Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista mexicana de ciencias agrícolas
versión impresa ISSN 2007-0934
Rev. Mex. Cienc. Agríc vol.7 no.4 Texcoco may./jun. 2016
Articles
Mycorrhizae in Capsicum annuum L. to promote growth and biosecurity against Phytophthora capsici L.
1 Instituto de Investigaciones Agropecuarias y Forestales-Universidad Michoacana de San Nicolás de Hidalgo, Carretera Morelia-Zinapécuaro, km 9.5, Col. El Trébol. 58880 Tarímbaro, Michoacán, México. Tel. 01 (443) 2958323. (eyesnator@hotmail.com).
2 Biotecnología Vegetal, Centro de Investigación y Asistencia en Tecnología y Diseño del Estado de Jalisco (CIATEJ). Av. Normalistas No. 800, Colinas de la Normal. 44270, Guadalajara, Jalisco. México. Tel: 01 (33) 33455200. (equinones@ciatej.mx; grincon@ciatej.mx).
In order to evaluate the promotion of growth and bio consortia arbuscular mycorrhizal fungi (HMA) against Phytophthora capsici (PC) in pepper plants (Capsicum annuum L.) var. poblano and serrano, in 2014 in the IIAF of the UMSNH an experiment was conducted under greenhouse conditions to evaluate seven inoculates HMA, a positive control (Rhizophagus intraradices) and a negative control (without HMA). At 56 days after inoculation (DDI) of the plants with the HMA, plant height (AP), stem diameter (DT), number of leaves (NH), percentage of mycorrhizal colonization (PCM) and density was recorded HMA spores. At 75 DDI, the plants were infected with a zoospore suspension of PC, to the level of 95 DDI plant damage was assessed by an ordinal six levels of severity qualitative scale and fresh biomass was recorded. The data were subjected to analysis of variance and Tukey test (p≤ 0.05). The PCM found to 93.3%. In serrano pepper plants, the mycorrhizal consortium Cerro del Metate (CM) recorded the highest values of AP, DT and NH, poblano pepper while there were only differences in AP. The inoculated with CM in both varieties of pepper, plants showed a severity level 1 and increased biomass fresh air (15.4 and 6.95 g) compared to non-mycorrhizal plants (8.23 g and 2.83 g). The results showed that CM promoted growth and had a bioprotector effect against PC.
Keywords: Rhizophagus intraradices; antagonism; biological control; mycorrhizae; pepper wilt
Con el objetivo de evaluar la promoción del crecimiento y la bioprotección de consorcios de hongos micorrízicos arbusculares (HMA) contra Phytophthora capsici (PC) en plantas de chile (Capsicum annuum L.) var. poblano y serrano, en el año 2014 en el IIAF de la UMSNH se realizó un experimento en condiciones de invernadero evaluando siete inóculos de HMA, un control positivo (Rhizophagus intraradices) y un control negativo (sin HMA). A los 56 días después de la inoculación (DDI) de las plantas con los HMA, se registró altura de planta (AP), diámetro del tallo (DT), número de hojas (NH), porcentaje de colonización micorrízica (PCM) y densidad de esporas de HMA. A los 75 DDI, las plantas se infectaron con una suspensión de zoosporas de PC, a los 95 DDI se evaluó el nivel de daño de las plantas mediante una escala cualitativa ordinal de severidad de seis niveles y se registró la biomasa fresca. Los datos se sometieron a análisis de varianza y prueba de Tukey (p≤ 0.05). Se encontraron PCM de hasta 93.3%. En plantas de chile serrano, el consorcio micorrízico Cerro del Metate (CM) registró los mayores valores de AP, DT y NH, mientras que en chile poblano sólo hubo diferencias en AP. Las plantas inoculadas con CM en ambas variedades de chile, presentaron un nivel de severidad de 1 y mayor biomasa fresca aérea (15.4 y 6.95 g) en comparación con plantas no micorrizadas (8.23 g y 2.83 g). Los resultados mostraron que CM promovió el crecimiento y tuvo un efecto bioprotector contra PC.
Palabras clave: Rhizophagus intraradices; antagonismo; control biológico; marchitez del chile; micorrizas
Introduction
Wilt caused by Phytophthora capsici (PC) is the main disease that attacks the cultivation of pepper (Capsicum annuum L.). The level of damage of this disease in Mexico is very high, performance can drop between 25 and 80% (Garcia-Rodriguez et al., 2010). To control this disease they have been developed different strategies based on the application of chemicals, which however fail to satisfactory results on the phytopathogenic addition they generate resistance (Perez-Moreno et al., 2003). In recent years there has been an increased interest in finding alternative control that are environmentally friendly and sustainable. The most common is biological control, which involves the application of natural enemies, mainly antagonistic microorganisms against plant pathogens. Among the most studied they are: Trichoderma harzianum (Ezziyyani et al., 2004), Bacillus subtilis (Jong-Hui and Sang-Dal, 2010), and some strains of arbuscular mycorrhizal fungi (Hu-zhe et al., 2005).
