Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista mexicana de ciencias agrícolas
versión impresa ISSN 2007-0934
Rev. Mex. Cienc. Agríc vol.6 spe 12 Texcoco nov./dic. 2015
Articles
Effect of exogenous application of benzoic and salicylic acid on growth of tomato, tomatillo and pepper seedling
1Universidad Autónoma Agraria Antonio Narro-Departamento de Horticultura. Buenavista, Saltillo, Coahuila, México, C. P. 25315. Tel. 528444110303.
2Universidad Autónoma Agraria Antonio Narro- Cátedras CONACYT-Departamento de Horticultura.
3Universidad Autónoma Agraria Antonio Narro-Departamento de Fitomejoramiento. (lgvsk8@hotmail.com; qfb_sgm@hotmail.com; luisalonso_va@hotmail.com; godramf@gmail.com)
Benzoic acid and its derivatives, such as salicylic acid are metabolic components that perform critical functions in plants. The aim of the study was to verify the effect of foliar application of benzoic and salicylic acid on seedling growth and mineral composition of tomato, tomatillo and pepper. Seedlings were grown under greenhouse in polystyrene containers using as substrate peat moss and perlite (80:20). Irrigation was performed with Steiner nutrient solution. Treatments consisted on weekly foliar applications of AB and AS at concentrations 10-4, 10-5, 10-6 M and a control with water. The variables were height and stem diameter, leaf area, aerial and root biomass and mineral content. The experimental design was completely randomized with 25 replications per treatment, being the experimental unit a seedling. The application of AS and AB in tomato and pepper seedlings gave positive responses in some growth variables and mineral composition. In tomatillo most variables responded negatively to AS and AB. The response pattern of aerial and root biomass from the three species was not systematic or predictable, so that if a variable increased another could decrease. Zn and Ca content increased in tomato and pepper seedlings respectively, when applying AS and AB, while in tomatillo all mineral concentrations decreased when these compounds were applied.
Keywords: mineral absorption; organic acids; phenylpropanoids benzoates; salicylates
El ácido benzoico y sus derivados tales como el ácido salicílico, son componentes metabólicos que realizan funciones críticas en las plantas. El objetivo del estudio fue verificar el efecto de la aplicación foliar de los ácidos benzoico y salicílico sobre el crecimiento y composición mineral de plántulas de tomate, tomatillo y pimiento. Las plántulas fueron cultivadas en invernadero y crecidas en contenedores de poliestireno utilizando como sustrato peat moss y perlita (80:20). Los riegos se realizaron con solución nutritiva Steiner. Los tratamientos consistieron en aplicaciones foliares semanales de AB y AS en concentraciones 10-4, 10-5, 10-6 M y un testigo con agua. Las variables analizadas fueron altura y diámetro de tallo, área foliar, biomasa aérea y de raíz y contenido de minerales. El diseño experimental utilizado fue completamente al azar con 25 repeticiones por tratamiento, siendo la unidad experimental una plántula. La aplicación de AS y AB en plántulas de tomate y pimiento dio lugar a respuestas positivas en algunas variables de crecimiento y de composición mineral. En el tomatillo casi todas las variables respondieron de forma negativa frente al AS y AB. El patrón de respuesta de la biomasa aérea y de raíz de las tres especies no fue sistemático ni predecible, de manera que si una variable aumentaba otra podía disminuir. El contenido de Zn en las plántulas de tomate y de Ca en el pimiento aumentó al aplicar AS y AB, en cambio en el tomatillo todos los minerales disminuyeron su concentración al aplicar estos compuestos.
Palabras clave: absorción de minerales; ácidos orgánicos; benzoatos fenilpropanoides; salicilatos
Introduction
Plants are organisms that continually produce chemical, volatile and nonvolatile compounds that are used as signals to adapt and respond to changes in the environment (Avanci et al., 2010). Among these, plant hormones play an important role as they are involved in activities that promote growth and development by inducing an array of cellular, physiological and morphological processes (Ashraf et al., 2010). These are also involved in crucial processes related to plant development and survival, including defense mechanisms against biotic (Zhang et al., 2009) and abiotic (Ashraf et al., 2010) stress, secondary metabolism, reproductive process, fruit development and senescence (Wasternack, 2007). Among these compounds is the benzoic acid (AB) and derivatives such as salicylic acid (AS), originated from the metabolic pathway of phenylpropanoid (Qualley et al., 2012).
