Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista mexicana de ciencias agrícolas
versión impresa ISSN 2007-0934
Rev. Mex. Cienc. Agríc vol.6 spe 12 Texcoco nov./dic. 2015
Articles
Growth and nutritional status of tomato in response to organic substrates and mycorrhizal fungi
1Departamento de Horticultura, Universidad Autónoma Agraria Antonio Narro, Saltillo, Coahuila, C. P. 25315. (avilaperalta@universitarios.com; luisalonso_va@hotmail. com; hernandez865@hotmail.com).
2Departamento de Ciencias Básicas, Universidad Autónoma Agraria Antonio Narro, Saltillo, Coahuila, C. P. 25315. (emrc@me.com).
3Departamento de Plásticos en la Agricultura, Centro de Investigación en Química Aplicada, Saltillo, Coahuila, C. P. 25294. (antonio.cardenas@ciqa.edu.mx).
The main function of mycorrhizal fungi is to facilitate water absorption, phosphorus (P) and nitrogen (N) to plant, in addition to improve physicochemical properties of soil and aggregate formation through particle adhesion due to protein exuded by the mycelium called Glomalin. The aim of this study was to evaluate the effect of two arbuscular michorrizal fungi consortia and a commercial species (Rhizophagus irregularis) combined with a substrate based on vermicompost or peat on development of tomato plants (Solanum lycopersicum. Rio Grande). The experimental design was a randomized complete block with a factorial arrangement 2x4, with 8 treatments and 6 replications each replication consisted of a container per plant. 1 g of commercial mycorrhizal (500 ea g-1), and 100 g (500 ea g-1) of native soil per plant was applied. The variables plant height, stem diameter, root length, root, shoot and total plant dry weight were affected by the substrates and fungi and the interaction between these factors. The mycorrhization percentage was also affected by interaction, roots plants grown in vermicompost and peat inoculated with CN1 had the highest percentage of mycorrhization, for the first sampling, plants grown in peat decreased the percentage of mycorrhization compared with those developed in vermicompost. Nutrients concentration decreased compared with control excluding nitrogen.
Keywords: Solanum lycopersicum; Rhizophagus irregularis; mycorrhization; phosphorus
La función principal de los hongos micorrízicos es facilitarle a la planta la absorción de agua, fósforo (P) y nitrógeno (N), además de mejorar las propiedades fisicoquímicas del suelo y la formación de agregados por medio de la adhesión de partículas debida a una proteína exudada por el micelio llamada glomalina. El objetivo de este estudio fue evaluar el efecto de dos consorcios de hongos micorrizicosarbuscularesy una especie comercial (Rhizophagus irregularis) combinadas con un sustrato a base de lombricomposta o turba ácida sobre el desarrollo de plantas de tomate (Solanum lycopersicum. Rio Grande). El diseño experimental fue un bloques completos al azar con un arreglo factorial de 2x4, con 8 tratamientos y 6 repeticiones, cada repetición consistió de un contenedor por planta. Se aplicó 1 g de micorriza comercial (500 ea g-1), y 100 g (500 ea g-1) de suelo nativo por planta. Las variables de altura de planta, diámetro de tallo, longitud de raíz, peso seco de raíz, y parte aérea y total de planta fueron afectadas por los sustratos y hongos y por la interacción entre estos factores.El porcentaje de micorrización también fue afectado por la interacción, las raíces de plantas desarrolladas en lombricomposta y turba ácida e inoculadas con CN1 tuvieron el mayor porcentaje de micorrización, para el primer muestreo, asimismo, las plantas desarrolladas en turba ácida disminuyeron el porcentaje de micorrización comparadas con las desarrolladas en lombricomposta. La concentración de los nutrimentos disminuyó en comparación con el testigo a excepción del nitrógeno.
Palabras clave: Solanum lycopersicum; Rhizophagus irregularis; fósforo; micorrización
Introduction
Proper nutrition of crop plants is not necessarily achieved with the application of chemical fertilizers, as there are others that produce the same effect but have less environmental impact and can sustain agricultural production to meet the growing demand for food worldwide. As a result of this arise agricultural inputs based on microorganisms and materials of organic origin, such as mycorrhizal fungi and vermicompost, options that constitute what is called sustainable agriculture (Pretty, 2008), due to the wide range of activities that develop, and exert a great influence on soil fertility and on the development and protection against plant pathogens.
The main function of mycorrhizal fungi is to facilitate plants water absorption, phosphorus (P) and nitrogen (N), in addition to improve the physical and chemical properties of soil and aggregate formation through particle adhesion due to a protein exuded by the mycelium called glomalin, also improves the structure and stability, increase water retention capacity and reduce soil erosion (Finlay, 2008). Furthermore, it also affects directly or indirectly in the absorption of other ion minerals such as potassium (K), calcium (Ca), magnesium (Mg), iron (Fe) and manganese (Mn), promoting plant growth, especially in soils where these nutrients are scarce (Koltai and Kapulnik, 2010). Cause greater tolerance to water deficit and protect roots against pathogens through various mechanisms of action, among which are: mycoparasitism, enzymatic lysis, antibiosis and competition for space or nutrients (Finlay, 2008).