Arbuscular mycorrhizal fungi (HMA) provide an effect as bioprotectors against plant diseases (Harriers and Watson, 2004) by improving nutritional status, which allows the plant to have greater tolerance to different biotic and abiotic stress. In addition, these fungi are able to compete with pathogens for space and nutrients in the root and mycorrhizosphere (Trotta et al., 1996). Also, mycorrhizae can change patterns of root exudation allowing the establishment of other plant pathogenic microorganisms antagonistic as in the case of plant growth promoting bacteria. Thus a barrier bioprotection is generated for the plant and network extrarradicales hyphae HMA serve as a functional structural compensation roots of infected plants, thereby reducing the severity of the disease (Azcon-Aguilar and Barea, 1996). HMA can also induce systemic resistance (RSI) in plants to turn your system on alert when colonize the root and activate metabolic pathways which are involved plant hormones such as jasmonic acid and ethylene leading to the synthesis of toxic substances for phytopathogenic as phytoalexins and pathogenesis-related proteins (Madriz, 2002).
Pozo et al. (2002) showed that mycorrhization in tomato plants with Rhizophagus intraradices AND Glomus mosseae reduced symptoms of the disease caused by PC inducing local and systemic resistance. In addition to the RSI, the mycorrhizal symbiosis stimulates sensory preconditioning response on the ground in the presence of a phytopathogenic agent, which reacted rapidly increasing their levels of defense and hindering its establishment. This mechanism is known as enhancer or "priming" (Jung et al., 2012). Hu-zhe et al. (2005) showed that pre-inoculation of R. intraradices in pepper plants (Capsicum annuum) the PC infected with increased activity of enzymes related to lignification,which was reflected in are duction inmortality of the root. Based on the above, the objective of this work was to evaluate the effect of different consortia HMA in promoting growth and biosecurity plant pepper serrano and pepper poblano (Capsicum annuum) against Phytophthora capsici under greenhouse conditions.
Materials and methods
This work was conducted jointly by the Institute of Agricultural and Forestry Research (IIAF) of the Universidad Michoacana de San Nicolas de Hidalgo (UMSNH) and the Laboratory of Plant Biotechnology Centre for Research and Assistance in Technology and Design State Jalisco (CIATEJ). The greenhouse experiment was established in the IIAF (19° 42' north, 1 012° 11' west longitude, 1 941 meters) in the municipality of Tarímbaro, Michoacan, Mexico between may and august 2014.
Microbiological material
Phytophthora capsici strain (PC): the strain CH11 provided by the Plant Pathology Laboratory of the IIAF- UMSNH was used. This strain was isolated from diseased plants wilting from a culture of pepper in the State of Michoacan, Mexico. The infectivity of the CH11 strain previously revived in plants serrano pepper, placing discs mycelium in the root system of plants causing infection and development of necrotic lesions, where he was re- isolated and purified in culture medium potato dextrose agar (PDA).
Inocula HMA: seven mycorrhizal consortia from soils with plantations of Agave cupreata the State of Michoacan, which were given a nomenclature according to the site which were used were extracted: El Huizachal (EH), Las Campesinas (LC), Rancho Carlos Rojas (RCR), Paso Ancho (PA), El limón (EL), Cerro del Metate (CM), Barranca de las Nueces (BN). The inocula previously were propagated for 12 months in pots in the greenhouse trap. Consortia were composed of different species of HMA, finding a total of 40 species in 13 genera, 9 families and 3 orders (Rincon-Enriquez et al., 2012; Trinidad-Cruz, 2014).
Vegetal material
Pepper varieties: the pepper varieties used in this work were: serrano pepper (cultivar Tampiqueña) and poblano pepper (cultivar San Luis). The seeds were sown in aluminum trays with wells containing 100 sterile river sand (120 °C for three h, three consecutive days). The plants were transplanted when they were four true leaves approximately 35 days after planting.
Establishment of the experiment
HMA and inoculation of substrate: the substrate containing the pots consisted of a mixture of clay loam soil with sand in the ratio 1:1 v/v sterilized (120 °C for three hours three consecutive days). The plants were transplanted to pots with 500 g substrate, then inoculated into the root system with 80 spores HMA contained in a given amount of sand as carrier material. Technique wet sieving and decanting of Gerdemann and Nicholson (1963) was used for extraction, identification and standardization of the number of spores of each inoculum in combination with the flotation technique sugar sucrose gradient (Walker, 1997). For counting a stereomicroscope it was used (VELAB).
