Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista mexicana de ciencias agrícolas
versión impresa ISSN 2007-0934
Rev. Mex. Cienc. Agríc vol.7 no.1 Texcoco ene./feb. 2016
Articles
Survey the natural enemies of Leucaena Psyllid and Onion Thrips on the harvest processes in different Leucaena genotypes
1Facultad de Ciencias de Medicina Veterinaria y Zootecnia-Universidad de Yucatán, Mérida, Yucatán, México.
2Department of Plant Protection, Faculty of Agriculture, Assiut University, Assiut (71526), Egypt.
3Department of Plant Protection and Integrated Pest Management, Faculty of Agriculture, Mu’tah University, Karak P. O. BOX 61710, Jordan.
The study was conducted in 2013 and 2014, at Xmatkuil Merida, Yucatan, Mexico to survey natural enemies and their effects on the population dynamics of Leucaena leucocephala (Lam.) de Wit pests: Leucaena Psyllids, Heteropsylla cubana, Crawford (1914) (Homoptera: Psyllidae) and Onion Thrips, Thrips tabaci, Lindeman (1889) (Thysanoptera: Thripidae) on four Leucaena genotypes: Cunningham, K636, Nativa and KX2. The juvenile leaves were collected, examined and the natural enemy’s counts were correlated with each pest numbers. Nine predators related to 5 orders, and one parasitoid controlled the populations of both pests. In the first season Nativa had the largest densities of Psyllid, and Cunningham for Thrips. In the second season, Nativa for both pests. The least numbers recorded in KX2 for both pests. The coefficient of correlation (r) was stronger between natural enemies and H. cubana than T. tabaci. In the first season three peaks of Psyllid were occurred, two before the first harvest during May and June and one after at December. Otherwise, four peaks of Thrips were recorded on Cunningham, Nativa, and K636 during May to Aug. and only two occurred on KX2 at May and June. In the second season the largest population of pests was recorded in Nativa, and the lowest were in KX2 for both pests (r) was negative between Thrips and natural enemies. Psyllid showed two peaks in February and April, and two for Thrips in April and June.
Keywords: biological control; Leucaena Psyllid; Leucaena pest’s dynamics; onion thrips
El estudio se llevó a cabo en 2013 y 2014, en Xmatkuil, Mérida, Yucatán, México a la encuesta enemigos naturales y sus efectos sobre la dinámica poblacional de Leucaena leucocephala (Lam.) de plagas Wit: Leucaena Psílidos, Heteropsylla cubana Crawford (1914) (Homoptera: Psyllidae) y cebolla trips, Thrips tabaci Lindeman, 1889 (Thysanoptera: Thripidae) en cuatro genotipos de Leucaena: Cunningham, K636, Nativa y KX2. Se recogieron las hojas juveniles, examinaron y cuenta del enemigo natural, se correlacionaron con cada uno de los números de plagas. Nueve depredadores relacionados con 5 órdenes, y uno parasitoide controlan las poblaciones de ambas plagas. En la primera temporada Nativa tuvo las mayores densidades de Psyllid, y Cunningham para Thrips. En la segunda temporada, Nativa para ambas plagas. Los números de menos registran en KX2 para ambas plagas. El coeficiente de correlación (r) fue más fuerte entre los enemigos naturales y H. cubana que T. tabaci. En la primera temporada se produjeron tres picos del psílido, dos antes de la primera cosecha en mayo y junio y una después en diciembre. De lo contrario, cuatro picos de trips se registraron en Cunningham, Nativa y K636 durante mayo a agosto y sólo dos ocurrieron en KX2 en mayo y junio. En la segunda temporada de la mayor población de plagas se registró en Nativa, y la más baja se encontraban en KX2 para ambas plagas (r) fue negativa entre trips y enemigos naturales. Psyllid mostró dos picos en febrero y abril, y dos para los trips en abril y junio.
Palabras clave: control biológico; dinámica de Leucaena plagas; Leucaena psílido; trips de la cebolla
Introduction
Undoubtedly, Leucaena leucocephala (Lam.) de Wit, (leguminosae: Mimosoideae) led to a revolution in animal food supplies. In the past, Leucaena was known by the absence of disease, insect pest’s infestation, and that was the reason behind its spread in the tropical world. But, recently Leucaena was more vulnerable to insect pests infestation. These pests are divided into major and minor pest according to the host range and the interaction between pest and Leucaena whether monophagous or oligophagous pest.
The monophagous, H. cubana is a major pest which exclusively feeds on L. leucocephala and to a lesser extent on closely related mimosoid leguminous trees at several world regions. The pest is native to Central and South America and spread to Africa, Asia and mainly exists with extensive damage to Leucaena Heydon and Affonso (1991); USAID (1991); FAO (2007); Rao et al. (2000). It is a typical example of pest risk for its devastating outbreaks in exotic L. leucocephala plantations across the tropics (Nair, 2007). Besides; it achieved international notoriety in the early 1980s when detected in Florida and Hawaii with an extremely rapid rate of spread. Therefore, it is reasonable to assume that sooner or later all areas where Leucaena is grown will be affected (Bray 1994; Geiger et al., 1995; Olckers 2011).