Vermicompost is an organic fertilizer that provides nutrients to plants; its application increases growth, development and productivity of a wide range of crops (legumes, cereals, vegetables, ornamental plants and flowers), which is attributed to the physical and chemical characteristics of the fertilizer (Moreno et al., 2008); it also provides N, P, K, Ca, Mg and carbon (Duran and Henriquez, 2010). Because of this, vermicompost can be used as growth media of horticultural species grown under greenhouse conditions (Cruz et al., 2010). Knowledge of the impact of arbuscular micorrizal fungi (AMF) on vegetables grown in fields is wide (Hernández and Chailloux, 2004); however, the effect of these in greenhouse crops has not been studied, not even in combination with organic fertilizers like vermicompost, a practice that should be incorporated into horticultural production systems (Nelson and Nelson, 2015; Oseni et al., 2010; Carpio et al., 2005).
Some reports indicate that inoculation of AMF in tomato increase nutritional status, fruit size and allows higher yield (Al-Karaki, 2006; Desgan et al., 2008; Oseni et al., 2010). However, the effect in protected environments depends on the strain of AMF, the cultivated plant species and growing conditions (Corkidi et al., 2004). On the other hand, commercial mycorrhizal species may be in disadvantage by competition with soil microorganisms, so it is advisable to use native strains (Caldera et al., 2013). Therefore, the present study aimed to evaluate the effect of two consortia of arbuscular mycorrhizal fungi and a commercial species (Rhizophagus irregularis) combined with a substrate based on vermicompost or peat on plant development of tomato (Solanumly copersicum) cv. Rio Grande, under the hypothesis that native AMF spores can outperform commercial species in growth and nutritional status of the plants.
Materials and methods
The experiment was carried out from March to June 2013 in a greenhouse from the Universidad Autonoma Agraria Antonio Narro (UAAAN) in Saltillo, Coahuila, Mexico, whose geographical coordinates are: 25° 27' north latitude and 101° 02' west longitude at an altitude of 1 610 masl. The minimum and maximum average temperature during the experimental period was 12 and 34 °C respectively, while relative humidity ranged between 25 and 72%.
Tomato seedlings cv. Rio Grande of 15 cm height was used, which were transplanted in a black polyethylene container of 5 L volume. The containers were filled with a substrate based on vermicompost, peat, sand and soil (Table 1); the soil was sterilized at 120 °C for 15 min in an autoclave for three times. Before the transplant 1 g of the product was inoculated with 500 spores from the commercial mycorrhizal fungi Endovit (Rhizophagus irregularis) (Ri).
Table 1 Treatments set with different composition of the substrate and type of inoculum used in tomato (Solanum lycopersicum L.) Var. Rio Grande under greenhouse conditions.

CN1 =Consorcio nativo 1, CN2=Consorcio nativo 2,Testigo 1 y Testigo 2 sin inocular y nutridos con la solución de Steiner.
However, for native mycorrhizal fungi 100 g of soil containing the same amount of spores were inoculated. Native mycorrhizal fungi were obtained in two soil types, one low in organic matter (1%) (native consortium 1 CN1) and the other high in organic matter (5%) (native consortium 2 CN2). 3 kg of soil with plant roots were collected for the extraction of spores of these fungi through wet sieving and decanting (Guerderman and Nicolson, 1963). CN1 consisted of spores of different polar and meridional diameter 209.31 and 189.89, 110.56 and 105.49, 129.13 and 142.63, 194.11 and 174.70 µm, while in CN2 these were 93.27 and 186.52, 229.56 and 275.98, 124.06 and 124.91, 222.72 and 179.04 µm. The diameter of the spores was measured by Dino Capture 2.0. version 1.3.8 software for Windows with the help of an optical microscope.
AMF spore types were evaluated in combination with two types of substrate (vermicompost and peat) containing the same proportion of soil (10%) and sand (30%), improved with the addition of vermicompost or peat (60 %), treatments evaluated are presented in Table 1. Vermicompost used has a pH= 8.4, EC= 2.6 dS cm-1, organic matter= 5%, bulk density = 0.67 g cm3, N= 0.62 meq L-1, P= 0.6 meq L-1 and K= 1.3 meq L-1 and irrigation water Ca= 4.2, Mg= 3.9, Na= 0.54, Cl= 1.38, SO4= 5.9 and HCO3 -= 5.9 meq L-1respectively. So the contents of both were considered for the formulation of nutrient solutions (SN), using in the control SN Steiner (1961) to 100%, and in the treatments inoculated the solution contained 50% phosphorus. Irrigation was carried manually applying 1 L SN per plant, managing to keep a fraction of 25% of leachate; irrigation was made every four days.
The experiment finished at 80 days after transplanting (DAT). To determine the percentage of mycorrhization two assessments (50 and 80 DAT) were performed using four plants per treatment, from which shoot and root was removed; to the latter was removed the excess of substrate with tap water, to determine the percentage of mycorrhization. Plant organs were dried in an oven at 65 °C for 72 h to obtain dry weight using a digital scale VE1000. Other variables evaluated were plant height and stem diameter.
Before determining the percentage of mycorrhization, roots were placed in test tubes by adding a solution of 10% KOH for 10 min at 60 °C, adding 10% H2O2 to remove KOH residues, resting for 5 min and finally added lactoglyserol with trypan blue to stain roots (Phillips and Hayman, 1970). The percentage of mycorrhization was obtained from roots dyed; roots were segmented in 1 cm length, placing it 10 sec on a slide, with three replicates for subsequent observation in optic microscope (10 and 40 x) (Sieverding, 1983).