AB is an aromatic carboxylic acid found naturally in plants, playing an important role in metabolism and growth; its salts and esters are known as benzoates. AB and its derivatives are structural elements in a large number of metabolites and natural products that perform critical functions in plants, such as growth regulators, repellent compounds and pollinator attractors (Qualley et al., 2012). In industrial processes are used as preservatives and flavor enhancers, analgesics, antiseptics and chemotherapy (Chipley, 2005). Reports indicate that exogenous application of this substance, modifies the anatomy and morphology of edible and ornamental species, induces stress tolerance and improves germination in saline mediums (Benavides-Mendoza et al., 2004; Ortega-Ortiz et al., 2007). Also, it has been documented that AB has positive effects on plants under growth conditions far from optimal, as it modifies the profile of mineral nutrients accumulation in the tissues. Furthermore, it has been reported that plants accumulate AB in the soil, where it works as an allelochemical (Kaur et al., 2005); that is, as a compound released to the environment by plants that interferes with the growth of competing organisms.
Meanwhile AS is a phenolic compound that was initially identified in the bark from willow (Salix sp) (Raskin, 1992), it was subsequently isolated from the metabolism of salicin, which was named salicyl alcohol glucoside (Hayat et al., 2007). AS acts as a non-enzymatic antioxidant and as plant growth regulator, playing an important role in regulating a number of physiological processes of the plant, including photosynthesis (Waseem et al., 2006; Arfan et al., 2007) and overall chloroplast activity, absorption and nutrient transport, causes changes in plant anatomy and increases yield (Khan et al., 2010; Purcărea and Cachita-Cosma, 2010).
Exogenous applications of AS on plants has shown to induce greater tolerance to stress conditions such as drought (Horváth et al., 2007; Xu and Tian, 2008), phytotoxicity (Metwally et al., 2003) and low temperatures (López Delgado et al., 1998; Janda et al., 1999; Farooq et al., 2008; Mora-Herrera and Lopez-Delgado, 2006). Among other reports, it is mentioned that foliar applications of this compound, increases biomass in soybean plants (Gutiérrez-Coronado et al., 1998) and wheat yield (López-Tejeda et al., 1998). Besides increasing enzyme activity (catalase and peroxidase) in tomato (Ortega-Ortiz et al., 2007) extends shelf life of banana (Srivastava and Dwivedi, 2000).
It is worth noting that compared to AS, AB is a compound that has been little studied regarding its agricultural applications (Valdez-Sepulveda et al., 2015). If it’s application exerts positive effects on plants subjected to biotic or abiotic stress, then it could become another alternative in the arsenal of natural compounds available for agricultural practice. However prior to this, it is necessary to carry studies regarding the effect of this compound on plant growth in agriculture. Therefore the aim of this study was to evaluate the effect of exogenous application of salicylic and benzoic acid on seedlings growth of tomato, tomatillo and pepper. AS was included in the study as a reference well known since a lot has been published about its beneficial effects on plants.
Materials and methods
The experiment was conducted under greenhouse conditions at the Universidad Autonoma Agraria Antonio Narro, located in Saltillo, Coahuila, Mexico, with warm semiarid climate according to Köppen (BSh). Tomato seed Saladette type (Solanum lycopersicum L.) variety "Rio Grande" tomatillo seeds (Physalis ixocarpa Brot) variety "Gran Esmeralda" and seed pepper (Capsicum annuum) variety "Sven RZ". These were germinated in a mixture of peat moss and perlite (80:20) in polystyrene containers. Irrigation was carried through drip using Steiner solution (Steiner, 1961) with an electrical conductivity of 1500 μS cm-1 three times a week in each treatment.
Treatments consisted in foliar applications of AB and AS at concentrations 10-4, 10-5, 10-6 M and a control with water. The applications initiated at 13 days after emergence on weekly basis; performing six applications. Seven days after the last spraying, the seedlings were harvested to determine treatments effect. The experimental design was completely randomized with 25 replications per treatment, being the experimental unit a seedling. During the course of the experiment three samplings of four seedlings were performed every 14 days.