Inoculation of PC: at 75 days after inoculation with HMA (DDI), at which time the plants had higher mycorrhizal colonization 50%, were inoculated with a zoospore suspension of Phytophthora capsici adjusted to a concentration of 1 x 104 zoospores ml-1 (Ristaino, 1990), using 1 mL per plant 500 g-1 substrate (20 zoospores g-1 substrate).
Response variables
Growth variables: to determine the effect of mycorrhizae on growth of pepper plants at 56 DDI, plant height (AP) was measured starting from the stem base to the apex of the last sheet (ruler), stem diameter (DT) storey (digital vernier), and the number of sheets (NH). A 95 DDI was terminated the experiment and proceeded to record total fresh biomass (root, stem, leaves and fruit) with an analytical balance (Mettler Toledo AT200).
Micorrización variables: the 56 DDI substrate samples and roots of each treatment were obtained to quantify the production of spores of HMA and mycorrhizal root colonization. Evaluating the number of spores it was made by wet sieving technique and decanting (Gerdemann and Nicholson, 1963) in combination with sugar flotation technique sucrose gradient (Walker, 1997). For counting a stereomicroscope (VELAB) it was used. Mycorrhizal colonization was determined by the technique described by Hayman and Phillips (1970) and using trypan blue was used to determine the percentage of colonization by the method described McGonigle et al. (1990).
Disease severity: the 20 days after inoculation of PC (95 DDI), was terminated the experiment and the level of damage was determined on the ground by recording the symptomatology of the disease according to the scale of virulence wilt in pepper plants described by Quinto-Alvarez (2014). This scale includes six zero values: 0= healthy plant; 1= plant with leaves partially collapsed; 2= plant with leaves collapsed and some detached, slightly inclined stem; 3= plant with leaves and chlorotic collapsed, with some detached stem slightly inclined; 4= plants with leaves collapsed, most of them detached, collapsed stems and necrosis at the base; 5= plants completely collapsed leaves, almost all detached, collapsed and presence of necrosis from the base to the middle of the stalk stalks; and 6= plants completely collapsed leaves detached most part, collapsed and presence of necrosis in more than 50% of the stem stems.
Experimental design and statistical analysis
Two experiments (one for each variety of pepper) under an experimental design of randomized complete block with four replications were made. In each experiment nine treatments for seven consortia HMA native rhizosphere Agave, a positive control which consisted of an inoculum of commercial HMA made from Rhizophagus intraradices and a negative control without mycorrhizal (S/HMA) were evaluated. The experimental units (UE) consisted of a potted plant chili with their respective treatment. A data obtained from the growth variables and mycorrhizae, was applied an analysis of variance and to determine statistical differences between treatments a comparison test of Tukey (p≤ 0.05) was performed. For fresh air biomass variable, data were transformed with the exponential function to meet the normality and homoscedasticity of variances. For the scale of virulence of the disease, non-parametric analysis with the Kruskal-Wallis and confidence interval for the median with the Dunn test was performed (p≤ 0.05). The Pearson correlation coefficients were calculated (p≤ 0.05) between mycorrhizal colonization number of spores of HMA and complete with the level of virulence of the disease fresh biomass. Statistical analyzes were performed with StatGraphics Centurion program (Statgraphics, 2005).
Results and discussion
The HMA as plant growth promoters in pepper
Analysis of variance showed significant differences (p≤ 0.05) for all variables in serrano pepper growth (Table 1). In AP (p= 0.0206) inoculum CM showed the highest values with 23.0 cm; DT (p= 0.012) with 4.05 mm and NH (p= 0.0003) with 33.5 leaves. In all of these variables CM proved to be statistically different from control without HMA where the lowest values were recorded. For poblano pepper, no significant differences in DT (p= 0.71) and NH (p= 0.63), only to AP, where the inoculum LC obtained 18.03 cm were found. These results agree with those reported by Alonso-Contreras et al. (2013), where different mycorrhizal consortia isolated from the rhizosphere of apple (Malus domestica B.) had a positive effect in promoting stem diameter and leaf area of C. annuum plants.
Table 1 Growth of chili effect of different inocula of arbuscular mycorrhizal fungi (HMA) under greenhouse conditions at 56 days after transplantation.
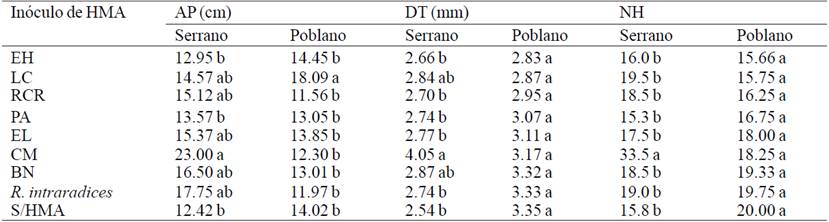
Diaz-Franco et al. (2013), in an experiment in pepper (C. annuum) under greenhouse conditions they found that the mycorrhizae with Rhizophagus intraradices promoted growth by increasing fruit weight by 30% compared to non-mycorrhizal plants. In another study, when evaluating mycorrhizal inoculants in tomato plants (Lycopersicon esculentum M.) a significant increase in height and dry biomass compared to non-mycorrhizal plants was reported (Fernandez et al., 2006).