In root and shoot the concentration of N, P and K was determined; N through micro-Kjeldahl method (Bremner, 1996), P by the method of ammonium molybdate and K by atomic absorption (AOAC, 1980). The treatments were established under an experimental design of complete randomized blocks with a factorial arrangement of 2 x 4 (2= vermicompost and peat; 4= CN1, CN2, Ri and Control) with 8 treatments and 6 replications. The experimental unit consisted of a container per plant, spacing 25 cm. Data was subjected to an analysis of variance (ANOVA) and mean comparison test Tukey (p≤ 0.05) using SAS (Statistical Analysis System) version 9.2.
Results and discussion
The percentage of mycorrhization in plants grown in peat and vermicompost was higher with inoculation from CN1 in sampling at 50 days (Figure 1A) and in control with vermicompost. In the second sampling, the trend was similar, excep plants grown in peat, as colonization was higher with CN1 and CN2 (Figure 1B). A higher percentage of mycorrhization was recorded with inoculation from spores of CN1 both in early and advanced stages of plant development coinciding with Velasco et al. (2001), who indicate that the highest mycorrhization in tomatillo (Physalisixocarpa Brot.) observed at 60 days after transplantation.
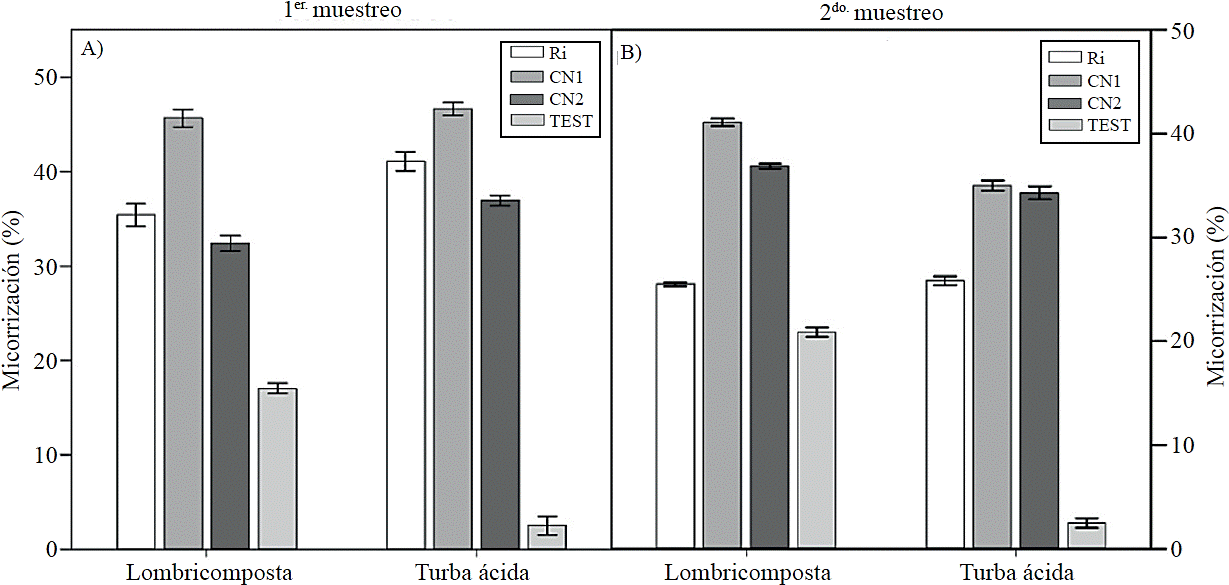
Figure 1 Effect of organic substrates and spores of mycorrhizal fungi inoculated on mycorrhization percentage in tomato (Solanum lycopersicum L.) cv. Rio Grande. Ri = Rhizophagus irregularis, native consortium CN1 = 1; CN2 = native 2 consortium; TEST = control. Bars indicate the standard error of the mean. (micorrización1 and 2; substrates ANOVA = ns, ANOVA fungi= p≤ 0.001, ANOVA substrates x = p≤ 0.001 fungi) coefficient of variation; = 8.93 and 6.3).
In control plants grown in a substrate based on vermicompost, despite not being inoculated, showed greater colonization than those grown with peat, indicating that this substrate facilitates colonization, probably due to the presence of humic substances in the same since establishment of arbuscular mycorrizal fungi and functionality of the symbiosis are favored by the application of certain amounts of humic substances (Gryndler et al., 2005; Pinheiro et al., 2014). It has been reported that vermicompost is comprised by bacteria and fungi capable of degrading compounds like lignin and hemicellulose (Aira et al., 2007).
However, it has not been stated that the vermicompost contains mycorrhizal fungi spores, so mycorrhization may be due to a contamination by irrigation water; moreover, peat can contain mycorrhizal fungi as has already been pointed out by Callejas-Ruiz et al. (2009). The lowest colonization in plants grown in the substrate based on peat may be because this substrate has high water retention capacity, which can be up to 90% (Adams, 2004), implying lower aeration in root zones and therefore lower oxygenation; some reports indicate that optimal concentrations of oxygen are between 12 and 16% for mycorrhization and beneficial expression of symbiosis on plant growth (Callejas-Ruiz et al., 2009).