Variables evaluated were: height, measured with a ruler from stem base to the terminal apex of the seedling; stem diameter, using a digital vernier on the mid area of the stem (Autotec Caliper 150mm) and leaf area, using a leaf area meter (L1-3100, LI-COR). Subsequently, the four seedlings were sectioned in leaves, stems and roots. The fresh leaf weight (PFH), stem (PFT) and root (PFR) was obtained with an analytical scale (Ohaus Pioneer) and dry weight of leaves (PSH), stems (PST) and roots (PSR), these were dehydrated in a drying oven with forced air circulation (Lindberg Blue) at a temperature of 70 °C for 72 h. The mineral content (K, Ca, Mg, Na, Fe, Zn, Cu and Mn) was analyzed in four seedlings through atomic absorption spectrophotometry (Varian AA-1275) (Fick et al., 1976); P was determined through visible spectrophotometry (AOAC, 1980b) and N was quantified by the micro Kjeldahl (AOAC, 1980a). The results were analyzed through an analysis of variance and means comparison by LSD (p≤ 0.05) with the statistical package SAS (2001).
Results and discussion
The results indicate that exogenous application of AB and AS in tomato, tomatillo and pepper seedlings caused significant changes in seedling height, leaf area and stem diameter with some of the concentrations but not for the three variables mentioned simultaneously; that is, a positive response in one of the variables are not necessarily accompanied by the same response in the other two (Table 1). It is known that depending on the concentration of AS and AB, induces certain responses that do not occur consistently or predictable. Therefore it is possible to find an answer in a given concentration, that is, 10-6 M, but not observed at 10-5 M, since the cellular components that respond to a threshold of 10-6 M are not necessarily the same activated at 10 -5 M.
Table 1 Effect of different concentrations of AB and AS sprayed on tomato seedlings, tomatillo and pepper variables seedling height, stem diameter and leaf area.
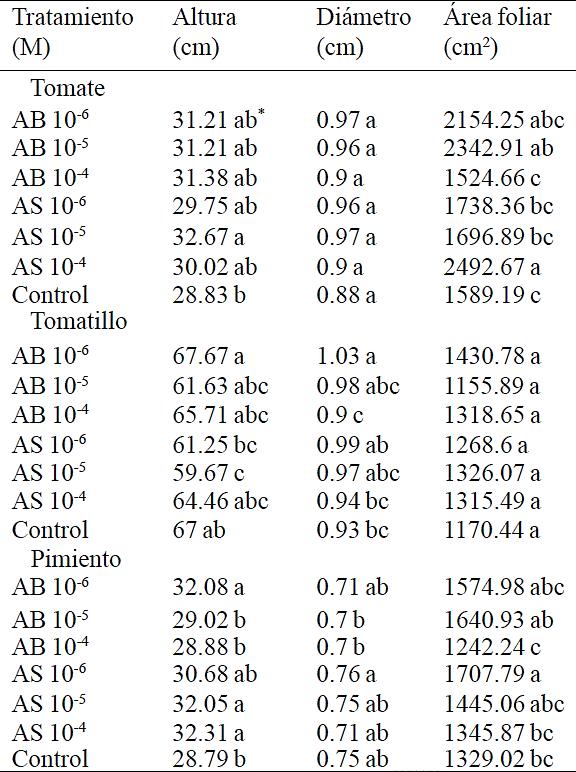
*Valores con la misma literal dentro de columnas para cada especie son iguales de acuerdo a la prueba LSD (p≤0.05). Cada valor es la media de 4 repeticiones.
Similarly, the application 10-4 M is likely to trigger different responses to those observed in 10-6 M, because the cellular components activated or deactivated are different. Currently there are many physiological and biochemical studies on the effect of AS in plants, but it lacks of a full model of genomic or transcriptomic response to these compounds (Rivas-San Vicente and Plasencia, 2011). Therefore, morphological or composition responses are complex and difficult to fit into a simple model.
The effect of exogenous application of AS in plant growth depends on the species, stage of development and concentration applied (Rivas-San Vicente and Piacenza, 2011). The height of tomato seedlings, increased 13.32% compared to control applying AS 10-5 M; these results are similar to those achieved by Larqué-Saavedra et al. (2010) who reported a height increase of 14.8% applying 1 µM AS. Among the beneficial effects of AS are those reported in chrysanthemums, favoring plant growth in diameter and height (Villanueva-Couoh et al., 2009).