This reflects that the HMA is a biological resource of importance in organic-biological fertilization of plants of economic interest, which could even be an alternative to other types of management and chemical fertilization (Armenta-Bojorquez et al., 2010).
For variables mycorrhizal (Table 2) statistical differences in mycorrhizal colonization (p= 0.00) and spore density (p= 0.00) in both varieties of chili at 56 days after inoculation (DDI) met the HMA . Not mycorrhizal colonization was observed in the negative control, the inoculates recorded higher mycorrhizal colonization of roots in serrano pepper were BN and PA with 84.93% and 83.43%, Rhizophagus intraradices showed only 33.6% of colonization, the rest of inoculates were statistically equal the CM inoculum promoted further growth in serrano pepper recorded only 44.96% of mycorrhizal colonization, therefore it is not a very infective inoculum but effective in promoting plant growth. The concepts of infectivity, defined as the ability to colonize the root and effectiveness, ability to promote plant growth, are not related and are known to exist HMA inocula that colonize the root lesser percentage (15-40%) and have an excellent effect on plant nutrition and growth (Tapia-Gone et al., 2010).
Table 2 Mycorrhization and spore density of arbuscular mycorrhizal fungi (HMA) in pepper plants under greenhouse conditions at 56 days after transplantation.
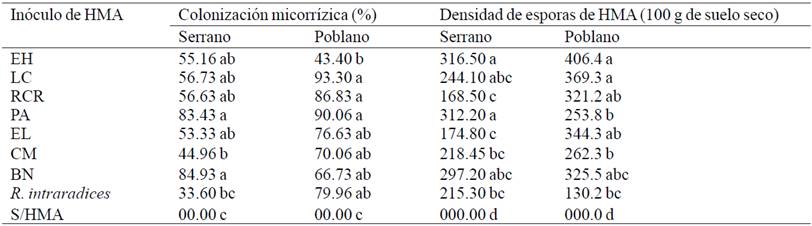
The high percentage of mycorrhizal colonization found in this study is similar to that shown by Lucas-Santoyo (2011) who reported a 92.92% colonization up in plants guajillo (C. annuum L.) inoculated with mycorrhizal different consortia to 150 days after transplantation; also Tanwar et al. (2013) report a high percentage of colonization in the root (93.98%) in pepper, however this was even greater in the presence of Pseudomonas fluorescens (97.62%) which was reflected in increased growth. The high percentages of colonization found in the present study suggest that these varieties of pepper are highly micotróficas and not self-regulate the degree of colonization in their roots.
Regarding the density of spores of HMA analysis of variance showed statistically significant differences (p≤ 0.05). In serrano pepper where all treatments were inoculated HMA recorded statistically similar values between them however were higher than the negative control. In poblano pepper with R. intraradices control showed the lowest density of spores (130 in 100 g of dry soil), the highest spore density was recorded in the inoculum EH with 406.4 in 100 g of dry soil that was statistically equal to LC, PA and BN. correlation coefficients of Pearson significant between mycorrhizal colonization and number of spores in poblano (r= 0.5469) and serrano (r= 0.7475) were found, this is consistent with that reported in the literature, which indicates that high densities of spores foster greater mycorrhizal colonization (Vazquez et al., 2010). However, both variables are not always necessarily dependent and depending on the species or plant variety and species of HMA used (Mendoza et al., 2002).
The spore density is a variable that indicates the abundance of propagules of HMA that could compete against plant pathogens, providing for space and nutrients directly to the plant biosecurity (Azcon-Aguilar and Barea, 1996). In this case, CM inoculum was the best in reducing the severity of disease caused by PC (Figures 1 and 2) but not the densest produced spores. This might suggest that the sickest plants being in a situation of greater biotic stress, could have induced greater sporulation of the HMA. This effect is related to survival mechanisms of HMA, which may be able to increase the amount spores being threatened carbon supplies the plant. Some authors suggest that some species of Glomus are strategists "r"; i.e. increase reproduction with a larger number of spores in the presence of a limiting survival factor (Ijdo et al., 2010).