The increase in the percentage of mycorrhization in plants inoculated with CN1 compared with Ri may be due to consortia of native fungi have the ability to establish symbiotic relationships with microbes from the environment and edapho-climatic conditions, while commercial mycorrhizal (Ri) have passed through a selection process, the above is consistent with that reported by Jargeat et al., (2005), who note that mycorrhizal fungi are associated with endosymbiotic bacteria bacillus shaped and cannot develop an independent life phase, thus eliminating these bacteria seriously compromises the presymbiotic development and growth of the fungus (Lumini et al., 2007). This suggests the importance of selecting native mycorrhizal fungi strains to lead a specific condition in order to obtain satisfactory results (Trejo et al., 2011).
In Figure 2 A, B, and C it is shown that plants grown in peat showed a decrease in plant height, stem diameter and root length when inoculated with native mycorrhizae or with Ri a decrease in root length may be due to increased synthesis of hormones made by the same plant and by the fungus, as they are important in the early stages of colonization, as was reported by Foo et al. (2013) and Bucher et al. (2014), who point out that plant hormones interact to regulate the establishment and operation of the symbiosis.

Figure 2 Effect of substrates orgánicosy inoculation of mycorrhizal fungi spores in height, stem diameter and root length of tomato plants (Solanum lycopersicum L.) cv. Rio Grande. Ri = Rhizophagus irregularis, native consortium CN1 = 1; CN2 = native 2 consortium; TEST = control. Bars indicate the standard error of the mean. (Height, stem diameter, root length; substrates= p≤ 0.001 ANOVA, ANOVA fungi= p≤ 0.001, ANOVA yeast substrates x p= 0.001 coefficient of variation; Height = 3.23 = 4.60 stem diameter and root length = 2.39).
For its part Pozo et al. (2015) report that some hormones control the first steps of the pre-symbiotic stage, while others regulate root morphological adaptations for the fungus to adapt and control colonization and functionality, these results contrast with those reported by Velasco et al. (2001), who points out that tomatillo plants height was higher in those grown with vermicompost and inoculated with Glomusintraradices, and by Alvarado et al. (2014) in tomato plants cv. Cid inoculated with Rhizophagus irregularis. Moreover salicylates, ethylene and cytokinins have negative effects in the early stages of penetration or root colonization by the fungus (Foo et al., 2013). In general, plant growth and mycorrhizal colonization increased when a substrate based on vermicompost was used, although this response was affected by the interaction with the type AMF spore inoculated.
Plants grown in vermicompost obtained greater root dry weight with inoculation from CN1, CN2 and in control plants (Figure 3A), while shoots (Figure 3B) and total (3C) was greater in plants inoculated only with CN1. CN1 and CN2 increased total biomass in plants developed with vermicompost, mainly due to increase in aerial biomass, which can be due to mycorrhizal fungi in combination with vermicompost increases photosynthetic activity, as reported by Velasco et al. (2001).

Figure 3 Effect of organic substrates and spores of mycorrhizal fungi inoculated on dry matter of root and aerial part of plants of tomato (Solanum lycopersicum L.) cv. Rio Grande. Ri = Rhizophagus irregularis, native consortium CN1 = 1; CN2= native 2 consortium; TEST = control. Bars indicate the standard error of the mean. (dry matter of roots, aerial and overall ANOVA substrates= p≤ 0.01, ANOVA fungi= p≤ 0.01, ANOVA yeast substrates= p≤ x 0.01 coefficient of variation; dry matter root = 2.21, aerial dry matter= 6 and matter total dry= 4.02).
Similar results were described in banana (Musa paradisiaca) clone Horton (Barrera et al., 2012) and in maize (Zea mays L.) (Zhu et al., 2012) as it obtained higher total dry matter with native mycorrhizae and commercial mycorrhizal respectively. Decreasing biomass accumulation in plants inoculated with native consortia and developed in peat can be due to these are receiving chemical fertilization, affecting the activity of mycorrhizal fungi; this coincides with those reported by Ortega-Larocea et al. (2008), who reported that mycorrhizal fungi are significantly affected when employed in high fertility conditions, as in the case of hydroponic crops.
N concentration in root showed no changes by effect of AMF spores inoculated in plants grown with vermicompost, while those grown in peat recorded higher concentration, when inoculated with Ri (Figure 4A). N concentration in shoot was higher in non-inoculated plants, whether developed in vermicompost or peat, although the effect was more pronounced in those developed in the latter (Figure 4B). P concentration in root and shoot was higher in those plants not inoculated, whereas in inoculated plants decreased regardless of the type of substrate, but this effect was more pronounced on the plants grown in peat (Figure 4C-D).
