Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista mexicana de ciencias agrícolas
versión impresa ISSN 2007-0934
Rev. Mex. Cienc. Agríc vol.6 spe 12 Texcoco nov./dic. 2015
Articles
Effect of alkamides as tolerance inductors to biotic stress in tomato
1Universidad Autónoma Agraria Antonio Narro- Departamento de Horticultura. Cátedras CONACYT.
2Universidad Autónoma Agraria Antonio Narro-Departamento de Ciencias Básicas.
3Universidad Autónoma Agraria Antonio Narro-Departamento de Parasitología Agrícola. Calzada Antonio Narro 1923. Buenavista Saltillo, Coahuila C. P. 25315. Tel: 844 4110303. (sgonzalezmo@conacyt.mx; emlaugaren@gmail.com; edmundo. rdz@uaaan.mx: aflooli50@gmail.com).
The aim of this study was to determine the histological effects and those related to vigor with the application of an extract of Heliopsis longipes on tomato seedlings inoculated with Fusarium oxysporum f. sp. lycopersici (FOL). H. longipes extract was sprayed at a concentration of 300 mgL-1 in tomato seedlings subsequently inoculated with FOL by root immersion. Twenty days later incidence and severity data were taken, finding that the application of the extract caused a decrease of 15% and 80% respectively for these variables whereas for plant height there was an increase of 14.3%. In root length and dry weight of leaves and stem there was a decrease in the results; however, these values were higher than those obtained for plants inoculated with FOL. Histological changes were found by effect of FOL infection as well as for extract application, which consisted in modifying the distribution of vascular bundles in root, as well as increasing the relative area of root and stem cortex and reducing the number of xylem vessels in root. These results indicate that the application of the extract has an effect on wilting symptoms caused by FOL.
Keywords: affinin; Heliopsis longipes; Fusarium oxysporum f. sp. Lycopersici and Solanum lycopersicum
El objetivo de este estudio fue conocer los efectos histológicos y los relacionados a vigor con la aplicación del extracto de Heliopsis longipes sobre plántulas de tomate inoculadas con Fusarium oxysporum f. sp. lycopersici (FOL). El extracto de H. longipes se asperjó a una concentración de 300 mgL-1 en plántulas de tomate, posteriormente se realizó la inoculación con FOL por inmersión de raíces. Veinte días después se tomaron los datos de incidencia y severidad, encontrándose que la aplicación del extracto provocó una disminución de 15% y 80% respectivamente para estas variables mientras que para altura de la planta se presentó un incremento de 14.3%. En longitud de raíz y peso seco de hojas y tallo se observó un decremento en los resultados; sin embargo, estos valores estuvieron por encima de los obtenidos para plantas inoculadas con FOL. Se encontraron cambios histológicos ocurridos por la infección de FOL así como por la aplicación del extracto, que consistieron en la modificación de la distribución de los haces vasculares en la raíz, así como en el incremento del área relativa del córtex de raíz y tallo y la disminución del número de vasos del xilema de la raíz. Estos resultados indican que la aplicación del extracto tiene un efecto sobre los síntomas de marchitamiento causados por FOL.
Palabras claves: afinina; Heliopsis longipes; Fusarium oxysporum f. sp. Lycopersici y Solanum lycopersicum
Introduction
Tomato is one of the most important crops from an economic and productive point of view. It has highlighted in recent years by the constant incorporation of technologies for its production (Rabie and Humiany, 2004). Within the production technologies of this vegetable are the use of chemicals for disease control, because yields are largely affected by the incidence of diseases caused by soil fungi (Jiménez and Sanabria, 2008).
One of the most important diseases affecting this crop is vascular wilt caused by Fusarium oxysporum f. sp. lycopersici (FOL), causing 60% decrease in yield and affecting product quality (Cai et al., 2003; Srivastava et al., 2010). Three races of the pathogen are recognized for this disease, which distinguish by their virulence on differential materials of tomato and contain different resistance genes (Masato et al., 2005). Resistant cultivars are so far the best control strategy, as these pathogens remain in the soil for decades (Reis et al., 2004).
In their need to counter the attack of pests and maximize production, modern agriculture is highly dependent on inputs and synthetic pesticides. This has brought enormous problems for animal and human health with a growing environmental impact. A potential method to reduce the severity of diseases caused by pathogens is the induction of plant resistance (Baysal et al., 2003). This activity is associated with numerous defense responses activated by the host after the contact with the pathogen (Flores et al., 2009) or with any biotic or abiotic inductor (Valueva and Mosolov, 2004).
Among the variety of metabolites studied are the alkamides that are widely distributed in different plant families, being in higher amount in roots of Heliopsis longipes (common name Chilcuague), being affinin (N-isobutyl-2E, 6Z, 8E -decenamide) its main bioactive constituent and the molecule responsible for the main biological effects (Molina and García, 2001; Morquecho and López, 2007).
In plants it has been shown that these molecules play an important role in regulating morphogenesis and adaptation processes (López et al., 2006), just as has been expressed in vitro assays where affinin promotes growth of Arabidopsis thaliana seedlings, as its importance in signaling and cell proliferation, it is also mentioned its role in reshaping the architecture of roots and aerial part through DHM1 proteins (Pelagio et al., 2013).
Therefore, the objective of this research was to observe the effect of spraying the ethanol extract of H. longipes on tomato seedlings inoculated with FOL, measuring the degree of severity of the disease, variables related to vigor and histological changes in stems and roots associated to tolerance response to FOL in plant.
Materials and methods
Fusarium oxysporum f. sp. lycopersici. The strain used in this study was isolated from roots of diseased tomato plants obtained in the area of Silao, Guanajuato, Mexico (González et al., 2011).
H. longipes extract. The extract was prepared from dried and ground roots of this plant, collected in Sierra Gorda, Guanajuato. To obtain it, a ratio 1:10 was used this is 1 g of macerated root per each 9 ml of solvent, which was 98% ethanol. The extract was obtained by immersing the macerated tissue in the solvent at room temperature, maintaining it for 30 days. The crude extract was filtered with Whatman filter paper no. 1 and concentrated in a Rotavapor (IKA). Determining the concentration of affinin was conducted by HPLC according to Ramírez et al. (2000).
In vivo testing. It was conducted in tomato seedlings from 5 to 6 true leaves of the variety Rio Grande, sown in plastic trays with peat: perlite at a ratio 1:1. The test was divided into six treatments: absolute control (TA), control infested with FOL (FOL), plants sprayed with extract of H. longipes (HL), plants sprayed with extract of H. longipes and inoculated with FOL (HL + FOL), plants sprayed with a commercial product recommended to induce systemic resistance in plants based on salicylic acid, which was denominated as positive control (C+) and plants sprinkled with positive control and inoculated with FOL (C+ + FOL).
