 días de aclimatación.
Palabras clave: Agave americana var. Oaxacensis L.; clorofila; micropropagación; sustrato; solución Steiner
Introduction
The Agave americana L. var. Oaxacensis L. is a wild species used for mezcal, which is a strategic activity for the economy of the state of Oaxaca, Mexico. Small populations, small and fragmented; are in isolated areas and have been diminished considerably, to the extent not officially appear in the production figures Mezcal Regulatory Board (CRM) 2015.
The producers appreciate the size of the stem of more than 100 kg and sugar content, so it is a pressing need to generate a schema propagation to ensure producers have enough raw material of this kind in a span of less time than the traditional scheme involves. According Monja-Mio (2015), in vitro culture represents an effective method for mass production of pathogen-free plants and at the same time allows the selection of the most vigorous regard, individuals Aureoles et al. (2008), and Dominguez et al. (2008), adding that in some cases allows rescue, conservation and multiplication of threatened or endangered.
The in vitro culture is only a partial achievement in the scheme spread as the plants obtained show morphological and functional characteristics according to the favorable conditions prevailing, so they are not prepared to deal with sudden changes involving its air conditioning during ex vitro to be achieved survival. And then the adaptation of new individuals in a nursery, to successfully meet their establishment in the field. Several authors (Martinez et al., 2005; Abreu et al., 2007; Monja-Mio, 2015) alike say they are within the characteristics shown by its low photosynthetic capacity due to low content of pigments as chloroplasts present granas disorganized; the absence of waxy cuticles, very functional stomata due to alteration in the shape of the guard cells, different from the normal distribution, inefficiency tissues livelihood due to the reduced presence of clorenchyma and esclerenquima, absorption and transport of inefficient water due to incomplete or weak vascular connection between the root and the bud, which differ from those plants obtained by conventional methods.
Therefore, the acclimatization of plants is the decisive stage due to the stress they are subjected plants, it depends on the success or failure of the whole process (Ortiz, 2000; Olmos et al., 2004). During acclimation no conditions of lower relative humidity, greater variation in temperature, high radiation, and reduced availability of nutrients, so should be considered: 1) their establishment in a greenhouse; 2) use substrates with good moisture retention, good drainage and aeration; 2) maintain the temperature in the range of 15 to 28 °C, 3) change gradually high (90%) to low (40- 60%) relative humidity during 30 to 50 days; 4) increase solar radiation 50% at baseline to full sunlight; and 5) an adequate supply of nutrients. The above conditions encourage plants to develop bodies with physical and functional characteristics that allow them to survive in the field (Enriquez et al., 2005; Martinez et al., 2005). Therefore, the objective of this study was to evaluate the effect of the substrate and fertilization in the adaptation and development of micro-propagated plants Agave americana var. Oaxacensis L.
Materials and methods
The study was conducted in the laboratory of plant tissue culture, the greenhouse acclimatization and the greenhouse adaptation of the Technological Institute of Oaxaca Valley, located in Xoxocotlan, Oaxaca, Mexico. The in vitro propagation scheme described by Murashige (1974), as amended by Deberg and Maene (1981) was followed. For multiplication of propagules stem tissue that were established in flasks of 145 cm3 with 25 mL of a culture medium prepared with mineral salts of Murashige and Skoog (1962) were used, 0.4 mg L-1 thiamine, 100 mg L-1 myo-inositol, 30 g L-1 sucrose, 1 mg L-1 benzyladenine. The pH of medium was adjusted to 5.8 before adding agar. The cultures were incubated for seven weeks under white fluorescent light at 2000 lux intensity, with photoperiod of 16 hours and temperatures between 6 and 30 °C.
After this period in each explant had formed groups of 5 to 12 adventitious shoots, those shoots that had 4 to 5 cm are individually separated to establish three in each flask of 145 cm3 total volume, which had 20 cm3 of medium culture to induce the formation of adventitious roots. The composition of the culture medium was used to that in the previous stage but without adding benzyladenine and 0.5 mg L-1 of indole butyric acid (AIB). The cultures were incubated for four weeks under similar conditions as the previous step. The 400 were obtained in vitro plants Agave americana var. oaxacensis of which 260 were selected to ex vitro they were transferred, washed with tap water to remove traces of agarand were immersed for five minutes in a solution of fungicide 5% to prevent the proliferation of fungi in the greenhouse. The experiment was set according to a completely randomized design factorial arrangement 4 x 5 (substrates by fertilization) with, so 20 treatments with 10 repetitions had. The experimental unit was a plant. The corresponding analysis of variance after the first five weeks made, at 210 and 245 days in addition to the comparison of means (Tukey, p= 0.05).