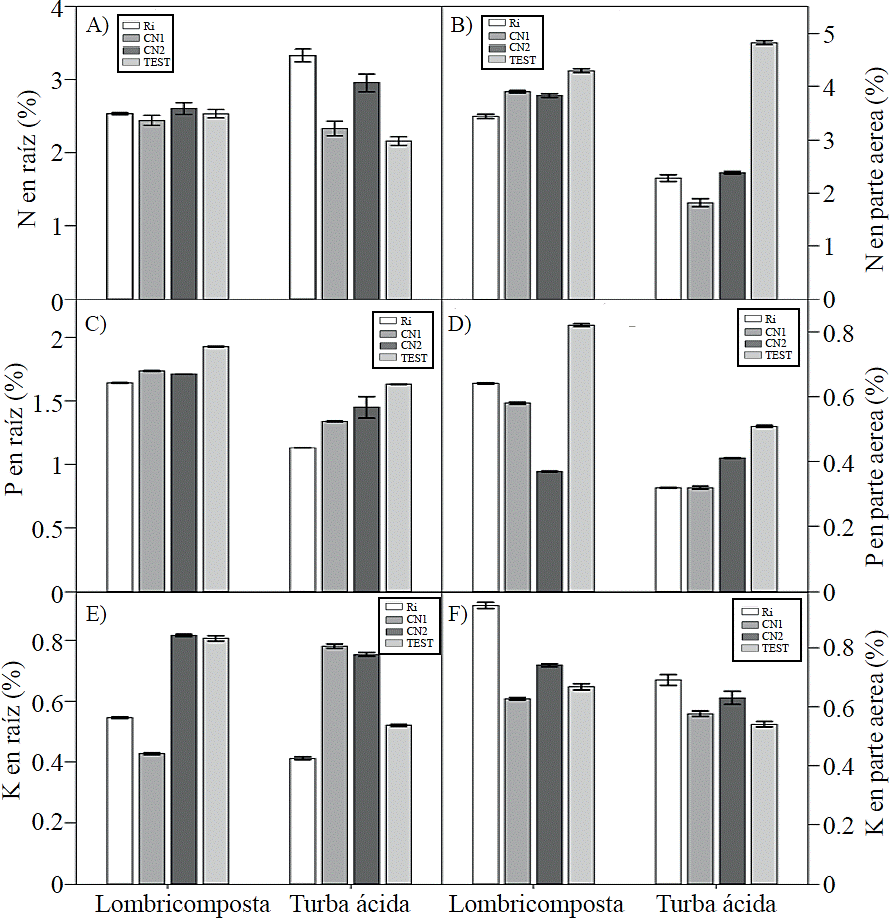
Figure 4 Effect of organic substrates and mycorrhizal fungi esporasde inoculated in the concentration of N, P and K in the roots and aerial parts of plants of tomato (Solanum lycopersicum L.) cv. Rio Grande. Ri = Rhizophagus irregularis, native consortium CN1 = 1; CN2 = native 2 consortium; TEST = control. Bars indicate the standard error of the mean. N root; ANOVA substrates p= ns, ANOVA yeast = p≤ 0.003, ANOVA substrates x = p≤ 0.01 fungi, aerial part N, P and K in root and aerial part; p≤ substrates ANOVA = 0.001, ANOVA fungi p≤ = 0.001, ANOVA substrates x = p≤ 0.001 fungi (ANOVA substrates = p≤ 0.001) and p aerial part (p≤ 0.01) (ANOVA fungi p≤ = 0.001, ANOVA substrates p≤ 0.001 x fungi = Coefficient of variation;. in root N = 12.48, N = 5.65 aerial part in root p = 1.05, p= 2.24 in aerial part, root K = 3.57 and K = 5.37 aerial part).
Mycorrhizal symbiosis favors the absorption of P and N, causing higher plant growth (Veresoglou et al., 2012; Velazco et al., 2001), which was confirmed in this study when tomato plants were inoculated with mycorrhizal fungi in combination with a substrate whose composition was based on vermicompost.
The decrease in root growth of plant developed in the substrate based on vermicompost could be due to a lower concentration of P and K in this organ, essential nutrients for its development (Ferrol and Perez-Tienda, 2009), while higher biomass accumulation in shoot may explain the decrease in the concentration of N and P due to a dilution effect. However, these results may also be due to vermicompost had an average contribution of P, as it has been reported that the application of high doses of this element, as well as N, have negative effects on the absorption of N, P, K , Fe, Mn and Zn in mycorrhized plants (Castro et al., 2009). In contrast with this result, Wang et al. (2008) note that in cucumber seedlings inoculated with three arbuscular micorrhizal fungi increased the concentration of N and P in roots and magnesium (Mg), copper and zinc in shoots.
A micorrhization between 20% and 35% negatively affected the height and the concentration of P and N in shoot, probably due to mycorrhizal plants have a negative effect on the synthesis of strigolactones, which is synthesized in the presimbiotic stage and it is able to inhibit stem branching (Gomez-Roldan et al., 2008) and affect root growth by inducing elongation and decreasing the number and length of root hairs (Ruyter-Spira et al., 2011).
The decrease in the concentration of P could be by effect of chemical fertilization, mycorrhizal fungi are affected by synthetic materials (Millaleo et al., 2006), plus phosphate as synthetic fertilizer should be previously hydrolyzed before being absorbed by the plant (Mäder et al., 2000); under this context it is possible to suggest that mycorrhizal fungi could act more efficiently on P forms of lower lability (Borie and Rubio, 2003), as well as organic P without the presence of synthetic substances. The decrease in N concentration due to mycorrhization results extremely interesting to note the effect of mycorrhizal fungi is not always beneficial, since plant response may vary depending on the degree of dependence between endophytes and the host plant as well as the degree of colonization (Ferrera-Cerrato and Alarcon, 2008). It has been indicated indicated that the variation of environmental conditions influencing plant physiology and therefore in mycorrhizal effectiveness, so it is likely that although mycorrhization is noted, not all fungal structures are active, affecting nutrient translocation (Varela and Estrada-Torres, 1997).