For its application the ethanol extract of H. longipes was standardized to provide 300 mg L-1 of affinin, adding 150 ml to each plant. The commercial positive control (the active ingredient is salicylic acid, and is a product recommended to induce resistance to biotic stress in plants) was applied at a concentration of 1.25 ml L-1 water adding 150 ml to each plant both treatments were applied by foliar spray.
Four days after the application of ethanol extract H. longipes, plants were inoculated with treatments FOL, HL + FOL and C ++ FOL, using the technique of root immersion in a spores solution of FOL 106 spores/ml for 15 minutes (Benhamou et al., 1998).
Twenty days after inoculation, severity and incidence data were taken for this variable using a scale reported by Diener and Ausubel, 2005; as well as samples to measure dry weight of roots, stem and leaves, plant height and root length using 20 replications per treatment.
Diener’s scale is defined: 0 Dead plant (100%); 1 dead old leaf and young leaves stunted (80%); 2 old chlorotic leaves and stunted young leaves (60%); 3 old leaves with vascular chlorosis and stunted young leaves (40%); 4 leaf petioles with stunted growth (20%); 5 without visible symptoms (0%).
Histopathological study. Samples for histopathological tests were collected twice, counting each time with 10 replications per treatment. The first sampling took place two days after application of the extract H. longipes and the second to three days after inoculation with FOL corresponding to six days after application of the extract. For this analysis the sample were dehydrated followed by paraffin embedding, microtome sectioning and safranin-fast green staining was performed (Hernández, 1984; Fuentes et al., 2005; Rivero et al., 2007). Samples were analyzed in a light microscope (Carl Zeiss) with a camera and were observed at 4 and 10 (x) using Pixera Wiewfinder Pro program. Measurements of total area of the lumen from xylem, cortex area, marrow and cell wall thickness were made with AxioVision LE Rel. 4.5 program. The xylem vessels count were made taking into account a quarter of the image per sample.
Statistical analysis. The experimental design was completely randomized, considering one plant as experimental unit, using 20 replicates per treatment to evaluate incidence, severity, plant height, root length and dry weight of root, stem and leaves. While for analyzing relative cortex area, cell wall thickness, in root and stem, considering 10 replications per treatment, for the variable number of xylem vessels root four replicates per treatment were considered. The data was subjected to an ANOVA test and mean comparison test Tukey (p> 0.05) using the statistical software Minitab 16.
To corroborate the assumptions of the statistical model was conducted a residual analysis of variable lumen area from root and stem xylem, resulting in the non-normality of the data, which is why transformation of Box --COX was performed, by which graphs in Figure 9 A and B are from the transformed data (Y) of these variables.
Results
Incidence and severity. The values obtained for incidence (Figure 1) show that spraying tomato plants with H. longipes extract had a positive effect by reducing 15% the incidence of wilt on plants sprayed with H. longipes (HL + FOL) regarding to FOL. This was also observed when evaluated the severity of symptoms caused by FOL (Figure 1), where a significant difference was found, which decreased about 80% with the application of the extract (HL + FOL) and using the positive control (C + + FOL) regarding to the infested control (FOL).
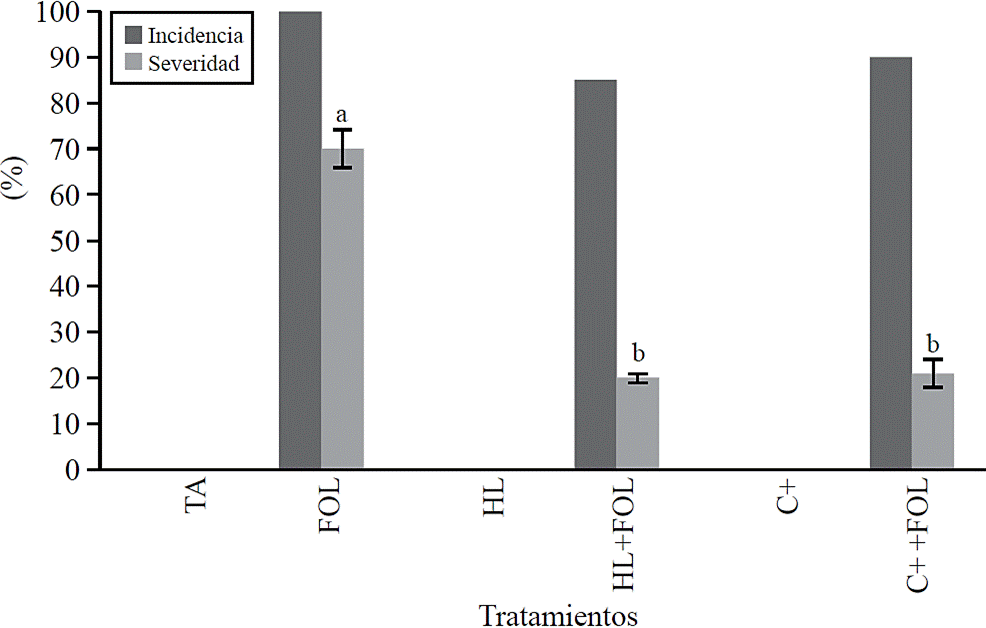
Figure 1 Effect of applying H. longipes extract in the incidence and severity of tomato seedlings. Means with the same literal are not statistically different (Tukey, p> 0.05).
Fungi pathogens, from genus Fusarium, are some of the agents causing root and basal stem deterioration, resulting in plant wilting (Ignjatov et al., 2012). For this study the use of the extract of H. longipes did not inhibit wilt by FOL; however, it decreased the severity of its symptoms; this is consistent with Molina et al. (2004) who suggest that affinin works as a potential inductor factor of defense, as in A. thaliana leaves stimulated resistance to B. cinerea as a result of physiological changes, as well as activation of genes related to development and defense related to stress, through the accumulation of jasmonic acid (Méndez et al., 2011).
Growth variables. The variables plant height and root length showed significant differences between treatments (Figure 2), observing an increase of 56% in plant height with the application of H. longipes extract and 14.3% when the extract was sprayed on tomato plants inoculated with FOL, for root length the increase with the use of the extract accounted for 84% and when applied to plants infected with FOL a decrease in this variable 41% was present regarding absolute control.