To feed the seedlings, were transplanted into pots of 6 x 6 x 5.5 cm (198 cm3) containing one of the four substrates: sand (1), sand: vermiculite (1:1), sand: peat (1:1) and vermiculite: peat (1:1:1). The seedlings were placed for 49 days in a greenhouse that was under high relative humidity (80-90%), which remained daily through an intermittent irrigation nebulization of 10 s every 12 min. to 11:00 to 14:00 h performing the cultural care recommended for cultivation (Murguia, 1993); 50% solar radiation decreased. The total number of plants in each substrate were separated into five groups to apply daily irrigation condition: 1) water (control); 2) nutrient solution SN) 25%; 3) SN to 50%; 4) SN to 75 %; y 5) SN to 100% liquid solution fertilization Steiner (1984), using 15 mL plant-1 to substrate ground- level. For five weeks and after 210 and 245 days under ex vitro variables were recorded: height blade longer (cm), leaf number, crown diameter (cm) and chlorophyll content (mg cm-2). After 49 days of acclimatization in the first green house they were transferred to the greenhouse adaptation, which is tunnel-type with polyethylene cover where they were exposed for 196 days at full solar radiation, ventilation and relative humidity lower (40-60%); plants no longer received irrigation misting but the risks to substrate level spaced every two days. When they passed the height of the longest leaf, leaf number, crown diameter and chlorophyll content for these features increases over the past 35 days recorded.
Results and discussion
Initial growth during acclimatization
When he finished the stage of invitro cultivation of plants of Agave americana these, were on average 4.6 leaves, 10.1 cm in the longest leaf, 8.5 cm in diameter crown, and contained 20.1 g cm-2 of chlorophyll sheet foliar. After five weeks the plants showed statistically significant differences produced by the main factors. Both the substrate and the fertilization and the interaction of both influenced significantly (p≤ 0.01) on increasing the height of the longest leaf (AHML), the number of sheets (NH) and the diameter of the crown (DC). While chlorophyll content (CL) showed only significant (p≤ 0.01) dose fertilization (Table 1).
Table 1 Mean squares and significance of increased variables at the end of the first five weeks of acclimatization of micropropagated plants Agave americana var. Oaxacensis L.
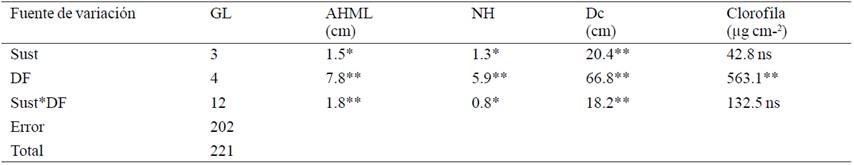
This may be due to the natural process of acclimatization, since plants are obliged to respond in a stressful environment, even if they are under some protection where nutrient availability has more restrictions than in the in vitro environment, which responds better dose fertilization, using minerals are provided and diluted in chemical forms usable for them to be diluted in irrigation water. Given that has little ability to change their morphology, the only way to increase their photosynthetic capacity it is increasing its light gathering area, so the chlorophyll content remains unchanged. About Salazar et al. (2009) mention that achieved adapting Agave cocui Trelease and plants had normal morphology after one week.
As for the effect of the substrate it was observed that the plants established in the sand-peat substrate without fertilization showed greater increases in height of the longest leafand number of leaves (Table 2). The foregoing suggests that the content of organic matter in the soil promotes plant nutrition as it stabilizes the pH, increased water retention, drainage, cation exchange capacity and availability of nutrients, which is reflected in growing dedicated to photosynthesis organs to ensure their survival, on the other hand it is important to consider that possibly net photosynthesis is zero or may even have negative values due to the lack of control experienced by vitro plants not being prepared to face conditions outside lab. However, nutrients available are not sufficient to trigger further accumulation of reserve substances and dry matter accumulation.