The oligophagous, T. tabaci is a minor pest for its ability in completing its life cycle on different alternative host plants than Leucaena, the thing that makes it difficult to be controlled. Previously, T. tabaci has been recorded from more than 300 species of host plant scattered through diverse plant groups (Sakimura 1932; Ghabn 1948). In this work it is recorded as a minor pest on Leucaena for the first time.
In the field of pest management, quitting pesticides with such animal fodder tree and using biological control agent still the first demand around the world, because it automatically regulates pest population such as the predators of Coleoptera: Coccinellidae: Curinus coeruleus (Mulsant, 1850) Valenciaga et al. (1999) ;Singh (2004), Olla v-nigrum (Mulsant, 1866) and the parasitoids of Hymenoptera: Psyllaephagus yaseeni Noyes, 1990 and Tamarixia leucaenae Boucek, 1988 which have met with partial success (Napompeth 1994; Shivankar et al. 2010).
Study of Leucaena pest’s population dynamics, H. cubana and T. tabaci under the effect of their natural enemies is considered as a corner stone to set management recommendations for pest control. From this point, survey of natural enemies which habit Leucaena is an important step to study their effects on the population dynamics of the most dangerous Leucaena pests.
Materials and methods
The present experiment was conducted throughout two seasons (2013 and 2014) at farm located in Xmatkuil community at Merida, Yucatan, Mexico. The experimental farm (Figure 1) contains four plots (replications), which maintained free weeds by hand weeding without using weedicides. The plots size is 9 m X 6 m, each genotype planted in row (four rows per plot), the spacing between rows is 2 m, and 1 m within trees. Each genotype had 10 trees per row. In the first season (2012-2013) at the beginning of the experiment, the trees had (2 years and 3 months old) and weren’t harvested till the end of October, 2013. Then, Successive harvests were done each two months in order to assess the effect of chemical compositions of new plant leaves during the months of the second season (2013-2014). The trees were allowed to grow in a month, and sampled in the second month. The harvest process took place at 1 m height from the soil according to the traditional agriculture processes of Leucaena at Merida, Yucatan, Mexico. Harvests were done at the end of the following months: 1st harvest in October, 2013; 2nd December 2013; 3nd in February, 2014; 4th in April, 2014; 5th in June, 2014, and 6th in August, 2014. To survey the natural enemies of the most abundant Leucaena pests: H. cubana and T. tabaci, and study their effects on the population fluctuation on new and old Leucaena leaves of four Leucaena genotypes: two cultivars of Leucaena, Cunningham and K636 (L. leucocephaIa), and two hybrids Nativa and KX2 (L. leucocephala x L. pallida). Two trees from each row were tagged to collect the samples from their terminal fresh leaves, as most of heavy infestations occurred in the terminal shoots; up to 3 000 nymphs and adults were recorded in the terminal (15 cm) of shoot by Nair (2007), also the Psyllid populations boomed in the presence of young Leucaena leaves (Geiger and Andrew, 2000). The tagged trees were divided into three levels upper, middle and lower. Two leaves / level were collected weekly from each selected tree (192 samples per week) pesticides-free.

Figure 1. Distribution of Leucaena genotypes in the experimental blocks of Xmatkuil farm, Merida, Yucatan, Mexico.
The leaves were carefully cut and collected into separate plastic bags and transferred to the refrigerator and kept in fridge at 4-7ºC for well examination in the laboratory of FMVZ nutrition lab. at Faculty of Veterinary Medicine and Animal Science (FMVZ), University of Yucatan (UADY), Yucatán, Mexico. The upper and lower surfaces of each leaf were well examined by a binocular microscope [Nikon (SMZ645), C-W 10x22], and the numbers of H. cubana and T. tabaci and their natural enemies were recorded. It had been stated that using biological control agents is the best way to control Leucaena Psyllid (Shivankar et al., 2010). The unknown natural enemies were mounted on Resina Sintetica Media and sent to specialists for identification and classification.
The simple correlation coefficient was calculated between sums of monthly mean numbers of natural enemies and the monthly mean numbers of each pest separately in each Leucaena genotype (Table 2).
Table 1. A partial taxonomic list of H. cubana and T. tabaci natural enemies on four Leucaena genotypes; Cunningham and K636, Nativa, and KX2 at Xmatkuil farm during (2012/13 -2013/14) seasons.
| No. | Order | Family | Scientific name | N. E. type |
|---|---|---|---|---|
| 1 | Neuroptera | Chrysopidae | Chrysoperla carnea (Stephens) | Predator |
| Coniopterygidae | Conwentzia barretti | Predator | ||
| 2 | Hemiptera | Anthocoridae | Anthocoris nemoralis | Predator |
| 3 | Coleoptera | Coccinelidae | Chilocorus stigma (Say) | Predator |
| Olla v-nigrum Muslant | Predator | |||
| Curinus coeruleus Mulsant | Predator | |||
| 4 | Orthoptera | Mantidae | Archimantis latistyla (Serville) | Predators |
| Mantis religiosa | ||||
| 5 | Hymenoptera | Encyrtidae | Psyllaephagus yaseeni | Parasitoid |
| 6 | Acari | Phytosiidae | Amblyseius cucumeris | Predator |
N. E: Natural enemies.
Table 2. Monthly mean numbers of leucaena pests H. cubana, T. tabaci and their correlations with natural enemies, on the four Leucaena genotypes at Xmatkuil farm during (2012-2013).