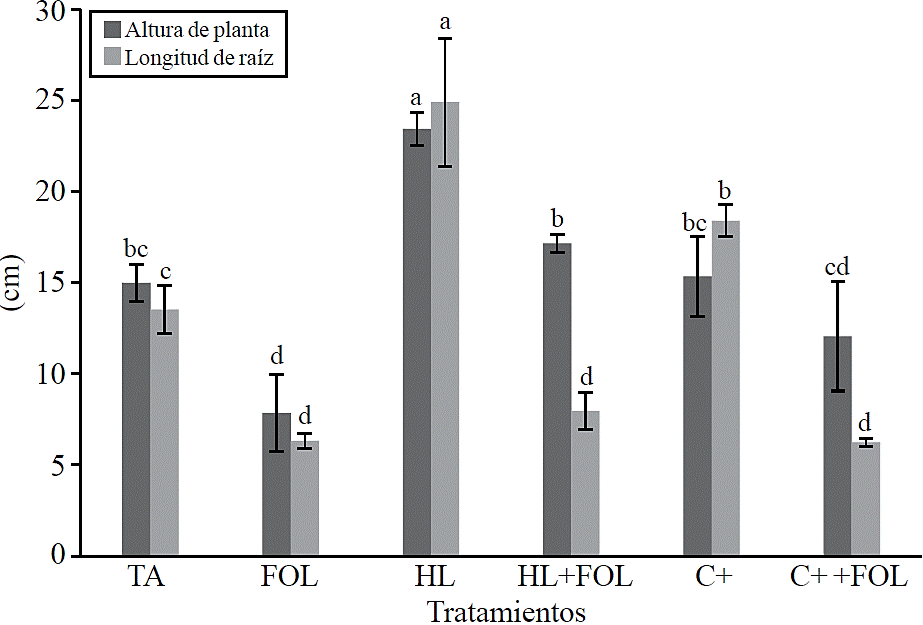
Figure 2 Effect of the application of the extract of H. longipes in plant height and root length of tomato seedlings. Means with the same literal are not statistically different (Tukey, p> 0.05).
Significant differences for dry weight of leaves, stems and roots (Figure 3) were found, observing increases of up to 185%, 177% and 503% respectively when plants were subjected to foliar application of H. longipes, contrary effect was found when the extract was applied to plants inoculated with FOL, showing a decrease of 27%, 18.5% and 17.6% in dry weight values of leaf, stem and root; however, this decrease was lower than that obtained in plants inoculated with FOL, which had about 60% reduction in these variables. The effect of the extract H. longipes on biomass development of leaves, stem and root is attributed to the biological action of alkamides present in the extract, especially affinin. López (2007) indicates that for their physiological and cellular effects, alkamides are capable of altering different aspects of plant life and has been suggested to be considered as growth regulator or plant hormone. This effect coincides with that reported by Campos et al. (2008) who found that alkamides alter the system, root architecture and shoots; it also affects biomass productivity according to the applied dose.
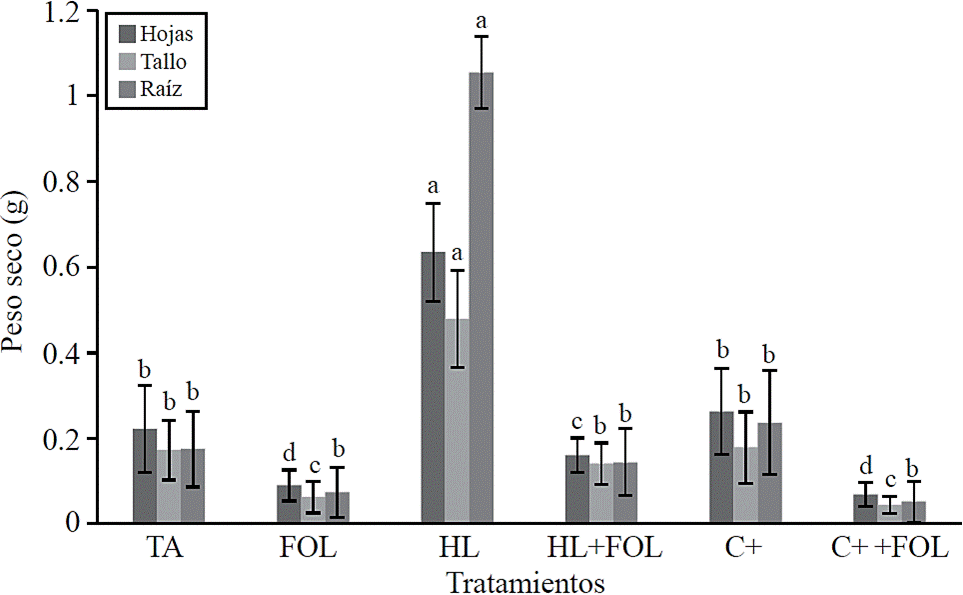
Figure 3 Effect of applying the H. longipes on the dry weight of leaf, stem and root tomato seedlings. Means with the same literal are not statistically different (Tukey, p> 0.05).
Histological changes. In photomicrographs made to the root structure (Figure 4) the absolute control (A), H. longipes (B) and the commercial product (C), there was a difference in stele or vascular cylinder organization, where an alteration is appreciated in the distribution of vascular bundles when the extract of H. longipes was applied. Furthermore, in photomicrographs from treatments inoculated with FOL (D), it can be seen a change in stele presenting a difference in the distribution of vascular bundles and the presence of a darker color in staining vascular bundles; which could be associated to necrosis of these tissues caused by FOL. In the treatment extract application to plants inoculated with FOL (E) and the application of the commercial product to plants inoculated with FOL (F) shows changes in distribution within the vascular cylinder.