Table 2 Means of treatments increases for the first five weeks of acclimatization of Agave americana var. Oaxacensis L.
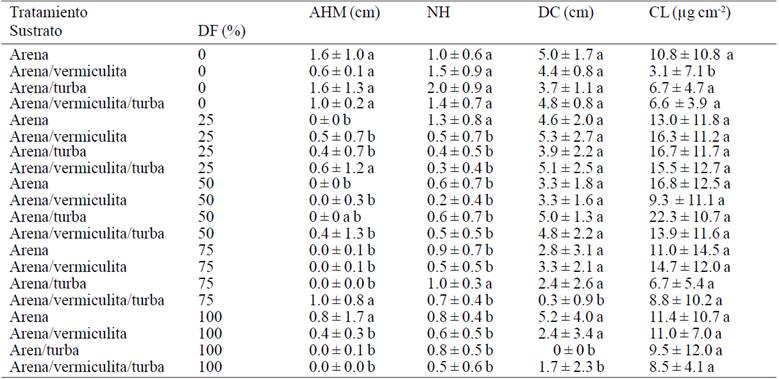
It is noteworthy that the highest value for the diameter of the crown, was observed when plants were established in a sand substrate:- vermiculite and also received irrigation with a 25% solution of Steiner, the accumulation of dry matter can due to the vermiculite it contains potassium (K), magnesium (Mg) and calcium (Ca), although such plants relatively decreased increased height and number of leaves as the highest value for the last variable mentioned was recorded in the vermiculite substrate sand- plus 25% of nutrient solution. These results coincide with Enriquez et al. (2005), who mentioned that micropropagated plants of Dendranthema grandiglora not require the application of some mineral solution to show growth, draws attention to the results above seem to be influenced by the combination of sand and peat in some proportion in the substrate. This behavior seems logical considering that the tested plants are subject to some degree of stress caused by the sudden change in environmental conditions, because although apparently they change gradually to establish plants outside the lab, suffer a total lack of control, because they do not have mechanisms that allow them to regulate basic functions such as gas exchange, which also affects their water status.
It was observed that the number of sheets, up to the largest sheet, crown diameter and chlorophyll content were continuously increased during the first five weeks later the plants began to lose the older leaves during the period between 35 and 70 days of acclimatization. These results agree with those reported by Abreu et al. (2007), who mentioned that fourcroydes Agave plants micropropagated had a positive response in terms of growth and development after 30 days of acclimatization. Moreover Monja-Mio et al.(2015) mention that plants Agave angustifolia cultured in vitro, experience severe changes in morphology and leaf surface during acclimatization, the more noticeable are the development of stomatal, deposition epiticulares waxes, formation protuberances and calcium oxide crystals in the leaf epidermis, which achieved after three months. Which was partially coincides with the results of this study because despite the effort made by the plants to acclimatize, showed growth in AHM and NH, and the dry matter accumulation by increasing the DC during the first five weeks. Moreover, some authors like Pospisilova et al. (1999); Obledo et al. (2004); Abreu et al. (2007); Enriquez (2008) and Monja-Mio et al. (2015) agree that plants lose leaves during the process of acclimatization due to stress they suffer when they are transferred from the in vitro conditions to greenhouse conditions or field, as these plants do not have the anatomical and physiological features operate efficiently, preventing showing significant increases in growth and development, so that their efforts are channeled to the minimum processes to survival during this very critical stage. Regarding the acclimatization stage, Morales et al. (2009), indicate that is a period of time during which the plants are more susceptible to environmental stress. Preece and Sutter (1991), agree that should be adapted to have a continuous growth and development, to new environmental conditions such as lower relative humidity, with more light and septic substrates.
In many species of plants, leaves formed in vitro are unable to be further developed under greenhouse conditions and are replaced by new leaves (Pospisilova et al., 1999). Enriquez (2008) mentions that plants Agave angustifolia replaced the leaves that had formed in the laboratory by new leaves in the acclimatization stage. This phenomenon occurs in plants generated in vitro, they are explained by Taiz and Zeiger (2002), as a specific response that plants have to get rid of non-essential parts; that is, taking into account that the leaves were formed in vitro under controlled conditions where it is not necessary to develop any adaptation strategy, so they are not able to respond to changes involving their acclimatization, so it is functionally preferable translocate useful components and form new leaves with morphological and functional characteristics that replace the previous ones.