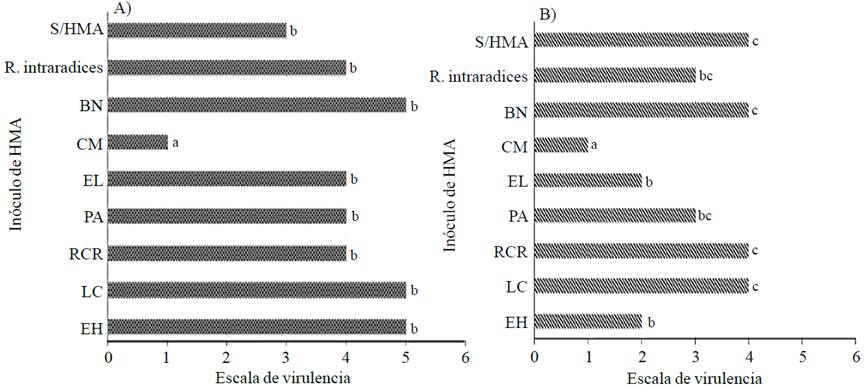
Figure 1 Severity of wilting caused by PC plants serrano pepper (A) and poblano (B) inoculated with HMA under greenhouse conditions at 95 days after transplantation. El Huizachal (EH); Las Campesinas (LC); Rancho Carlos Rojas (RCR); Paso Ancho (PA); El Limón (EL); Cerro del Metate (CM); Barranca de las Nueces (BN); without HMA (S/HMA). Different letters indicate statistically significant differences according to Dunn's test (p≤0.05).

Figure 2 Viewing the severity of wilt caused by Phytophthora capsici in plants serrano pepper (1) and poblano pepper (2) to 95 DDI under greenhouse conditions. Cerro del Metate (level 1: a and d); without HMA (level 3: b; Level 4 = f); The Huizachal (level 5= c); Paso Ancho (level 3= e).
This coincides with that reported by Schalamuk et al. (2014), where on wheat plants (Triticum sp.) That were severely affected by septoria caused by Mycosphaerella graminicola the spore density was higher than in plants selectively with lower disease damage.
Bioprotector effect of HMA against Phytophthora capsici L. (PC) in pepper plants
After inoculation of PC, all plants showed symptoms of the disease, however the degree of severity of the inoculated mycorrhizal consortium depended. The nonparametric Kruskal-Wallis statistical analysis showed significant differences in serrano pepper (statistic= 15.6042, value- p= 0.0484086) and poblano (statistic= 15.6959, value- p= 0.0469459) for the scale of virulence. In both varieties of pepper all inoculates HMA except bioprotector CM did not exert effect against wilting plants meeting the same level of disease than the negative control (Figure 1).
According to the scale of virulence, plants inoculated with CM they found a severity index of one, indicating partially collapsed leafy plants with erect stem (Figure 2). This shows that the mycorrhizal consortium CM is able to bioproteger chili plants against Phytophthora capsici given the reduction in the severity of wilting. This is consistent with that reported by Ozgonen and Erkilic (2007) who by inoculating spores of Glomus etunicatum, Gl. fasciculatum and Gigaspora margarita in pepper plants, found a decrease in disease caused by PC up to 91.7% under greenhouse conditions and 57.2% under field conditions. On the other hand, plants inoculated with CM had the highest fresh biomass radical, which could give them a larger area of exploration for nutrients and water, which could favor their nutritional status, development and protection against Phytophthora capsici. This is consistent with that reported by Gomez-Dorantes et al. (2008), who found a larger size of the root in tomato plants inoculated with HMA, which showed less damage by Phytophthora capsici compared to non-mycorrhizal plants which showed a significant loss of root biomass (p≤ 0.05).
The synthesis of phytoalexins is documented as a plant response to attack by pathogens which are involved jasmonic acid and ethylene that promote systemic resistance can be induced by HMA (Carreon-Abud et al., 2008). Meanwhile Gómez-Dorantes et al. (2008) attributed the reduced susceptibility to PC in tomato to improve the nutritional status of plants and competition for carbon compounds in the root. Considering these results it is possible that the bioprotector effect found in this study by the HMA, reflected in a lower severity of illness, it is due among other things to the induction of biochemical signaling to give defense responses prompted by the activity of plant hormones such as salicylic and jasmonic acid (Kapoor, 2008).
This bioprotective effect has been reported in Tomato plants where inoculation with G. mosseae induced systemic response against Phytophthora parasitica in a root system divided, where resistance was associated with the accumulation of phenolic compounds, thickening of the cell wall and proteins related pathogenesis (PR-1) (Cordier et al., 1998). Tomato systemic response against P. parasitica was also reported by Pozo et al. (1999) where hydrolytic enzymes isoforms possibly related to this response were found. The protection PC via protein synthesis PR peroxidase activity has been reported in pepper plants inoculated with Trichoderma harzianum, where colonization by the antagonist induces systemic resistance that can trigger the mechanism of hypersensitive defense (Ezziyyani et al., 2005).