In plants grown with vermicompost K concentration increased in roots inoculated with CN2 and uninoculated (Figure 4E), while plants grown in peat the concentration of this nutrient was higher with inoculating spores from CN1 and CN2 (Figure 4E). In shoot K concentration increased in plants inoculated with Ri in both substrate types (Figure 4F).
The highest plant height was between 30 and 32% of mycorrhization (Figure 5A) and the highest concentration of P in shoot was with 15% of mycorrhization (Figure 5B); however, when the percentage of this was above 35% and 20%, respectively, both tended to decrease. Also, the increase in mycorrhization was associated with a decrease in the concentration of N (Figure 5C), while the concentration of K is higher with increasing percentage of mycorrhization (Figure 5D). So our results are consistent with Kalyanne-Fernandez (2010) and George et al. (1995).
Literatura citada
Adams, P. 2004. Aspectos de nutrición mineral en cultivos sin suelo en relación al suelo. In: tratado de cultivo sin suelo. Mundi-Prensa. 3a edición. Madrid, España. 95-104 pp. [ Links ]
Aira, M.; Monroy, F. and Domínguez, J. 2007. Eiseniafetida (Oligochaeta: Lumbricidae) Modifies thes tructure and physiologicalcapabilities of microbial communities improving carbon mineralization during vermicomposting of pig manure. Alemania. Microbial Ecol. 54(4):662-671. [ Links ]
Alvarado, C. M.; Díaz, F. A. y Peña del Río M. de los Á. 2014. Productividad de tomate mediante micorriza arbuscular en agricultura protegida. México. Rev. Mex. Cienc. Agríc. 5(3):513-518. [ Links ]
AOAC (Association of OficialAnalitical Chemists). 1980. Official methods of analysis. 13th edition.Washington. 1084 p. [ Links ]
Barrera-Violeth J. L.; Oviedo-Zumaque, L. E. and Barraza-Álvarez F. V. 2012. Evaluación de micorrizas nativas en plantas de plátano Hartón (Musa AAB Simmonds) en fase de vivero. Colombia. Acta Agronómica. 61(4):315-324. [ Links ]
Borie, F. and Rubio R. 2003. Total an organic phosphorus in Chilean volcanic soils. Chile. Gayana Botanica. 60(1):69-73. [ Links ]
Bremner; J. M. 1996. Total nitrogen. In: Sparks, D. L. (Ed.). Methods of soil analysis. Part II. Chemical. Methods. American Society of Agronomy. Soil Science Society of America. Madison, WI. USA. 1085-1086 pp. [ Links ]
Bucher, M.; Hause, B.; Krajinski, F. and Kuster, H. 2014. Through the doors of perception to function in Arbuscular mycorrhizal symbioses. Inglaterra. 204(4):833-840. [ Links ]
Caldera, E.; Acosta, K.; Garcés, G.; Petit, B.; Gutiérrez, W. and Pérez C. 2013. Response of a catatumbo variety bean crop (Vignaun guiculata L. Walp) to native and commercial mycorrhizal inoculant sunder controlled conditions. Venezuela. Redie luz. 3(1 y 2):157-164. [ Links ]
Castro, A.; Henríquez, C. y Bertsch, F. 2009. Capacidad de suministro de N, P y K de cuatro abonos orgánicos. Costa Rica. Agronomía Costarricense. 33(1):31-43. [ Links ]
Corkidi, L.; Allen, E. B.; Merhaut, D.; Alle, M. F.; Downer, J.; Bohn, J. and Evans M. 2004. Assessing the infectivity of commercial mycorrhizal inoculants in plant nursery conditions. Corea. J. Environ. Hort. 22(3):149-154. [ Links ]
Callejas-Ruiz, B. A.; Castillo-González, A. M.; Colinas-León, M. T.; González-Chávez, M. del C., Pineda-Pineda, J. and Valdez-Aguilar, L. A. 2009. Sustratos y hongos micorrízicos arbusculares en la producción de nochebuena. México. Revista Chapingo. Serie horticultura. 15(1): 57-66. [ Links ]
Carpio, A. L.; Davies, F. T. and Arnold, M. A. 2005. Arbuscularmycorrhizal fungi, organic and inorganic controlled-release fertilizers: effect on growth and leachate of container-grown bush morning glory (Ipomoea carnea ssp. fistulosa) under high production temperatures. Estados Unidos. J. Amer. Soc. Hort. Sci. 130(1):131-139. [ Links ]
Cruz, E.; Osorio, R.; Martínez, E.; Lozano del Río J.; Gómez, A. y Sánchez R. 2010. Uso de compostas y vermicompostas para la producción de tomate orgánico en invernadero. México. Interciencia. 35(5): 363-368. [ Links ]
Desgan, Y. H.; Kusvaran, S. and Ortas, I. 2008. Responses of soilless grown tomato plants to Arbuscular mycorrhizal fungal (Glomus fasciculatum) colonization in recycling and open systems. Sudafrica. Afr. J. Biotech. 7(20):3606-3613. [ Links ]