Such plants require a process of adaptation to the new environment which, during the period of time that are more susceptible to environmental stress to have a continuous growth and development face (Morales et al., 2009). Abreu et al. (2007) mentions that plants grown in vitro, alter their morphology, anatomy and physiology as a result of the environment in vitro, so during the acclimation present inability to control various processes, among which water loss, so their rate of photosynthesis is reduced, so that a specific response of plants is to get rid of non-essential parts and recover the nutrients present in these parts, of which melts to essential organs for them.
Growth at the end of the adaptation stage
Plants with age 210 to 245 days showed that the factor fertilization had no significant effect on the growth of the height of the longest leaf (AHML), however influenced the increase in crown diameter, number of leaves and chlorophyll content (p≥ 0.05). Plants that were established in the sand-peat substrate and fertigation to 50% concentration of nutrients of the Steiner solution showed greater increases in height of the longest leaf and number of leaves that plants were established in other substrates and were applied to other doses of fertilizers in regard to the number of sheets, they had higher concentrations increased with 75% solution. Furthermore, the plants established vermiculite substrate and irrigated with water only showed greater increases in crown diameter. These results agree with those reported by Enriquez et al. (2005), which mentioned that during the acclimation of plants Dendranthema grandiglora micropropagated and settled on a substrate with high content of organic matter not required to be applied mineral solution to show growth, while plants fertigation 50% had further increases of chlorophyll in the leaves (Table 3). This means that the more nutrients has the blade, will have more concentration, but without excess nutrients from the substrate as mentioned Enriquez et al. (2005).
Table 3 Increase in the characteristics of plants during the 210-245 days of acclimatization levels according main factors.
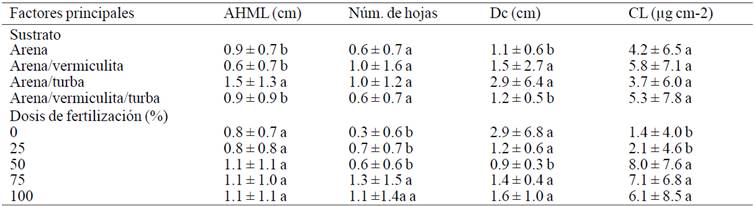
Established plants in peat substrate showed greater increases in height of the longest and crown diameter blade, this is because the peat has characteristics that are close to an ideal substrate: aeration capacity, 10 to 20%; water retention, 55 to 70%; granulometry 2.25 mm 0.25; bulk density <0.4 g cm-3; total pore water readily available 48% and 93% space (Urresatarazu, 2000, Iskander, 2002). While established plants in vermiculite substrate, showed that the number of irrigated leaves with 75% Steiner solution and the content of irrigated chlorophyll with 50% had greater increases, therefore neither the substrate had a high concentration of nutrients nor solution dose was very high. Which can be attributed to the characteristics of vermiculite, and that has a high cation exchange capacity which facilitates mobility and absorption of ions in chemical forms to keep available.
In the period of 210-245 days after transplantation, the plants no longer showed senescence of leaves as occurred during the period between 40 to 60 days, because at this age already had new leaves that had developed in the ex vitro environment (Martinez et al., 2005). Therefore, also it increased crown diameter, the height of the largest sheet and therefore chlorophyll content was constant.
Gonzalez et al. (2007), they worked with fourcroydes Agave and report that the 42 days of acclimatization, the leaves were already considered functional with normal stomata an waxes on its cover. These results agree with those found in this study, as Agave americana acclimation lasted 49 days, therefore, in the following days, the plants were already considered functional. All results indicate that the tested treatments increased the height of the largest leaf, leaf number, crown diameter and chlorophyll content, not being the same treatment for each increase more. According to the results of this study and consideration of other authors, it is clear that the plant needs are different, depending on the environment in which are located, so a change of environment will result, adaptations different scale depending on the speed and magnitude of change. Which is observed in any species when its cultivation in vitro is established, and unlike when to acclimatize again to ex vitro conditions, however evolutionary processes give them that ability through a "memory" long-term, the species preserves and allows you to develop best suited to survive according to the environment in which you find features, and this process can reverse it and adapt it as often as necessary.