| Sampling date months | Mean numbers of individuals | Grand mean | ||||||||||||
| Cunningham | Nativa | K636 | KX2 | |||||||||||
| H | T | N | H | T | N | H | T | N | H | T | N | H | T | |
| Feb., 2013 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Mar. | 11.0 | 211.5 | 41.5 | 7.3 | 171.5 | 7.8 | 10.0 | 149.5 | 14.8 | 1.3 | 62.0 | 1.8 | 29.6 | 594.5 |
| Apr. | 159.0 | 544.8 | 171.8 | 137.8 | 372.5 | 158.3 | 87.0 | 258.0 | 114.0 | 95.8 | 272.5 | 79.5 | 479.6 | 1447.8 |
| May | 244.8 | 745.0 | 155.0 | 220.0 | 567.3 | 108.3 | 200.3 | 469.0 | 88.3 | 110.5 | 409.0 | 97.5 | 775.6 | 2190.3 |
| Jun. | 194.8 | 837.5 | 95.5 | 101.8 | 918.3 | 49.3 | 111.0 | 715.0 | 32.3 | 54.0 | 621.3 | 21.8 | 461.6 | 3092.1 |
| Jul. | 47.5 | 850.5 | 69.5 | 43.5 | 809.0 | 123.5 | 23.5 | 412.0 | 27.8 | 6.0 | 265.3 | 134.0 | 120.5 | 2336.8 |
| Aug. | 65.0 | 479.0 | 54.8 | 42.8 | 483.0 | 48.5 | 53.8 | 463.3 | 19.8 | 17.3 | 234.8 | 72.0 | 178.9 | 1660.1 |
| Sept. | 113.3 | 386.3 | 148.5 | 65.0 | 270.3 | 96.3 | 23.5 | 263.3 | 98.3 | 11.0 | 235.3 | 100.8 | 212.8 | 1155.2 |
| Oct. | 15.8 | 112.0 | 4.5 | 19.8 | 136.3 | 1.8 | 16.8 | 86.8 | 0.0 | 24.0 | 109.8 | 4.3 | 76.4 | 444.9 |
| Nov. | * | * | * | * | * | * | * | * | * | * | * | * | * | * |
| Dec., 2013 | 7422.3 | 0.0 | 0.0 | 8465.0 | 171.5 | 0.0 | 3866.0 | 0.0 | 0.0 | 2829.0 | 0.0 | 0.0 | 22582.3 | 171.5 |
| Grand total | 8273.3 | 4166.5 | 741.0 | 9102.8 | 3899.5 | 593.5 | 4391.8 | 2816.8 | 395.0 | 3148.8 | 2209.8 | 511.5 | ---- | ---- |
| Grand mean | 827.3 | 416.7 | 74.1 | 910.3 | 390.0 | 59.4 | 439.2 | 281.7 | 39.5 | 314.9 | 221.0 | 51.2 | ---- | ---- |
| Correlations | 0.80 | 0.47 | ---- | 0.61 | 0.35 | ---- | 0.47 | 0.06 | ---- | 0.16 | 0.17 | ---- | ---- | ---- |
* Trees were harvested; H= H. cubana; T= T. tabaci; N= natural enemies; Shaded figures represent peak counts.
Results
Survey of natural enemies:
This study is focusing on a comprehensive understanding of the surveyed natural enemies of Leucaena pests on the new shoots of four Leucaena genotypes: Cunningham, Nativa, K636, and KX2. The surveyed natural enemies associated with H. cubana and T. tabaci is shown in Table 1. Data of survey study and frequent field observations indicated the presence of 9 predators related to 5 Orders, and one parasitoid.
Population fluctuation of H. cubana and T. tabaci:
This part of the current investigation concentrate on the population dynamics of the most dangerous and abundant insect pests, H. cubana and T. tabaci under the effect of the natural enemies on different Leucaena genotypes (Cunningham, Native, K636, and KX2) were shown in Table 2 and 3 and illustrated graphically in Figure 2 and 3.
Table 3. Monthly mean numbers of leucaena pests H. cubana, T. tabaci and their correlations with natural enemies, on the four Leucaena genotypes at Xmatkuil farm during (2013-2014).