Duran, L. Y. and Henriquez C. 2010. El vermicompost: su efecto en algunas propiedades del suelo y la respuesta en la planta. Costa Rica. Agrono. Mesoam. 21(1):85-93. [ Links ]
Ferrol, N. and Pérez-Tienda, J. 2009.Coordinated nutrient exchange in arbuscular mycorrhiza. In: Azcón-Aguilar, C.; Barea J. M.; Gianinazzi, S.; Gianinazzi-Pearson, V. (Eds.). Mycorrhizas - functional processes and ecological impact. Springer-Verlag Berlin, Heidelberg. 73-87 pp. [ Links ]
Ferrera-Cerrato, R. and Alarcón, A. 2008. Biotecnología de los hongos micorrízicos arbusculares. In: Díaz, A. y Mayek, N. (Eds.). Biofertilización como tecnología sostenible. Plaza y Valdéz / CONACYT. México. 25-38 pp. [ Links ]
Finlay, R. D. 2008. Ecological aspects of mycorrhizal symbiosis: with special emphasis on the functional diversity of interactions involving the extraradical mycelium.Reino Unido. J. Exp. Bot. 59(5):1115-1126. [ Links ]
Foo, E.; Ross, J. J.; Jones, W. T.and Reid, J. B. 2013. Plant hormones in arbuscular mycorrhizal symbioses: an emerging role for gibberellins. Reino Unido Ann. Bot. 111(5):769-779. [ Links ]
Gerdemann, J. W. and Nicolson, H. T. 1963. Spores of mycorrhizal endogone species extracted from soil by wet sieving and decanting. Reino Unido. Transactions of the British Mycological Society. 46(2):235-244. [ Links ]
George, E.; Marschner, H. and Jackobsen I. 1995. Role of arbuscularmycorrhizal fungi in uptake of phosphorus and nitrogen form soil. Reino Unido. Critical Reviews in Biotechnology. 15(3-4): 257-270. [ Links ]
Gomez-Roldan, V.; Fermas, S.; Brewer, P. B.; Puech-Pagès, V.; Dun, E. A.; Pillot, J. P.; Letisse, F.; Matusova, R.; Danoun, S.; Portais, J. C.; Bouwmeester, H.; Bécard, G.; Beveridge, C. A.; Rameau, C. and Rochange, S. F. 2008.Strigolactone inhibition of shoot branching. Reino Unido. Nature. 455(1):189-194. [ Links ]
Gryndler, M.; Hrselová, H.; Sudová, R.; Gryndlerová, H.; Řezácová, V. and Merhautová, V. 2005. Hyphal growth and mycorrhiza formation by the arbuscular mycorrhizal fungus Glomus claroideum BEG23 is stimulated by humic substances. Alemania. Mycorrhiza. 15(7):483-488. [ Links ]
Hernández, M. y Chailloux, M. 2004 Las micorrizas arbusculares y las bacterias rizosféricas como alternativa a la nutrición mineral del tomate. Cuba. Cultivos tropicales. 25(2): 5-12. [ Links ]
Jargeat, P.; Cosseau, C.; Ola'h, B.; Jauneau, A.; Bonfante, P.; Batut, J. and Bécard, G. 2005 Isolation, freeliving capacities, and genomestructure of ‘Candidatus Glomeri bactergigas porarum’, the endocellular bacterium of the mycorrhizal fungus Gigaspora margarita. Estados Unidos. J. Bacteriol. 186(20): 6876-6884. [ Links ]
Koltai, H.; Dor, E.; Hershenhorn, J.; Joel, D. M.; Weininger, S.; Lekalla, S.; Shealtiel, H.; Bhattacharya, C.; Eliahu, E.; Resnick, N.; Barg, R. and Kapulnik, Y. 2010. Strigo actones' effect on root growth and root-hair elongation may be mediated by auxin-efflux carriers. EstadosUnidos. J. Plant Growth Reg. 29(2): 129-136. [ Links ]
Koltai, H. and Kapulnik. Y. 2010. Arbuscular micorrhyzas: physiology and function. Second Edition springer, London New York, US. 323 p. [ Links ]
Kalyanne-Fernández, M. C. 2010. Micorrización in vitro e in vivo de plántulas de papa (Solanum tuberosum var. Alfa). Cuba. Cultivos Tropicales. 31(2): 21-31. [ Links ]
Lumini, E.; Bianciotto, V.; Jargeat, P.; Novero, M.; Salvioli, A.; Faccio, A.; Bécard, G. and Bonfante, P. 2007. Presymbiotic growth andsporal morphology are affected in the arbuscular mycorrhizal fungus Gigaspora margarita cured of its endobacteria. Reino Unido. Cellular Microbiology. 9(7):1716-1729. [ Links ]
Mäder, P.; Edenhofer, S.; Boller, T.; Wiemken, A. and Niggli, U. 2000. Arbuscular mycorrhizae in a longtermfield trial comparing low-input (organic, biological) and high-input (conventional) farming systems in a crop rotation. Italia. Biol. Fertil. Soils. 31(2):150-156. [ Links ]
Millaleo, M. R.; Montecinos, U. C.; Rubio, H. R.; Contreras, N. A. y Borie, B. F. 2006. Efecto de la adición de compost sobre propágulos micorrícicos arbusculares en un suelo volcánico del centro sur de chile. Chile. J. Soil Sc. Plant. Nutr. 6(3):26-39. [ Links ]