In addition, the treatments were tested (Table 4) showed that in the last five weeks that were 210 to 245 days after transplantation to ex vitro, the treatment showed the largest increase taking into account all the variables was the combination of sand-vermiculita-peat with 50% fertilization.
Table 4 Average of increments by treating the last five weeks of acclimatization of Agave americana var. Oaxacensis L.
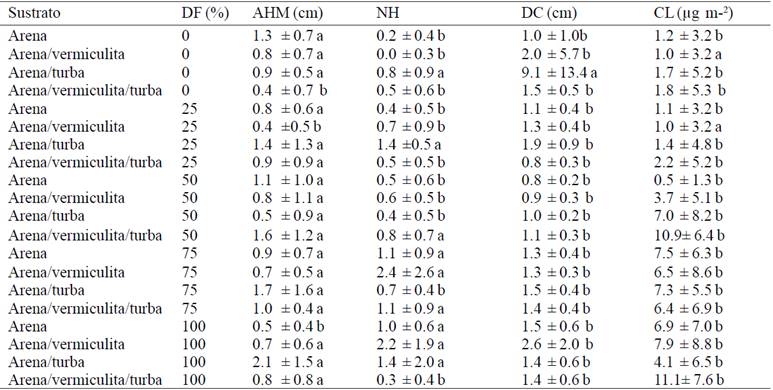
Comparing both periods, it was noted that in the first five weeks established plants in sand substrate had higher increase irrigated with water unlike plants were irrigated with a dose; while in the last five weeks (between 210 and 245 days), plants that were established in the peat substrate irrigated with 50% mineral solution had a higher increase. This may be because the plants during the first few weeks did not require as supplemented irrigation and water only is more than enough to survive the average ex vitro, since at that time perform their duties at the expense of reserves acquired under in vitro conditions. It is important to note that once achieved adaptation and capacity to develop photosynthetic processes, exposure to greater light as in the nursery is favorable to increase growth rates and plant growth factor, therefore the stage after acclimatization plants and needed a further supplemented irrigation, because they no longer had reservations throughout the plant.
Conclusions
The acclimatization stage can be concluded at seven weeks when Agave americana plants have stabilized their growth and development processes.
Micropropagated plants are replacing sheets between 35 and 70 days of acclimatization.
Established plants in sand-peat substrate showed higher growth increments without applying a dose of solution.
The presence of organic matter in the soil favors the acclimation process plant Agave americana var. Oaxacensis L.
Literatura citada
Abreu, E.; González, G.; Ortiz, R.; Rodríguez, P.; Domech, R. y Garriga, M. 2007. Evaluación de vitroplantas de henequén (Agave fourcroydes lem) durante la fase de aclimatización. Cuba. Cultivos Tropicales. 28: 5-11. [ Links ]
Aureoles, F.; Rodríguez-de la O, J. L.; Legaria- Solano, J. P.; Sahagún- Castellanos, J.; Peña Ortega J. y Peña-Ortega, M. 2008. Propagación in vitro del "maguey bruto" (Agave inaequidens Koch.), una especie amenazada de interés económico. México. Rev. Chapingo. Ser. Hortic. 14: 263-269. [ Links ]
Debergh, P. C. and Maene, L. J. 1981. A scheme for commercial propagation of ornamental plants by tissue culture. Sci. Hortic. 14:335-345. [ Links ]
Domínguez, R. M. S.; González, M. L.; Rosales, C.; Quiñones, C.; Delgadillo, L.; Mireles, J. y Pérez, B. E. 2008. El cultivo in vitro como herramienta para el aprovechamiento, mejoramiento y conservación de especies del género Agave. España. Investigación y Ciencia. 16:53-62. [ Links ]
Enríquez, J. R.; Velásquez, B.; Vallejo, A. y Velasco, V. 2005. Nutrición de plantas de Dendranthema grandiflora obtenidas in vitro durante su aclimatación en invernadero. México. Rev. Fitotec. Mex. 28: 377-383. [ Links ]
Enríquez, J. R. 2008. La propagación y crecimiento de agaves. Instituto Tecnológico del Valle de Oaxaca. México. 46 p. [ Links ]