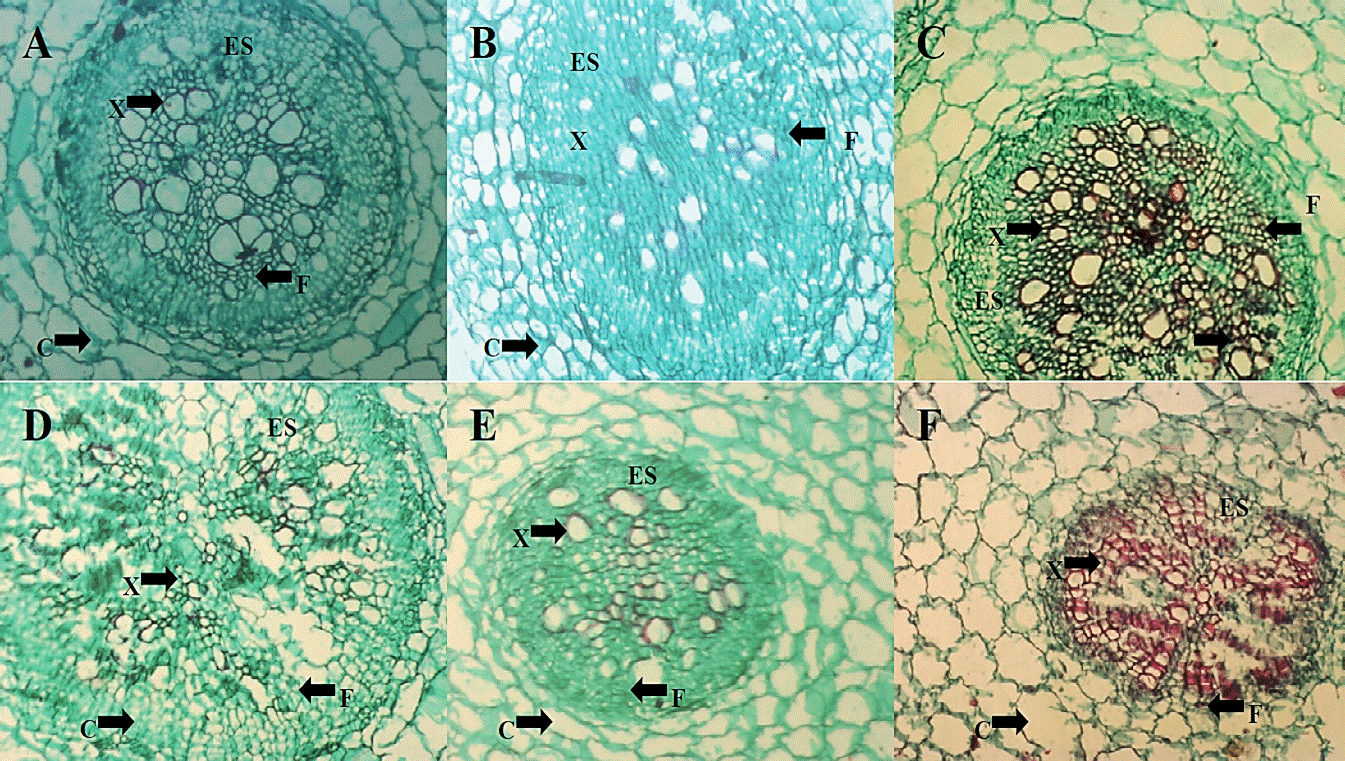
Figure 4 Photomicrographs of cross-sections of tomato seedling root (4x) of the TA treatment (A), HL (B) and C + (C) two days after application of the extract, as well as treatments FOL (D), HL + FOL (E) and C ++ FOL (F) at six days after application of the treatments, showing differences in cell organization. C (cortex), ES (wake) X (xylem), F (phloem).
For stem (Figure 5) there were no qualitative changes with the application of treatments compared to absolute control. Comparing photomicrographs from control (A and D) in the two evaluation dates there were no changes in the structure perhaps due to this time interval the fungus has not colonized yet this tissue.
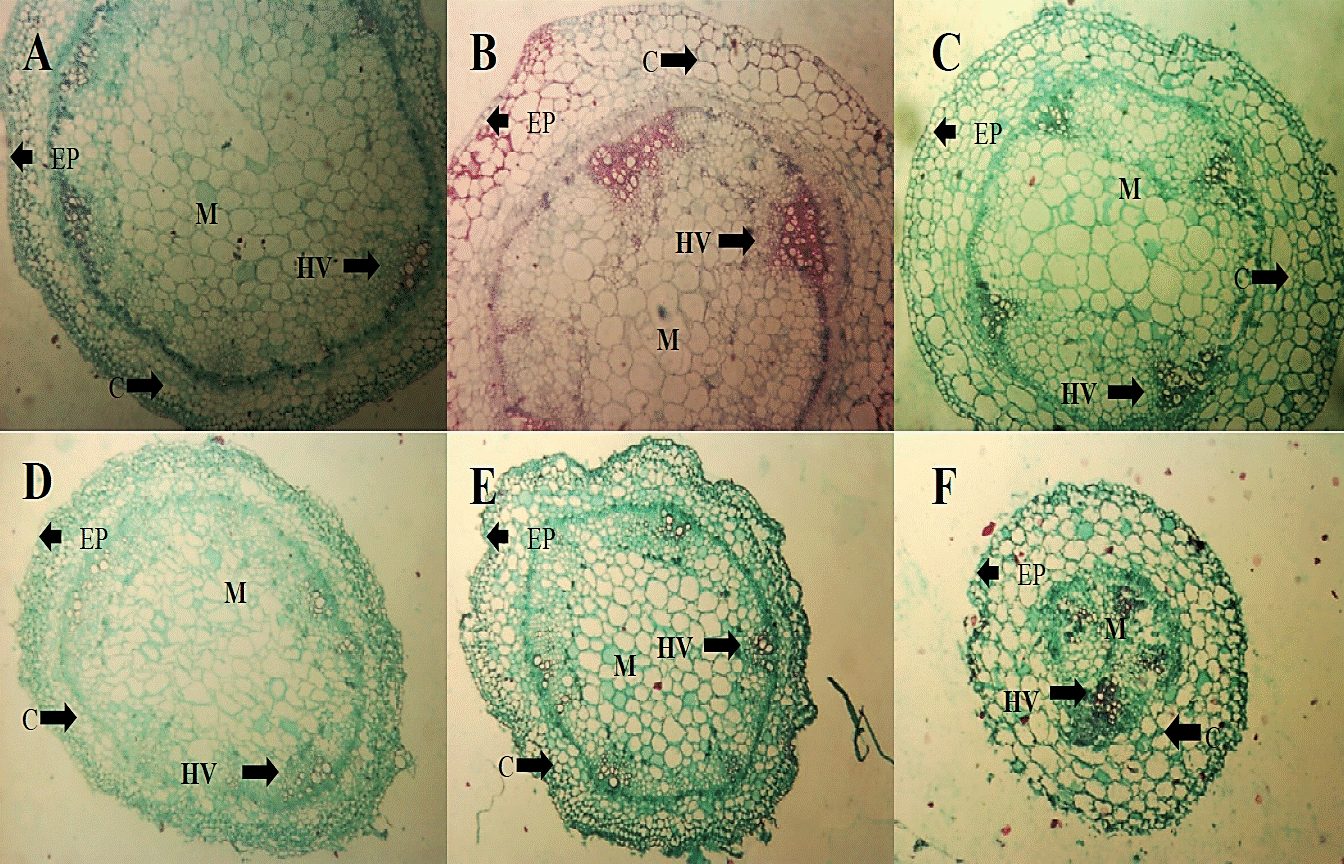
Figure 5 Micrographs of cross sections of tomato seedlings stalk (2.5 x) TA (A), HL (B) and C + (C) treatments within two days of the application of the extract, as well as treatments FOL (D), HL + FOL (E) and C ++ FOL (F) to 4X six days after application of the treatments, showing differences in cell organization. C (cortex), EP (epidermis), M (bone) and HV (vascular bundles).