The stimulatory effects of AS on plant growth have also been reported in soybean (Zhao et al 1995; Gutiérrez-Coronado et al, 1998), wheat (Shakirova et al., 2003), corn (Gunes et al., 2007) and chamomile (Kováčik et al., 2009). Similarly, leaf area from tomato seedlings increased by treatments reaching 56.85 and 47.43% compared to control, with AS 10-4 M and AB 10-5 M respectively. These results are superior to those obtained by Larqué-Saavedra et al. (2010), who managed an increase of leaf area by 38.6% compared to control, applying 1µM AS. It is worth noting the number of applications needed to produce positive effects on plants, as in the present study AB and AS were applied six times, while in the study from Larqué-Saavedra et al. (2010) two applications were made.
In contrast, on tomatillo seedlings AS 10-5 M reduced height by 7.33 cm compared to control. However, on this vegetable the application of AB 10-6 M, increased stem diameter 10.75%. The response pattern of this variable indicates that low concentrations of AB, has positive effects on growth, confirming that exogenous application of this substance, modifies the anatomy and morphology of edible species (Ortega-Ortiz et al., 2007).
Finally, pepper seedlings were affected positively by increasing its height in a range of 11 to 12% regarding to control by treatments AB 10-6, AS 10-5 and AS 10-4 M. Studies made by Sánchez-Chávez et al. (2011) reported a significant increase of leaf biomass in jalapeño pepper using AS 0.1 and 0.2 mM. Similarly, leaf area from seedlings increased 28.50% compared to control with treatments AS 10-6 M. It is worth mentioning that there was no information of response patterns from AB applied to Capsicum annuum and the current information highlights that it is a sensitive specie to these growth regulators.
AS has natural variations in concentration resulting from developing events such as different phenological and flowering stages (Abreu and Munne, 2009). Therefore, it is expected that the concentration of AB will vary in plant tissue. It is also known that constant adjustments take place in the concentration of AS according to temperature and irradiation conditions (Mateo et al., 2006). Table 2, shows the effect of foliar applications of AB and AS in tomato, tomatillo and pepper seedlings on aerial and root biomass. Again, depending on the amount applied of AB and AS, certain responses are promoted that do not fit to a linear pattern. Currently there is a lack of models on genomic or transcriptomic response towards these compounds, leading to carry studies and applications under a system of trial and error. Therefore morphological responses or composition are complex and difficult to frame in a simple model.
Table 2 Effect of foliar application of AB and AS on biomass and root seedlings of tomato, tomatillo and pepper.
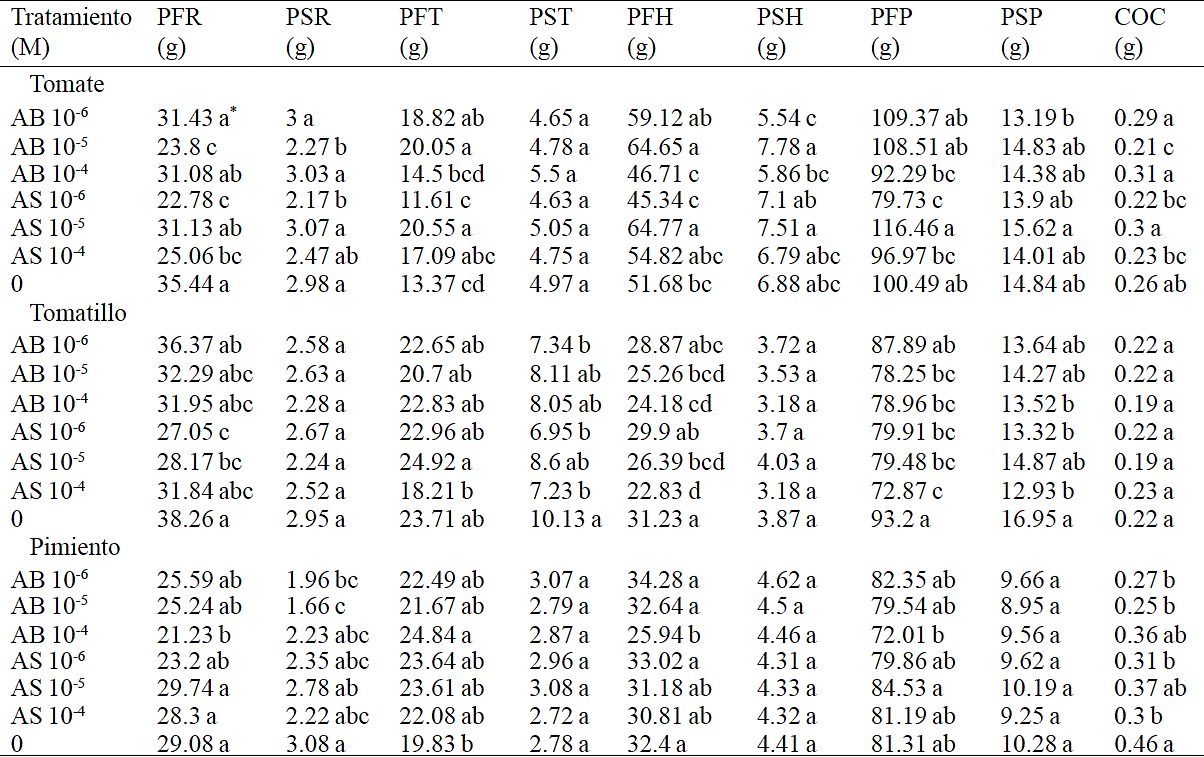