| Sampling date months | Mean numbers of individuals | Grand mean | ||||||||||||
| Cunningham | Nativa | K636 | KX2 | |||||||||||
| H | T | N | H | T | N | H | T | N | H | T | N | H | T | |
| Jan., 2014 | * | * | * | * | * | * | * | * | * | * | * | * | * | * |
| Feb. | 8056.75 | 3.25 | 126.75 | 8880.75 | 0 | 82 | 0 | 131.25 | 580 | 0 | 152 | 22222.75 | 3.25 | |
| Mar. | * | * | * | * | * | * | * | * | * | * | * | * | * | * |
| Apr. | 17.5 | 560.25 | 0 | 13.5 | 659.75 | 0 | 17.75 | 480.25 | 0 | 27 | 300.25 | 0 | 75.75 | 2000.5 |
| May | * | * | * | * | * | * | * | * | * | * | * | * | * | * |
| Jun. | 298.75 | 759.75 | 52.5 | 199 | 975.25 | 39.75 | 189.5 | 827 | 40.5 | 81 | 688.5 | 51.5 | 768.25 | 3250.5 |
| Jul. | * | * | * | * | * | * | * | * | * | * | * | * | * | * |
| Aug. | 174.5 | 477.5 | 131.5 | 136.25 | 415 | 63.75 | 139 | 213.5 | 46 | 42.75 | 81.5 | 63.75 | 492.5 | 1187.5 |
| Sept. | * | * | * | * | * | * | * | * | * | * | * | * | * | * |
| Oct., 2014 | 75.75 | 123.75 | 3 | 82.75 | 129.75 | 10.75 | 62 | 108.25 | 13.75 | 47.25 | 116.75 | 9.5 | 267.75 | 478.5 |
| Grand total | 8623.25 | 1924.5 | 313.75 | 9312.25 | 2179.75 | 196.25 | 5113.5 | 1629 | 231.5 | 778 | 1187 | 276.75 | ---- | ---- |
| Grand mean | 1724.65 | 384.9 | 62.75 | 1862.45 | 435.95 | 39.25 | 1022.7 | 325.8 | 46.3 | 155.6 | 237.4 | 55.35 | ---- | ---- |
| Correlations | 0.572 | - 0.22 | ---- | 0.70 | - 0.35 | ---- | 0.94 | - 0.45 | ---- | 0.91 | - 0.36 | ---- | ---- | ---- |
*Trees were harvested; H= H. cubana; T= T. tabaci; N= natural enemies; Shaded figures represent peak counts.
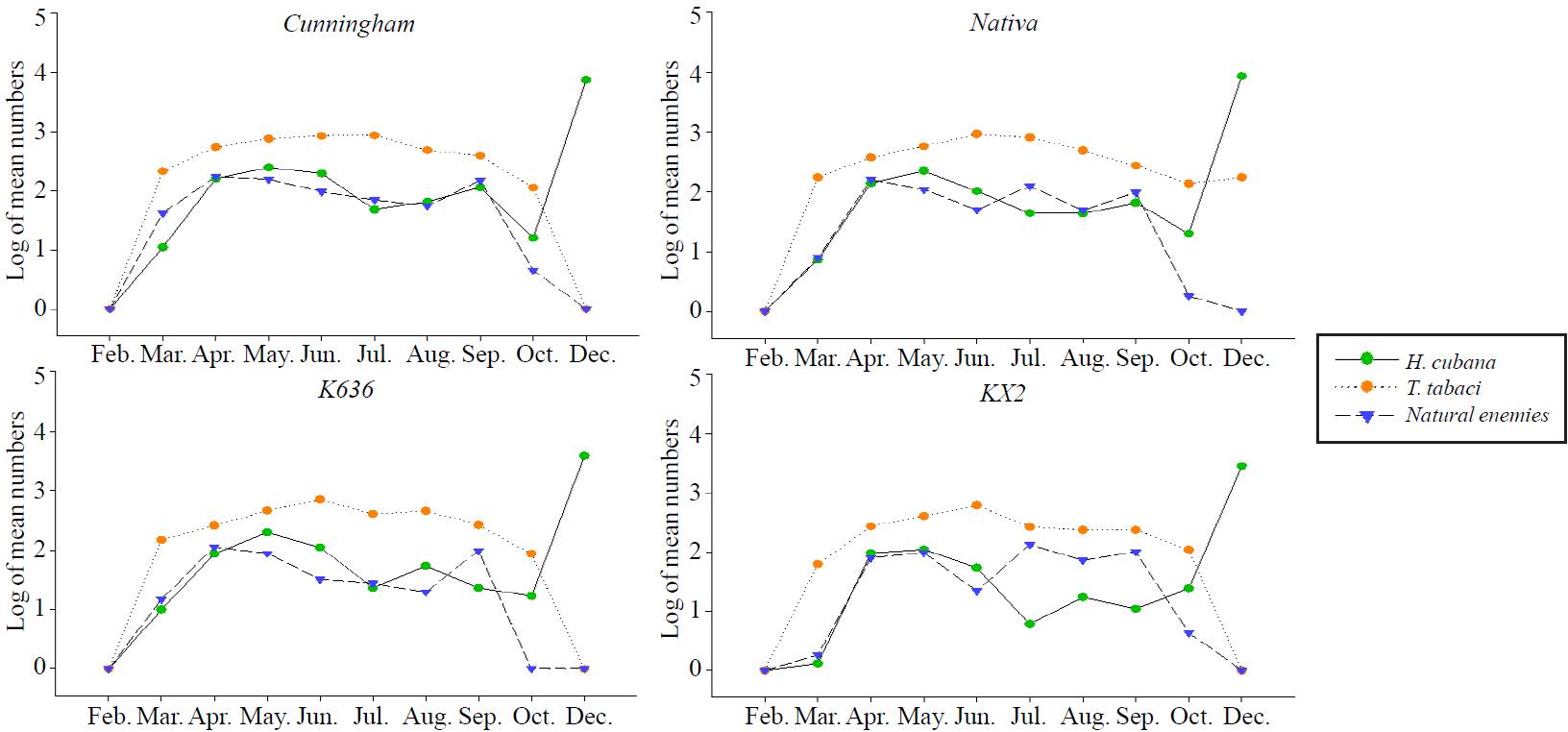
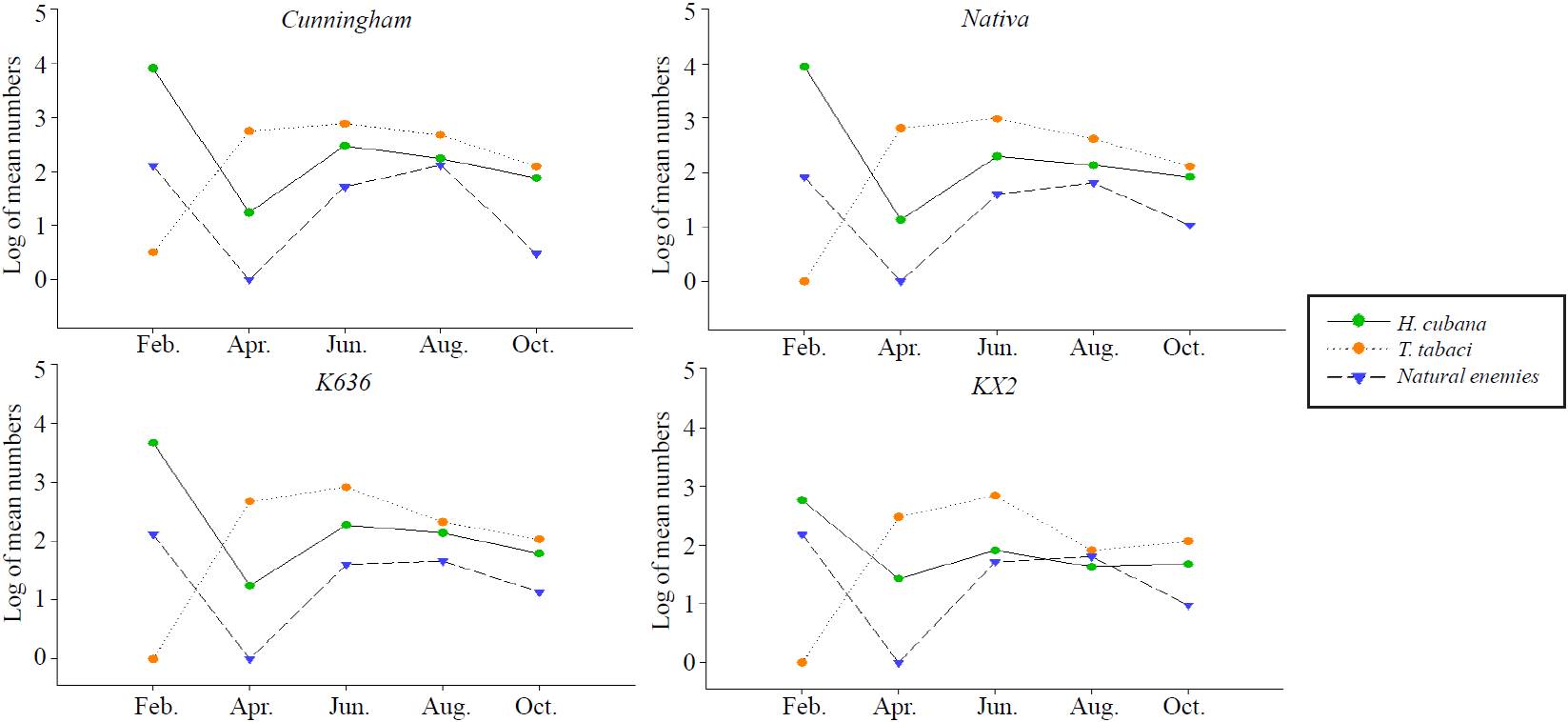
Figure 2. Population dynamics of H. cubana, T. tabaci, and their natural enemies on Leucaena genotypes during 2012/2013 at Xmatkuil farm, Yucatan, Mexico.
Population fluctuations of H. cubana:
In the first season (2012-2013):