Quantification of histological variables. In consideration to the relative area occupied by the cortex, it was observed that root samples (Figure 6A) evaluated in the second day of the application of the extract H. longipes showed a significant difference, which reflected in a decrease of 7% when the extract is sprayed and 2% when plants were treated with C +, in contrast during the second treatment evaluation showed increases in this variable corresponding to 174% with spraying H. longipes, 217% with the use of positive control, 67% in plants inoculated, 173% in those sprayed with extract and inoculated and 178% in plants sprayed with positive control and inoculated.
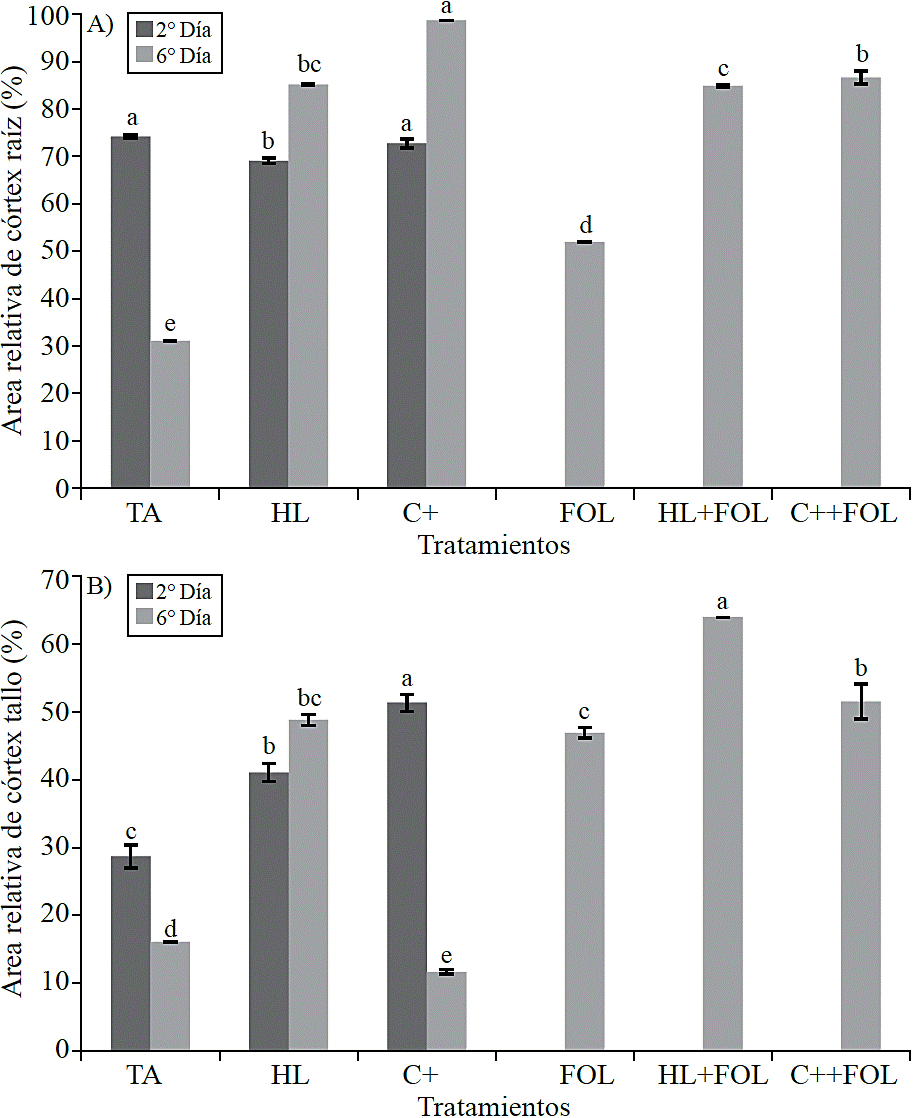
Figure 6 Effect of applying the H. longipes on the relative area of root cortex samples (A) and stem (B) of tomato seedlings for two evaluation dates. Means with the same literal are not statistically different (Tukey, p> 0.05).
For the relative cortex area of the stem there were significant differences (Figure 6B), presenting increases of 43% and 79% with the application of the extract and positive control. A similar effect was observed during the second evaluation date where the increases were 203% for plants sprayed with the extract, 192% in plants inoculated, 298% for those plants treated with H. longipes and infected with FOL and 220% when the commercial product was used, while when this was applied to non-inoculated plants a 27% decrease was observed in this variable.
The effect in the increase of relative cortex area with the application of H. longipes could be beneficial for the plant, since this thickening would delay the entry of the pathogen into the vascular cylinder, thereby preventing the rapid spread of the disease, since the vascular system is affected for being obstructed by FOL, preventing water and nutrients flow through it, which results in vascular wilting of the plant. Pharand et al. (2002) studied root samples of tomato plants treated with BTH (benzothiadiazole) and found evidence that the growth of the fungus (F. oxysporum f. sp. radicis-lycopersici) occurs mainly in the epidermis and in the outside of the cortex , so this effect could be similar to that reported in Silene vulgaris plants when grown under drought and stress, since a thicker cortex is important to improve the resistance to dehydration, because in this way, the internal tissues to endoderm are relatively protected against dehydration at the expense of the cortex.
By assessing the number of xylem vessels of the root showed significant differences (Figure 7), where the first evaluation found that the application of the commercial product increased the number of xylem vessels at 47% compared to absolute control, while in plants sprayed with the extract of H. longipes showed a decrease in this variable corresponding to 41%. For the second evaluation fewer xylem vessels were observed for all treatments compared to control, being the treatment with the commercial product which had 24% less.