Regarding the air fresh and radical biomass PC infected plants at 95 days after transplantation significant differences (p≤ 0.05) between treatments (Table 3) were found. In serrano pepper CM inoculum biomass promoted as fresh air with 15.4 g was statistically superior to the negative control (S/HMA) which registered 8.26 g. For poblano pepper trend was the same, the plants inoculated with CM showed the air fresh biomass of 7.95 g and statistically different treatments without HMA (2.83 g). In the radical fresh biomass HMA different consortiums including CM, they failed to promote root growth since in both varieties of pepper were statistically equal to the negative control without HMA.
Table 3 Fresh aerial and radical biomass in plants and poblano pepper serrano inoculated with different arbuscular mycorrhizal fungi (AMF) and infected with Phytophthora capsici at 95 days after transplantation under greenhouse conditions.
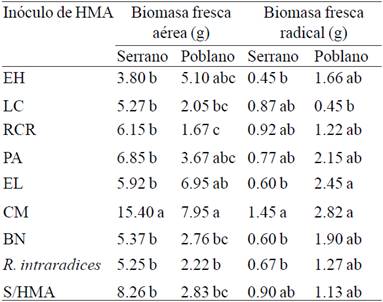
However, increased air fresh biomass by the CM consortium plants serrano pepper poblano was reflected in the lowest level of severity of the disease who had plants of this treatment (Figure 2), which was corroborated by making a correlation analysis between the total fresh biomass and the level of severity of wilting in plants of both varieties where a significant negative correlation in both cases (-0.47 and -0.37) was found. Finally, it is important to study the biological effectiveness in promoting growth and biocontrol of plant pathogens by consortia of HMA isolated from soil A. cupreata because it allows to know the associated species and their potential biotechnological application in this and other plant species of economic importance such as chili.
Conclusions
The mycorrhization in plants and poblano pepper serrano favored the increase in plant growth reflected in increased plant height, stem diameter, number of leaves and fresh aerial biomass. Furthermore mycorrhization showed a bioprotective effect against Phytophthora capsici to reduce the severity of wilting compared to non-mycorrhizal plants. In both varieties of pepper, the mycorrhizal consortium CM decreased the severity of Phytophthora capsici L. causing wilting to be inoculated at transplanting. Which, demonstrates the potential of this consortium in controlling plant pathogen; however, you need to conduct new greenhouse experiments to validate the results obtained after the consortium to use in field conditions and validate the bioprotector effect.
Literatura citada
Alonso-Contreras, R.; Aguilera-Gómez, L. I.; Rubí-Arriaga, M.; González-Huerta, A.; Olalde-Portugal, V. y Rivas-Manzano, I. V. 2013. Influencia de hongos micorrícicos arbusculares en el crecimiento y desarrollo de Capsicum annuum L. Rev. Mex. Cienc. Agríc. 4(1):77-88. [ Links ]
Armenta-Bojórquez, A. D.; García-Gutiérrez, C.; Camacho-Báez, J. R.; Apodaca-Sánchez, M. A.; Montoya, L. G. y Nava-Pérez, E. 2010. Biofertilizantes en el desarrollo agrícola de México. Ra Ximhai. 6(1):51-56. [ Links ]
Azcón-Aguilar, C. and Barea, J. M. 1996. Arbuscular mycorrhizas and biological control of soil-borne plant pathogens - an overview of the mechanisms involved. Mycorrhiza. 6(6):457- 464. [ Links ]
Carreón-Abud, Y.; Gómez-Dorantes, N. y Martínez-Trujillo, M. 2008. Las micorrizas arbusculares en la protección vegetal. Rev. Biol. 10(1):60-70. [ Links ]
Cordier, C.; Pozo, M. J.; Barea, J. M.; Gianinazzi, S. and Gianinazzi- Pearson, V. 1998. Cell defense responses associated with localized and systemic resistance to Phytophthora parasitica induced in tomato by an arbuscular mycorrhizal fungus. Mol. Plant Microbe Interac. 11(10):1017-1028. [ Links ]
Díaz-Franco, A.; Alvarado-Carrillo, M.; Ortiz-Chairez, F. y Grageda-Cabrera, O. 2013. Nutrición de la planta y calidad de fruto de pimiento asociado con micorriza arbuscular en invernadero. Rev.Mex.Cienc.Agríc. 4(2):315-321. [ Links ]