The population densities of H. cubana (Table 2 and Figure 2) were in low levels of abundance at the beginning of the season during March (monthly means of 11.0, 7.3, 10.0 and 1.3 individuals/sample of Cunningham, Nativa, K636, and KX2; respectively), then increased to moderate levels of abundance during April (monthly means 159.0, 137.8, 87.0, 95.8 individuals/sample). After that, two peaks were occurred before the first harvest during May (monthly averages were 244.8, 194.8, 220.0, and 101.8) and June (200.3, 111.0, 110.5 and 54.0 individuals/sample) on Cunningham, Nativa, K636, and KX2; respectively as shown in (Table 2; Figure. 2). Afterwards, the numbers of H. cubana rapidly declined during the next four months (July and October) till harvest in November. Then, the third peak was recorded in December directly after the harvest of November (monthly means 7 422.3, 8 465.0, 3 866.0, 2 829.0 individuals-sample) on Cunningham, Nativa, K636 and KX2; respectively.
In the second season (2013-2014):
An intensive invasion of Psyllid population (Table 3 and Figure 3) occurred in the beginning of the season with obvious differences between Psyllid densities on Leucaena genotypes. The first peak occurred in February for Leucaena genotypes (monthly means were 8 056.75, 8 880.75, 4 705.25 and 580 individuals-sample on Cunningham, Nativa, K636, and KX2; respectively). Afterwards, the populations of Psyllid were in lowest levels of abundance in April with monthly means 17.5, 13.5, 17.75 and 27 on Cunningham, Nativa, K636, and KX2; respectively. The second peak recorded in June (monthly means were 298.75, 199, 189.5 and 81 individuals/sample on Cunningham, Nativa, K636, and KX2; respectively). The moderate levels of infestations occurred in August, after that the population fluctuated slowly at October in Leucaena genotypes (Table 3 and Figure 3).