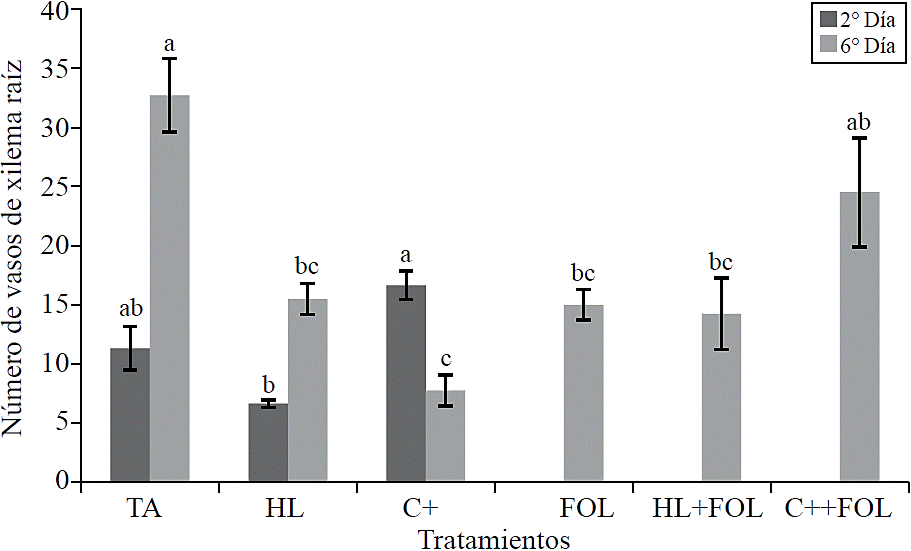
Figure 7 Effect of applying H. longipes extract on the number of xylem vessels in samples of tomato seedlings following two evaluation dates. Means with the same literal are not statistically different (Tukey, p> 0.05).
No statistical differences were found in the thickness of the cell wall of the xylem vessels (Figure 8) and the lumen area (Figure 9) corresponding to root and stem structure for none of the applied treatments.
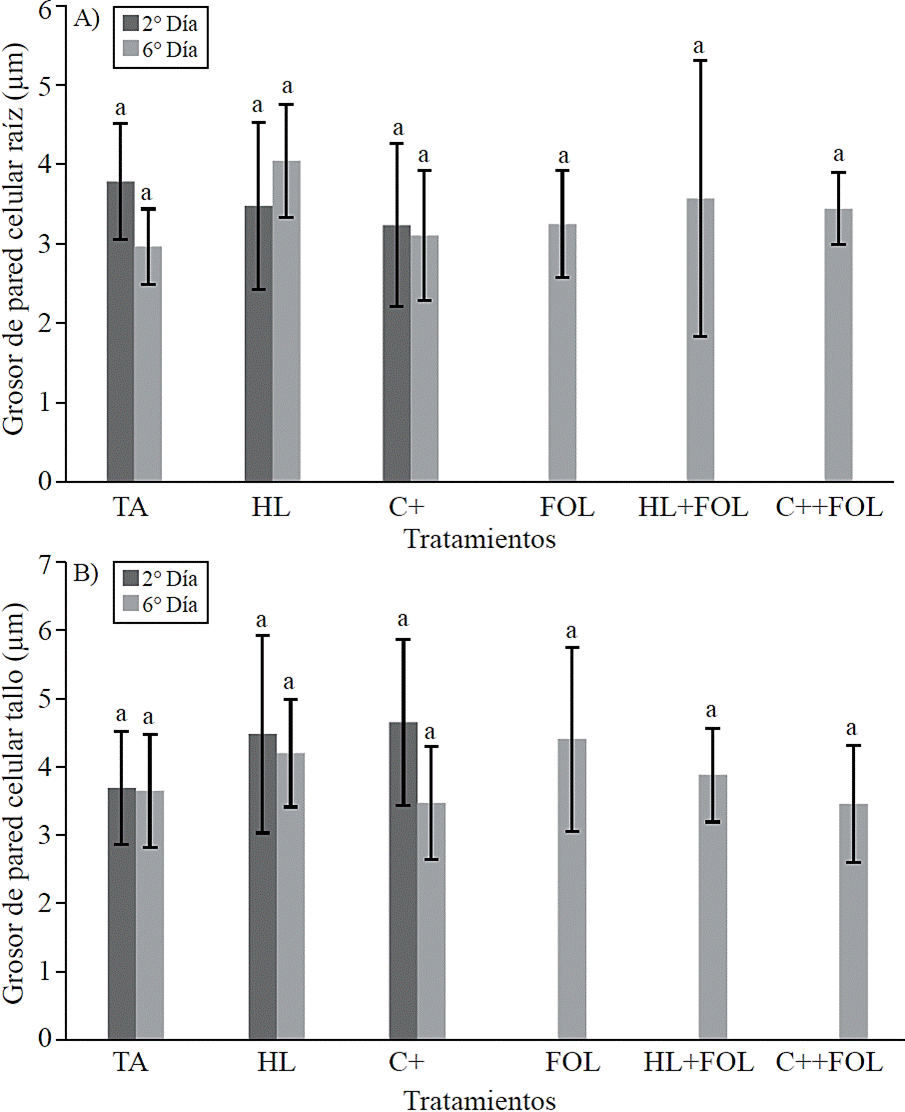
Figure 8 Effect of application of H. longipes extract on cell wall thickness of xylem Root samples (A) and stem (B) of tomato seedlings tested for two evaluation dates. Means with the same literal are not statistically different (Tukey, p> 0.05).