Moreno-Reséndez, A. L.; Gómez-Fuentes, P.; Cano-Ríos, V.; MartínezCueto, J. L.; Reyes-Carrillo, J. L.; Puente-Manríquez, L. and Rodríguez-Dimas, N. 2008. Genotipos de tomate en mezclas de vermicompost: arena en invernadero. México. Terra Latinoam. 26(2):103-109. [ Links ]
Nelson, J. C. y Nelson, J. M. A. 2015.Uso y manejo de hongos micorrízicos arbusculares (HMA) y humus de lombriz en tomate (Solanum lycopersicum L.), bajo sistema protegido. Cuba. Cultivos Tropicales. 36(1):55-64. [ Links ]
Ortega-Larrocea, M. P.; Morales, V. J. A. y García, S. R. 2008. Cultivos monospóricos de hongos Micorrízicos arbusculares. In: Álvarez- Sánchez, J. Monroy, A. A. (Comps.). Técnicas de estudio de las asociaciones micorrízicas y su implicación de la restauración. Distrito Federal, México. 69-83 pp. [ Links ]
Oseni, T.O.; Shongwe, N. S. and Masarirambi, M. T. 2010. Effect of arbuscular mycorrhiza (AM) inoculation on the performance of tomato nursery seedlings in vermiculite. Pakistan. Int. J. Agr. Biol.12: 789-792. [ Links ]
Phillips, J. M. and Hayman, D. S. 1970. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Reino Unido. Transactions of the British Mycological Society. 1970; 55(1): 158-161. [ Links ]
Pretty, J. 2008. Agricultural sustainability: concepts, principles and evidence. Reino Unido. Phil. Trans.R. Soc. B. 363:447-465. [ Links ]
Pinheiro, N. C.; Tavares, H. O. C.; Figueira, T. J. R.; Saggin, J. O. J.; Coutinho, F. H. M. A and Louro, B. R. L. 2013. Biostimulation of inoculation with Glomus proliferum and application of humic acid in the in vitro growth of Lunula riacruciata. Brasil. Acta Botánica Brasilica. 27(4):773-778. [ Links ]
Pozo, M. J.; López-Ráez, J. A.; Azcón-Aguilar, C. and García-Garrido, J. M. 2015. Phytohormones as integrators of environmental signals in the regulation of mycorrhizal symbioses. Reino Unido. New Phytologist. 205(4): 1431-1436. [ Links ]
Ruyter-Spira, C.; Kohlen, W.; Charnikhova, T.; van Zeijl, A.; van Bezouwen, L.; de Ruijter, N.; Cardoso, C.; López-Ráez, J. A.; Matusova, R.; Bours, R., Verstappen, F. and Bouwmeester, H. 2011. Physiological effects of the synthetic strigolactone analog GR24 on root system architecture in Arabidopsis: Another below ground role for strigolactones?. Estados Unidos. Plant Physiology. 155(2):721-734. [ Links ]
Steiner, A.A. 1961. A universal method for preparing nutrient solutions of a certain desired composition. Australia. Plant Soil. 15(2):134-154. [ Links ]
Sieverding E. 1983. Manual de métodos para la investigación de la micorriza vesículo-arbuscular en ellaboratorio. Ciat. PalmiraCali, Colombia. 120 p. [ Links ]
Trejo, D.; Ferrera-Cerrato, R.; García, R.; Varela, L. y Alarcón, A. 2011. Efectividad de siete consorcios nativos de hongos micorrícicos arbusculares en plantas de café en condiciones de invernadero y campo. Chile. Revista Chilena de Historia Natural. 84(1):23-31. [ Links ]
Varela, L. y Estrada-Torres, A. 1997. El papel de los microorganismos de la rizósfera y de la micorriza en la absorción de nutrimentos minerales y agua. In: Orellana, R.; Escamilla, J. A.; Larqué- Saavedra, A. (Eds.). Ecofisiología vegetal y conservación de recursos genéticos. Yucatán, México. 222 p. [ Links ]
Veresoglou, S. D.; Chen, B. and Rillig, M. C. 2012. Arbuscular mycorrhiza and soil nitrogen cycling. Australia. Soil Biology and Biochemistry. 46:53-62. [ Links ]
Velasco, V. J.; Ferrera, C. R. and Almaraz, S. J. J. 2001. Vermicomposta, micorriza arbuscular y Azospirillum brasilense en tomate de cáscara. México. Terra Latinoam. 19(3):241-248. [ Links ]
Wang, C.; Li, X.; Zhou, J.; Wang, G. and Dong, Y. 2008. Effects of arbuscular mycorrhizal fungi on growth and yield of cucumber plants. Reino Unido. Comm Soil Sci Plant Anal. 39(3-4):499-509. [ Links ]
Zhu, X. C.; Song, F. B.; Liu, S. Q.; Liu, T. D. and Zhou. X. 2012. Arbuscular mycorrhizae improves photosynthesis and water status of Zea mays L. under drought stress. Estados Unidos. Plant Soil Environ. 58(4)186-191. [ Links ]
Received: August 2015; Accepted: November 2015











 texto en
texto en 