Population fluctuations of T. tabaci:
In the first season (2012-2013):
T. tabaci showed low levels of initial infestation (Table 2 and Figure 2) in March 211.5, 171.5, 149.5, and 62.0 individuals/sample on Cunningham, Nativa, K636 and KX2; respectively. Then, the population density grew up slowly on all Leucaena genotypes, with observation of high mean numbers of Thrips on Cuninngham more than other genotypes during April (monthly means were 544.8, 372.5; 258.0 and 272.5 individuals/sample on Cunningham, Nativa, K636 and KX2; respectively). Four peaks of Thrips were recorded on Cunningham, Nativa and K636 during May to Aug. The monthly means of May were 745.0, 567.3, 469.0; means of June were 837.5, 918.3, 715.0; means of July were 850.5, 809.0, 412.0; and means of August were 479.0, 483.0, 463.3 individuals/sample on Cunningham, Nativa and K636; respectively. Besides, two peaks were occurred on KX2 in May and June with monthly means 409.0, 621.3 individuals/ sample; respectively. After that a sharp declines of population densities occurred throughout the following months. The pest totally disappeared in December after the 1st harvest.
In the second season (2013-2014):
Thrips population were in low levels of abundance (Table 3 and Figure 3) in February for Leucaena genotypes (monthly means were 3.25, 0.0, 0.0 and 0.0 individuals/sample of Cunningham, Nativa, K636, and KX2; respectively). Afterwards, the population of Thrips showed two peaks in April and June. The mean numbers in April were 560.25, 659.75, 480.25 and 300.25 on Cunningham, Nativa, K636, and KX2; respectively. The means of the second peak of June were 759.75, 975.25, 827 and 688.5 individuals/sample on the same respective genotypes. Thereafter, the moderate levels of abundance were recorded in August (monthly means 477.5, 415, 213.5 and 81.5 on Cunningham, Nativa, K636, and KX2; respectively), after that the populations were reduced at the end of the season in October on almost Leucaena genotypes.
The correlation study between natural enemies and pests:
The correlation coefficient was stronger between natural enemies and H. cubana than T. tabaci on Leucaena genotypes during the seasons of study.
During The first season (2012-2013):
The Leucaena Psyllid; H. cubana showed the strong correlation with natural enemies (Table 2) for Cunningham (r= 0.80), weak for KX2 (r= 0.16), and moderate (r= 0.61 and 0.47) for Nativa and K636; respectively. On the other side, the correlation was strong between T. tabaci and natural enemies for Cunningham (r= 0.47), weak for K636 (r= 0.06), and the moderate (r= 0.35 and 0.17) for Nativa and KX2; respectively.
During the second season (2013-2014):
The correlations (Table 3) between natural enemies and Psyllid were strong (r= 0.94 and 0.91, for K636 and KX2 respectively), the moderate relation was recorded for Nativa (r= 0.70), and weak for Cunningham (r= 0.572). However, the correlation between natural enemies and Thrips were negative in Leucaena genotypes.
Discussion
The slow population fluctuations of both pests H. cubana and T. tabaci were similar at the beginning of the season (Table 1) with a clear difference between their spatial distribution, due to the effect of natural enemy’s agents and the harvest processes (before and after harvest).
The main prevalence of both pests fell between March and December. The population densities of T. tabaci before harvest were higher than H. cubana (Table 2), because in the beginning of the experiment the trees weren’t young enough (2 years and 3 months old) and never harvested before and T. tabaci is not affected by the host age, in contrast with H. cubana which prefer only new leaves more than old leaves, the thing which encourage high population densities of T. tabaci to invade old Leucaena more than H. cubana. These results are in agreement with Geiger and Andrew (2000) and Chazeau et al. (1989) who reported that, H. cubana populations boomed only in the presence of young Leucaena leaves and new shoots. Therefore, an obvious increase of psyllid populations occurred during the successive harvests in the second season (Table 3) with the continued existence of the same differences between both pests populations.
H. cubana:
In the first season (2012-2013):
H. cubana individuals showed three generations a year, two before harvest in May and June, and the third peak in December after harvest. Because, an obvious reductions were observed in natural enemies populations during peaks times compared by the actual numbers of Psyllid. This could be due to the new generation overlapping and the effects of high temperatures in the dry seasons during May and June at Merida, Yucatan which reduce the populations of natural enemies. These results are in agreement with those reported by Funasaki et al. (1989) who stated that C. coeruleus failed to be established in many seasonal dry areas. Therefore, Psyllid numbers increased more than natural enemy’s agents, and this affected other biological control agents such as P. yaseeni which can’t overcome Psyllid peaks during high Psyllid numbers.
Geiger and Andrew (2000) stated that P. yaseeni never succeeded in regulating Psyllid populations in the presence of high Psyllid populations. Accordingly, the peaks were occurred when the Psyllid population was out of control during the absence of biological control agents, because natural enemy’s agents automatically control Psyllids populations, according to the findings of (Bray 1994; Napompeth 1994; Geiger et al. 1995; Valenciaga et al. 1999; Singh 2004; Shivankar et al. 2010). The third peak after harvest could be occurred due to the young leaves production of Leucaena, which supported about 98% of H. cubana infestation, because juvenile leaves encourage females for egg-laying. This is in accordance with those reported by (Chazeau et al., 1989).