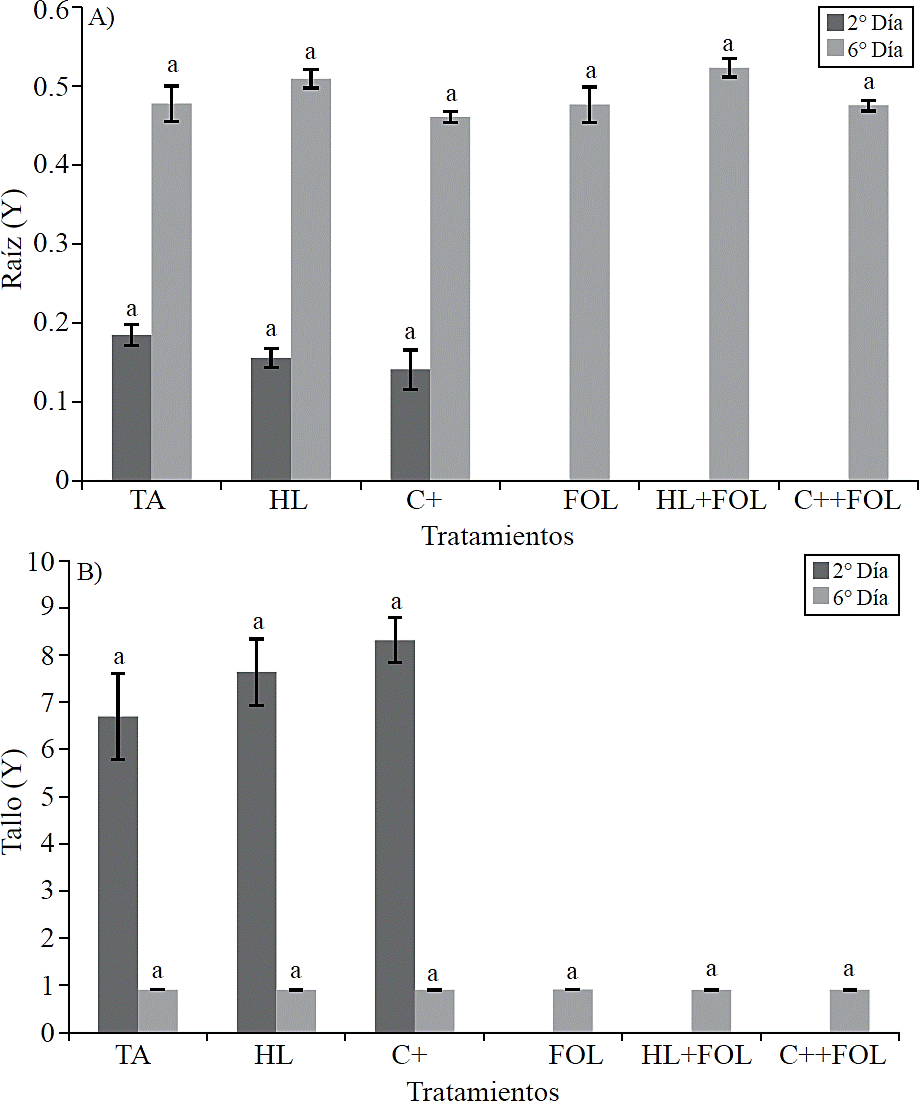
Figure 9 Data processed (Y) values obtained from the study of the application of the extract of H. longipes on the lumen area of root samples (A) and stem (B) of tomato seedlings for two testing dates. Means with the same literal are not statistically different (Tukey, p> 0.05).
Plants infected with F. oxysporum showed necrosis in xylem vessels, which can lead to possible alterations in carbohydrates distribution and causes difficulties in water transport, which leads to wilt symptoms (Norueg, 1983). It has been reported that Fusarium species cause wilt following a similar pattern of infection; penetrate through root and colonize the stem of the plant's vascular system (Turlier et al., 1994). Colonization is restricted to both resistant and susceptible cultivars to the region as initial entry of the pathogen, due to vessel occlusion by gels, callose and tylose deposition (Takken and Rep, 2010); however, in this study no structural barriers associated to passive defense response of plants related to the application of elicitor molecules were observed, this coincides with that described by Araujo et al. (2008), who evaluated banana plants inoculated with F. oxysporum f. sp. cubense treated with fungicides and plant extracts, who found no changes in the conducting tissues of root and stem, which was attributed to the products used, which did not allow these changes or the pathogen did not favor their formation; or it could be that continuous colonization promoted that gels calloses were degraded by effect of pectolytic enzymes from the pathogen and tyloses growth was inhibited (González et al., 2012).
The effect of H. longipes and C+ on the number of xylem vessels may have a positive role for the plant reducing xylem vulnerability against cavitation caused by the attack of pathogens that affect the vascular system (Sobrado, 2007; Pouzoulet et al., 2014).
Conclusions
The application of H. longipes extract reduces the severity of wilt symptoms in tomato seedlings inoculated with F. oxysporum f. sp. lycopersici. Similarly, plants treated with the extract exhibit a growth promoting effect, increasing height, root length and total biomass of the plant, besides morphological changes in root and stem on the cortex area and marrow. So the H. longipes extract could act inducing the defense system in tomato plants, besides its favorable phytohormone activity, promoting plant growth.
Literatura citada
Araujo, D.; Rodríguez, D. y Sanabria, M. E. 2008. Respuesta del hongo Fusarium oxysporum f. sp. cubense, causante del Mal de Panamá a algunos extractos vegetales y fungicidas. Venezuela. Fitopatología Venezolana. 21(2):2-8. [ Links ]
Baysal, Ö.; Soylu, E. M. and Soylu, S. 2003. Induction of defence-related enzymes and resistance by the plant activator acibenzolar-Smethyl in tomato seedlings against bacterial canker caused by Clavibacter michiganensis ssp. michiganensis. Reino Unido. Plant Pathol. 52(6):747-753. [ Links ]
Benhamou, N. and Belanger, R. 1998. Benzothiadiazole-mediated induced resistance to Fusarium oxysporum f. sp. radicis-lycopersici in tomato1. Estados Unidos. Plant Physiol. 118(4):1203-1212. [ Links ]
Cai, G.; Gale, I. R.; Scheider, R. W.; Kistler, H. C.; Davis, R. M.; Elias, K. S. and Miyao, E. M. 2003. Origin of race 3 of Fusarium oxysporum f. sp. licopersici at a single site in California. Estados Unidos. Phytopathology. 93(8):1014-1022. [ Links ]
Campos, C. J. C.; Pelagio, F. R.; Raya, G. J.; Méndez, B. A.; Ortiz, C. R. and López, B. J. 2008. Tissue culture of Arabidopsis thaliana explants reveals a stimulatory effect of alkamides on adventitious root formation and nitric oxide accumulation. Irlanda. Plant Sci. 174(2):165-173. [ Links ]
Diener, A. C. and Ausubel, F. M. 2005. Resistance to Fusarium oxysporum 1, a dominant arabidopsis disease-resistance gene, is not race specific. Estados Unidos. Genetics. 171(1):305-321. [ Links ]
Flores, J. B.; Puente, E. O. R.; Félix, E. A.; Ponce, J. F.; Cruz, M.; Juárez, O. G. y Ortega, A. M. G. 2009. Detección de Clavibacter michiganensis subespecie michiganensis en el tomate del estado de Sonora, México. México. Rev. Fitotec. Mex. 32(4):319-326. [ Links ]
Fuentes, L.; Mesa, A. R.; Ruíz, M. L.; Peláez, M. I. y Fernández, M. 2005. Estudio histológico en callos de Stylosanthes guianensis cv. CIAT-184. Cuba. Pastos y Forrajes. 28(3):199-209. [ Links ]
González, I.; Yailén, A. y Peteira, B. 2012. Aspectos generales de la interacción Fusarium oxysporum f. sp. lycopersici-tomate. Cuba. Rev. de Protección Vegetal. 27(1):1-7. [ Links ]
González, M. S.; Flores, L. M. L.; Benavides, M. A. y Flores, O. A. 2011. Actividad inhibitoria del extracto de Heliopsis longipes sobre Fusarium oxysporum f. sp. lycopersici. México. Rev. Mex. Fitopatol. 29(2):146-153. [ Links ]
Hernández, S. M. 1984. Manual de laboratorio de citología y citogenética. Universidad Autónoma Agraria Antonio Narro (UAAAN). Saltillo, Coahuila, México. 92 p. [ Links ]