Ezziyyani, M.; Pérez-Sánchez, C.; Sid-Ahmed, A.; Requena, M. A. y Candela, M. E. 2004. Trichoderma harzianum como biofungicida para el control de Phytophthora capsici en plantas de pimiento (Capsicum annuum L.). Rev. An. Biol.. 26:35-45. [ Links ]
Ezziyyani, M.; Requena, M. E. y Candela, M. E. 2005. Producción de proteínas-PR en la inducción de resistencia a Phytophthora capsici en plantas de pimiento (Capsicum annuum L.) tratadas con Trichoderma harzianum. Rev. An. Biol. 27:143-153. [ Links ]
Fernández, H. E.; Acosta, R. M.; Ponce, G. F. y Manuel, P. V. 2006. Manejo biológico de Phytophthora capsici Leo., Fusarium oxysporum Schelechtend (Fr.) y Rhizoctonia solani Kühn en jitomate (Lycopersicum esculentum Mill.). Rev. Mex. Fitopatol. 25(1):35-42. [ Links ]
García-Rodríguez, M. A.; Chiquito-Almanza, E.; Loeza-Lara, P. D.; Godoy-Hernández, H.; Villordo-Pineda, E.; Pons-Hernández, J. L.; González-Chavira, M. M. y Anaya-López, J. L. 2010. Producción de chile ancho injertado sobre criollo Morelos 334 para el control de Phytophthora capsici. Rev. Agrociencia. 44(6):701-709. [ Links ]
Gerdemann, J. W. and Nicholson, T. H. 1963. Spores of mycorrhizal endogene species extracted from soil by wet sieving and decanting. Trans. Br. Mycol. Soc. 46(2):235-244. [ Links ]
Gómez-Dorantes, N.; Carreón- Abud, Y. y Fernández-Pavia, S. P. 2008. Reducción de la susceptibilidad a Phytophthora capsici Leonian causante de la pudrición de raíz en jitomate (Solanum lycopersicum L). Rev. Biol. 10(1):100-108. [ Links ]
Harrier, L. A. and Watson, C. A. 2004. The potential role of arbuscular mycorrhizal (AM) fungi in the bioprotection of plants against soil-borne pathogens in organic and/or other sustainable farming systems. Pest. Manag. Sci. 60(2):149-57. [ Links ]
Hu-zhe, Z.; Chung-lan, C.; Yu-ting, Z.; Dan, W.; Yu, J. and Youg, K. K. 2005. Active changes of lignification-relatedenz y mesinpepper response to Glomus intraradices and/or Phytophthora capsici. J. Zhejiang Univ. Sci. 6(8):778-786. [ Links ]
Ijdo, M.; Schtickzelle, N.; Cranenbrouck, S. and Declerck, S. 2010. Do arbuscular mycorrhizal fungi with contrasting life-history strategies differ in their responses to repeated defoliation? Microbiol. Ecol. 72(1):114-122. [ Links ]
Jong-Hui, L. and Sang-Dal, K. 2010. Biocontrol of Phytophthora blight of red pepper caused by Phytophthora capsici using Bacillus subtilis AH18 and B. licheniformis K11 formulations. Korean Soc. Appl. Biol. Chem. 53(6):766-733. [ Links ]
Jung, S. C.; Martínez-Medina, A.; López-Ráez, J. A. and Pozo, M.A. 2012. Mycorrhiza induced resistance and priming of plant defenses. J. Chem Ecol. 38(6):651-664. [ Links ]
Kapoor, R. 2008. Induced resistance in mycorrhizal tomato is correlated to concentration of jasmonic acid. J. Biol. Sci. 8(3):49-56. [ Links ]
Li, Z.; Long, W.; Zheng, J. and Lei, J. 2007. Isolation and identification of Phytophthora capsici in Guangdong province and measurement of their patogenicity and physiological race differentiation. Front. Agric. China. 1(4):377-381. [ Links ]
Lucas-Santoyo, L. G. 2011. Fertilización fosfatada en chile guajillo (Capsicum annuum L.) y su interacción con hongos micorrízicos arbusculares. Tesis de Maestría. Colegio de Postgraduados en Ciencias Agrícolas. Montecillos, Estado de México, México. 155 p. [ Links ]
Madriz, O. K. 2002. Mecanismos de defensa en las interacciones planta-patógeno. Rev. Manejo Integrado de Plagas. 63:22-32. [ Links ]
McGonigle, T. P.; Miller, M. H.; Evans, D. G.; Fairchild, G. L. and Swan, A. 1990. A new method which gives an objective measure of colonization of roots by vesicular-arbuscular mycorrhizal fungi. New Phytol. 115(3):495-501. [ Links ]
Mendoza, R. E.; Goldmann, V.; Rivas, J.; Escudero, V.; Pagani, E.; Ozgonen, Collantes, M. y Marbán, L. 2002. Poblaciones de hongos micorrízicos arbusculares en relación con las propiedades del suelo y de la planta hospedante en pastizales de Tierra del Fuego. Ecol. Austral 12(2):105-116. [ Links ]