In the second season (2013-2014):
The low Psyllid population during non-harvested Leucaena genotypes at February, 2013of the first season, and the outbreak of pest in all Leucaena genotypes during February, 2014 emphasized that the harvest was the reason for the first peak, because the new and juvenile leaves of leucaena genotypes after harvest supported Psyllids females for egg-laying. This is in agreement with (Chazeau et al., 1989). Therefore, the natural enemies weren’t able to control the outbreak of Psyllid because there were clear differences between pest and natural enemies’ densities in all Leucaena genotypes (Table 3). However, this difference impedes the efficiency of many natural enemies. This is in accordance with (Geiger and Andrew 2000). The second peak of June which happened in the previous season under non-harvested trees occurred due to the normal time of overlapping generation. Consequently, the population reduced in August and October, due to the low temperatures during these months in Merida, Yucatán, Mexico. The same results were found by Anonymous (2014) where the reproduction and development of Psyllid were stopped or declined during cool weather and most species have 3 to 5 generations a year and some species may have one generation a year.
T. tabaci:
In the first season (2012-2013):
The four peaks of T. tabaci on Cunningham, Nativa, and K636 during May to Aug. and the two peaks of KX2 during May and Jun could be due to the numbers of overlapping generations which depend on the climatic factors (especially maximum temperature), host plant, and the numbers of natural enemies. (Bastos et al. 1981) recorded four population peaks of Thrips in Brazil which represented four generations of the pest. Nevertheless, the variation in the present results could be attributed to the differences between Leucaena genotypes. The highest grand total numbers of T. tabaci (Table 2) was occurred on the susceptible genotypes; Cunningham and Nativa. These are in agreement with Castillo et al. (1997), who found that, the most susceptible lines were Cunningham (L. leucocephala) and Nativa (L. leucocephala x L. pallida) which showed moderately resistance among different Leucaena accessions to pest infestation.
Besides, the differences between the fluctuations of the natural enemies on Leucaena genotypes, this may explain such variation in the numbers of peaks among Leucaena genotypes. Additionally, it seems that T. tabaci populations were out of control under the effect of natural enemies, because low population densities of the natural enemies were found on Cunningham, Nativa, and K636 compared with the numbers of T. tabaci during the four peaks, and as it known that natural enemies automatically regulating the numbers of Leucaena pests (Shivankar et al. 2010; Singh 2004; Bray 1994; Napompeth 1994).
In the second season (2013-2014):
The Thrips fluctuated slowly (Table 3) in the beginning of the season in February for Leucaena genotypes, because the high densities of natural enemies kept Thrips populations under control. Afterwards, the population of the pest increased due to the total absence of natural enemies which encouraged peaks formation on Leucaena genotypes during April. Likewise, in the next month of June the low population of natural enmies permits pest outbreak. These results are in agreement with Alston (2008) who reported that the plenty of natural enemies are adequate to suppress Thrips population.
Conclusion
Leucaena trees resulted from consequence harvests every two months were better than free growing leucaena trees, because it showed low Psyllid and Thrips population peaks. However, KX2 and K636 genotypes are recommended for plantation among the studied Leucaena genotypes for their pest’s resistance with an obvious low population’s dynamics of both pests. Natural enemies achieved satisfactory results in biological control field for pests, specially larvae and adults of Order: Coleoptera. Therefore, caring with natural enemies in Leucaena fields is the only important way for pest control and avoiding insecticides utilization with such animal fodder tree.
REFERENCES
Alston, D. G. 2008. Onion Thrips (Thrips tabaci). Available at: Available at: http://utahpests.usu.edu/IPM/htm/vegetables/vegetable-insect-disease/onion-thrips . (Accessed: September 2014). [ Links ]
Anonymous. 2014. Pests in Gardens and landscape, University of California Agriculture & Natural Resources. Available at: Available at: http://www.ipm.ucdavis.edu/PMG/PESTNOTES/pn7423.html (Accessed: September 2014). [ Links ]
Bastos, J.A .M.; Alves, V.P.O.; Magalhaes, B.J.A. and Oliveira, A.V.P. 1981. Survey of the population of the red grapevine Thrips Retithrips syriacus (Mayet, 1890) on grapevine. Fitossanidade. 5:1-6. [ Links ]
Bray, R. A. 1994. The leucaena psyllid. In: Forage Tree legumes in Tropical Agriculture Gutteridge, R.C. and H.M. Shelton (eds) (Department of Agriculture, The University of Queensland, Australia). CAB International, Oxford. 283-291 pp. [ Links ]