González, G.; Alemán, S.; Tujilo, R.; Kev, M.; Abreu, E.; Barredo, F.; Robert, M. L.; Ortiz, R. y Cordines, M. T. 2004. El cultivo in vitro como alternativa de la recuperación henequenera (Agave fourcroydes). Cuba. Biotecnología aplicada. 1:44-48. [ Links ]
Iskander, C. R. 2002. Manejo de sustratos para la producción de plantas ornamentales en maceta. Department of Horticultural Sciences Texas University. USA. 9 p. [ Links ]
Martínez, R. R.; Hazpíroz, H.; Rodríguez, J. L.; Cetina, V. M. y Gutiérrez, M. A. 2005. Aclimatación de plantas obtenidas in vitro Eucalyptus urophilla S. T. blake Eucalyptus grandis hill ex maiden. México. Revista Ra Ximhai. 3: 591-597. [ Links ]
Morales, C.; de la Fe, C.; Corbera, J. y Calaña, J. M. 2009. Estudio de la aclimatización de vitroplantas de anturio (Anthurium andreanum Lin.). Cuba. Cultivos Tropicales. 30: 48-51. [ Links ]
Monja-Mio M. K.; Barredo, P. F.; Herrera, H.; Esqueda, V. M. and Robert, L. M. 2015. Development of the stomatal complex and leaf surface of Agave angustifolia Haw. ‘Bacanora’plantlets during the in vitro to ex vitro transition process. Sci. Hortic. 189:32-40. [ Links ]
Murashige, T. 1974. Plant propagation through tissue cultures. Plant Physiol. 25:135-166. [ Links ]
Murashige, T. and Skoog, F. 1962. A revised medium for rapid growth and biassays with tobacco tissue culture. Physiol. Plantarum.15: 473-497. [ Links ]
Murguía, G. J. 1993. El cultivo de los anturios (Anthurium andreanum L.) Córdoba: Facultad de Ciencias Agrícolas Universidad Veracruzana. Veracruz. 28 p. [ Links ]
Obledo V. E. N.; Flores, V. N. y Cervantes, M. J. 2004. Detección del efecto de un extracto vegetal antimicrobiano sobre plantas de agave (Agave tequilana wever var. azul) cultivadas in vitro utilizando fluorescencia inducida por laser (LIF). Rev. Mex. Fitopatol. 22:328-332. [ Links ]
Olmos, S.; Luciani, G. y Galdeano, E. 2004. Métodos de propagación y conservación de germoplasma; biotecnología y mejoramiento vegetal. Argen Bio. Consejo Argentino para la Información y el Desarrollo de la Biotecnología. 161-172 pp. [ Links ]
Ortiz, R. 2000. Factores que afectan el desarrollo de vitroplantas de caña de azúcar en la fase adaptativa. Instituto Nacional de Ciencias Agrícolas. 36 p. [ Links ]
Pospisilová, J. I.; Tichá, P.; Kadlecek, D. and Plzáková, Y. S. 1999. Acclimatization of micropropagated plants to ex vitro conditions. Biol. Plantarum. 42: 481-497. [ Links ]
Preece, J. E. and Sutter, E. G. 1991. Acclimatization of micropropagated plants to greenhouse and field. In: Debergh, P. C. and Zimmerman, R. H. (Eds.). Micropropagation: technology and application. Kluwer Academic. Dordrecht. 71-93 pp. [ Links ]
Salazar, E.; González, P. y Hernández, C. 2009. Multiplicación in vitro de Agave cocui Trelease a través de yemas axilares. Agron. Trop. 59:129- 135. [ Links ]
Steiner, A. A. 1984. The universal nutrient solution. International Society For Soilless Culture (ISOSC). In: Sixth International Congress on Soilless Culture Lunteren. pp: 633-650. [ Links ]
Taiz, L. y Zeiger, E. 2002. Fisiología vegetal. Universitad Jaume. 3°edición. 581 p. [ Links ]
Urresatarazu, M. G. 2000. Manual de cultivo sin suelo. Editorial Mundi-prensa. Madrid, España. 465 p. [ Links ]
Received: January 2016; Accepted: May 2016











 texto en
texto en 