Ozgonen, H and Erkilic, A. 2007. Growth enhancement and phytophthora blight (Phytophthora capsici Leonian) control by arbuscular mycorrhizal fungal inoculation in pepper. Crop Prot. 26(11):1682-1688. [ Links ]
Pérez-Moreno, L.; Durán-Ortiz, L. J.; Ramírez-Malagón, R.; Sánchez- Palé, J. R. y Olalde-Portugal, V. 2003. Compatibilidad fisiológica y resistencia a fungicidas de aislamientos de Phytophthora capsici Leo. Rev. Mex. Fitopatol. 21:19-25. [ Links ]
Phillips, J. M. and Hayman, D. S. 1970. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Br. Mycol. Soc. 55(1):158-160. [ Links ]
Pozo, J. M.; Azcón-Aguilar, C.; Dumas-Gaudot, E. and Barea, J. M. 1999. β-1, 3-Glucanase activities in tomato roots inoculated with arbuscular mycorrhizal fungi and/or Phytophthora parasitica and their possible involvement in bioprotection. Plant Sci. 141(2):149-157. [ Links ]
Pozo, M. J.; Cordier, C.; Dumas-Gaudot, E.; Gianinazzi, S.; Barea, J. M. and Azcón-Aguilar, C. 2002. Localized versus systemic effect of arbuscular mycorrhizal fungi on defense responses to Phytophthora infection in tomato plants. J. Exp. Bot. 53(368):525-534. [ Links ]
Quinto-Álvarez, C. P. 2014. Nuevas alternativas de control biológico de Phytophthora capsici L. causante de la marchitez en el cultivo de chile (Capsicuum annuum L.). Tesis de Maestría. UMSNH. México. 54 p. [ Links ]
Rincón-Enríquez, G.; Quiñones-Aguilar, E. E.; Montoya-Martínez, A. C.; López-Pérez, L. y Hernández-Cuevas, L. V. 2012. Diversidad de hongos micorrízicos arbusculares de suelos de Agave cupreata Trel y Bergeren Michoacán. In: Blanco-Macías, F.; Bravo-Lozano, A.G.; Hernández-Martínez, J.; Lara-Herrera, A.; Magallanes-Quintanar, R.; Méndez-Gallegos, S. J. y Valdez-Cepeda, R. D. (Coords.). Tópicos edafológicos de actualidad. 1a edición. Universidad Autónoma de Zacatecas. Zacatecas, México. 11-15 pp. [ Links ]
Ristaino, J. B. 1990. Intraespecific variation among isolates of Phytophthora capsici from pepper and cucurbit fields in North Carolina. J. Phytopathol. 80(11):1253-1259. [ Links ]
Schalamuk, S.; Velázquez, S.; Simón, M. R. and Cabello, M. 2014. Effect of septoria leaf blotch and its control with commercial fungicides, on arbuscular-mycorrhizal-fungal colonization, spore numbers, and morphotype diversity. J. Plant Prot. Res. 54(1):9-14. [ Links ]
Statgraphics, 2005. StatGraphics Centurion: v. XV (user manual). Stat- Point, Inc. USA. 380 p. [ Links ]
Tanwar, A.; Aggarwal, A.; Kadian, N. and Gupta, A. 2013. Arbuscular mycorrhizal inoculation and super phosphate application influence plant growth and yield of Capsicum annuum. J. Soil Sci. Plant Nutr. 13(1):55-66. [ Links ]
Tapia-Goné, J. J.; Ferrera-Cerrato, R.; Varela-Fregoso, L.; Rodríguez- Ortíz, J. C.; Soria-Colunga, J. C.; Tiscareño-Iracheta, M. A.; Loredo-Osti, C.; Alcalá-Jáuregui, J. y Villar-Morales, C. 2010. Infectividad y efectividad de hongos micorrízicos arbusculares nativos de suelos salinos en el cultivo de lechuga (Lactuca sativa). Rev. Mex. Micol. 31:69-74. [ Links ]
Trinidad-Cruz, J. 2014. Hongos micorrízicos arbusculares asociados a la rizósfera de agave cupreata: riqueza de especies, cultivo in vitro, promotores del crecimiento y agentes de control biológico. Tesis de maestría. Centro de Investigación y Asistencia en Tecnología y Diseño del estado de Jalisco. Guadalajara, Jalisco. 107 p. [ Links ]
Trotta, A.; Varese, G. C.; Gnavi, E.; Fusconi, A.; Sampo, S. and Berta, G. 1996. Interactions between the soil-borne root pathogen Phytophthora nicotianae var. parasítica and the arbuscular mycorrhizal fungus Glomus mosseae in tomato plants. Plant Soil. 185(2):199-209. [ Links ]
Vázquez, B.; Rivera, R.; Fernández, K. y Rodríguez, Y. 2010. Caracterización del comportamiento micorrízico en Brachiaria decumbens inoculada con Glomus hoi-like. Rev. Cult. Trop. 3(31):01-10. [ Links ]
Walker, C.; Mize, C. W. and McNabb H. S. Jr. 1982. Populations of endogonaceous fungi at two locations in central Iowa. Can. J. Bot. 60:2518-2529. [ Links ]
Received: January 2016; Accepted: March 2016











 texto en
texto en 