Castillo, A. C.; Cuyugan, O. C.; Forgary, S. and Shelton, H. M. 1997. Growth, psyllid resistance and forage quality of Leucaena leucocephala, L. pallid, L. diversifolia and F1 hybrid of L. leucocephala x L. pallid. Tropical Grassland. 31:188-200. [ Links ]
Chazeau, J.; Bouye, E. and Bonnet de Larborgne, L.. 1989. Lutte biologique contre le psylle Heteropsylla cubana ravageur du faux mimosa Leucaena leucocephala en Nouvelle Calédonie. Orstom, Nouméa. [ Links ]
Food and Agriculture Organization of the United Nations (FAO). 2007. Forest Health & Biosecurity Working Papers, OVERVIEW OF FOREST PESTS MALAWI. Working Paper FBS/22E, FAO, Rome, Italy. 8 p. [ Links ]
Funasaki, G. Y.; Lai, P. Y. and Nakahara, L. M. 1989. Status of natural enemies of Heteropsylla cubana Crawford (Homoptera: Psyllidae) in Hawaii. In: Leucaena Psyllid: Problems and Management. Napompeth, B. and K.G. MacDicken (eds). Proceedings Bogor, Indonesia Winrock International, Bangkok, Thailand. 153-158 pp. [ Links ]
Funasaki, G.Y.; Lai, P.Y., and Nakahara, L.M. 1990. Status of natural enemies of Heteropsylla cubana Crawford (Homoptera: Psyllidae) in Hawaii, in Leucaena psyllid: problems and management. Proceedings of an International Workshop held in Bogor, Indonesia, January 1 6-21, 1989. F/FRED, IDRC, NFTA, Bangkok, Thailand, 153-158 pp. [ Links ]
Geiger, C. A.; Napompeth, B. and Van Den Beldt, R. J. 1995. An update on the status of the leucaena Psyllid in Southeast Asia. In: Leucaena: Opportunities and Limitations. Shelton, H.M., C.M. Piggin and J.L. Brewbaker. Canberra (eds). Australian Centre for Agricultural Research. 8-125 pp. [ Links ]
Geiger, C. A. and Andrew, G. P. 2000. Ecology of Heteropsylla cubana (Homoptera: Psyllidae): Psyllid Damage, Tree Phenology, Thermal Relations, and Parasitism in the Field. Environ. Entomol. 29:76-86. [ Links ]
Ghabn, A. A. A. E. 1948. Contribution to the knowledge of the biology of Thrips tabaci Lindeman in Egypt. Bulletin de la Societe Fouad Ier d'Entomologie. 32:123-174. [ Links ]
Heydon, D. and Affonso, M. 1990. Economic review of psyllid damage on leucaena in Southeast Asia and Australia. In: A report prepared for the Australian International Development Assistance Bureau (AIDAB) , CAB International Development Services, Wallingford, Oxford, U.K. 129 p. [ Links ]
Napompeth, B. 1994. Leucaena psyllid in the Asia-Pacific region: implications for its management in Africa. In: Workshop Proceedings. LeucaenaPsyllid: a Threat to Agroforestry in Africa. FAO Corporate Document Repository Food and Agriculture Organization of the United Nations, Rome, Italy. 1-15 pp. [ Links ]
Nair, K. S. S. 2007. Tropical Forest Insect Pests. Ecology, Impact, and Management. Book. by Dr. Nair, K. S. S. ISBN: 9780521873321. [Accessed 3 Jan., 2014]. Available from URL: Available from URL: http:/www.cambridge.org/9780521873321 . [ Links ]
Olckers, T. 2011. Biological Control of Leucaena leucocephala (Lam.) de Wit (Fabaceae) in South Africa: A Tale of Opportunism, Seed Feeders and Unanswered Questions. African Entomology. 19(2):356-365. [ Links ]
Rao, M. R.; Singh, M. P. and Day, R. 2000. Insect pest problems in tropical agroforestry systems: Contributory factors and opportunities for management. Kluwer Academic Publisher, printed in Netherlands. Agroforest Systems. 50:243-277. [ Links ]
Sakimura, K. 1932. Life-history of Thrips tabaci Lindeman, on Emilia sagittata and its host plant range in Hawaii. Journal of Economic Entomology. 25:884-891. [ Links ]
Shivankar, V. J. and Rao, C. N. 2010. Psyllid and pest management. Pest Management in Horticultural Ecosystems. 16:1-4. [ Links ]
Singh, S. P. 2004. Some Successes Stories in Classical Biological Control of Agriculture Pests in India. Asia-Pacific Association of Agricultural Research Institutions (APAARI) FAO-RAP, Maliwan Mansion 39 Phra Atit Road Bangkok 10200, Thailand. [Accessed 7 Feb., 2014]. Available from URL: Available from URL: http://http://www.apaari.org/wp-content/uploads/2009/05/ss_2004_02.pdf . [ Links ]
USAID. 1991. Environment Assessment of the classical biological control of the leucaena psyllid in Indonesia, Laos, Malaysia, Nepal, Philippines and Thailand. USAID Report, Washington DC. plus appendices. 94 p. [ Links ]
Valenciaga, N.; Barrientos, A. and Mora, C. 1999. Performance of the beneficial entomofauna in Leucaena leucocephala (Lam.) de Wit areas. Cuban Journal of Agricultural Science. 33:319-24. [ Links ]
Acknowledgement
I would like to express my deepest thanks to Consejo Nacional de Ciencia y Tecnología (CONACYT/ Mexico) which gave me the opportunity for obtaining a scholarship to conduct my Ph.D. researchers at Faculty of Veterinary Medicine and Animal Science, University of Yucatan (UADY), Merida, Yucatan, Mexico.
Received: September 2015; Accepted: January 2016











 texto en
texto en