Ignjatov, M.; Milošević, D.; Nikolić, Z.; Gvozdanović-Varga, J.; Jovičić, D. and Zdjelar, G. 2012. Fusarium oxysporum as causal agent of tomato wilt and fruit rot. Serbia. Pesticidi i fitomedicina 27(1):25-31. [ Links ]
Jiménez, C. y Sanabria, N. 2008. Población final de Trichoderma harzianum en el control de la marchitez vascular en tomate causado por F. oxysporum f. sp. lycopersici. Venezuela. Fitopatología Venezolana. 21:29-30. [ Links ]
López, B. J. 2007. Alcamidas hacia la nueva era agrícola. México. Revista Ciencia y Desarrollo. 33(205):60-67. [ Links ]
López, J. B.; Acevedo, G. H.; Ramírez, E. C.; Molina-Torres, J. T. and Herrera, L. E. 2006. Novel signals for plant development. Reino Unido. Current Opinion Plant Biol. 9(5):523-529. [ Links ]
Masato, K.; Yumiko, K.; Gen, O.; Isamu, Y.; Tohru T. and Tsutomu, A. 2005. Three evolutionary lineages of tomato wilt pathogen, Fusarium oxysporum f. sp. lycopersici, based on sequences of IGS, MAT1, and pg1, are each composed of isolates of a single mating type and a single or closely related vegetative compatibility group. Japón. J. General Plant Pathol. 71(4):263-272. [ Links ]
Méndez, B. A.; Calderón, V. C.; Ibarra, L. E.; Raya, G. J.; Ramírez, C. E.; Molina, T. J.; Guevara, G. A. A.; Bucio, B. J. and Herrera, E. L. 2011. Alkamides activate jasmonic acid biosynthesis and signaling pathways and confer resistance to Botrytis cinerea in Arabidopsis thaliana. Estados Unidos. PloS one. 6(11):e27251. [ Links ]
Molina, T. J. y García, C. A. 2001. Alcamidas en plantas: distribución e importancia. México. Avance y Perspectiva. 20:377-387. [ Links ]
Molina, T. J.; Salazar, C. C. J.; Armenta, S. C. and Ramírez, C. E. 2004. Fungistatic and bacteriostatic activities of alkamides from Heliopsis longipes roots: affinin and reduced amides. Estados Unidos. J. Agric. Food Chem. 52(15):4700-4704. [ Links ]
Morquecho, A. C. and López, J. 2007. Cannabinoid-like signaling and other new developmental pathways in plants. España. The Int. J. Develop. Biol. 1:34-41. [ Links ]
Pelagio, F. R.; Ortiz, C.R. and López, B. J. 2013. dhm1, an Arabidopsis mutant with increased sensitivity to alkamides shows tumorous shoot development and enhanced lateral root formation. Países Bajos. Plant Mol. Biol. 81(6):609-625. [ Links ]
Pharand, B.; Carisse, O. and Benhamou, N. 2002. Cytological aspects of compost-mediated induced resistance against fusarium crown and root rot in tomato. Estados Unidos. Phytopathology. 92(4):425-438. [ Links ]
Pouzoulet, J.; Pivovaroff, A.; Santiago, L. and Rolshausen, P. E. 2014. Can vessel dimension explain tolerance toward fungal vascular wilt diseases in woody plants? Lessons from Dutch elm disease and esca disease in grapevine. Suiza. Frontiers in Plant Sci. 5:1-11. [ Links ]
Rabie, G. H. and Humiany, A. A. 2004. Role of VA mycorrhiza on the growth of cowpea plant and their associative effect with N2 fixing and P-solubilizing bacteria as biofertilizer in calcareous soil. Finlandia. J. Food Agric. Environ. 2(3-4):186-192. [ Links ]
Ramírez, C. E.; Lucas, V. L.; Virgen, C. G. y Molina, T. J. 2000. Actividad fungicida de la afinina y del extracto crudo de raíces de Heliopsis longipes en dos especies de Sclerotium. México. Agrociencia. 34(2):207-215. [ Links ]
Reis, A.; Giordano, L. D. B.; Lopes, C. A. and Boiteux, L. S. 2004. Novel sources of multiple resistance to three races of Fusarium oxysporum f. sp. lycopersici in Lycopersicon germplasm. Brasil. Crop Breed. Appl. Biotechnol. 4:495-502. [ Links ]
Rivero, M. G. C.; Quirós, G.; Sánchez, U. A. B. y Sanabria, M. E. 2007. Morfoanatomía de sépalos y pedúnculo del fruto de Psidium guajava L., estructuras de preferencia del ácaro Brevipalpus phoenicis (Geijskes) (Acari: Tenuipalpidae). Venezuela. Rev. Fac. Agron. 24(1):135-140. [ Links ]
Sobrado, M. A. 2007. Relationship of water transport to anatomical features in the mangrove Laguncularia racemosa grown under contrasting salinities. Reino Unido. New Phytologist. 173(3):584-591. [ Links ]
Srivastava, R.; Khalid, A.; Sing, U. S. and Sharma, A. K. 2010. Evaluation of arbuscular mycorrhizal fungus, fluorescent Pseudomonas and Trichoderma harzianum formulation against Fusarium oxysporum f. sp. lycopersici for the management of tomato wilt. Estados Unidos. Biol. Control. 53(1):24-31. [ Links ]
Takken, F. and Rep, M. 2010. The arms race between tomato and Fusarium oxysporum. Reino Unido. Molec. Plant Pathol. 11(2):309-314. [ Links ]
Turlier, M. F.; Epavier, A. and Alabouvette, C. 1994. Early dynamic interactions between Fusarium oxysporum f. sp. liniand the roots of Linus usitatissimum as revealed by transgenic GUSmarked hyphae. Canada. Canadian J. Bot. 72(11):1605-1612. [ Links ]
Valueva, T. A. and Mosolov, V. V. 2004. Role of inhibitors of proteolytic enzymes in plant defense against phytopathogenic microorganisms. Rusia. Biochem. 69(11):1305-1309. [ Links ]
Received: May 2015; Accepted: August 2015











 texto en
texto en 