*Valores con la misma literal dentro de columnas para cada especie son iguales de acuerdo a la prueba LSD (p≤0.05). Cada valor representa la media de cuatro plantas. PFR: Peso fresco raíz; PSR: Peso seco raíz; PFT: Peso fresco tallo; PST: Peso seco tallo; PFH: Peso fresco hojas; PSH: Peso seco hojas; PFP: Peso fresco planta; PSP: Peso seco planta; COC: Cociente de la biomasa de la raíz y biomasa aérea.
In tomato seedlings, treatment AB 10-6 M decreased 5.45 g PFT and increased 0.03 g COC; treatment AB 10-5 M reduced 11.64 g PFR and 0.71 g PSR; instead it increased 6.68 g PFT and 12.97 g PFH; meanwhile treatment AB 10-4 M increased 0.05 g COC. Regarding AS applications, concentration 10-6 M reduced 12.66 g PFR and 0.81 g PSR and increased 20.76 g PFP; on the contrary treatment AS 10-5 M significantly increased 7.18 g PFT, 13.09 g PFH and 0.04 g COC; finally treatment AS 10-4 M reduced 10.38 g PFR. It can be seen that there is no systematic trend and shows that certain concentration increases or decreases some of the variables. Most of the literature indicates that the effect of AS on root growth is positive (Gemes et al., 2008; Umebese et al., 2009; Ahmad et al., 2013). Reports indicate that AS significantly increases soybean root growth (Gutiérrez-Coronado et al., 1998) and pine (Pinus patula) Schiede ex Schltdl & Cham (San Miguel et al., 2003). On the other hand, it has been reported (Ramírez et al., 2006) that applications of AB 10-6 M significantly increase PFR on cabbage (Brassica oleracea).
According to Salisbury and Ross (1994), modifications achieved in growth are due to AS favors the production of indole acetic acid and naphthalene acetic acid which are reported as key regulators of plant growth. It has been documented that AS levels are inversely proportional to lignin levels and to growth in some plants (Gallego et al., 2011).
Also, tomatillo seedlings were affected by the exogenous application of AS and AB. For AB, 10-6 M concentration decreased 2.79 g PST; 10-5 M concentration reduced 5.97 g PFH and 14.95 g PFP; a similar behavior occurred when applying AB 10-4 M where PFH decreased 7.05 g, 14.24 g PFP and 3.42 g PSP. Meanwhile, treatments with AS showed a response pattern similar to that described above. Treatment AS 10-6 M reduced 11.21 g PFR, 3.18 g PST, 13.29 g PFP and 3.63 g PSP; 10-5 M concentration decreased 10.09 g PFR, 4.84 PFH g and 13.72 g PFP and finally treatment AS 10-4 M reduced 2.9 g PST, 8.4 g PFH, 20.33 g PFP and 4.02 g PSP. These results show the importance of carrying out experiments to answer questions like concentration of AB and AS, and the number of applications needed to produce positive effects in this vegetables. Other reports have documented the positive effects when applying AS in other plant species; for example, in pine and chrysanthemum studies made on the use of different AS doses, showed increases in root production (San Miguel et al., 2003; Echeverría-Machado et al., 2007; Villanueva-Couoh et al., 2009 ).
In pepper seedlings, AB applications caused significant changes in some variables. Treatment AB 10-6 M decreased 1.12 g compared to control from PSR; a similar behavior was observed when applying AB 10-5 M decreasing 1.42 g compared to the control from PSR; meanwhile, the application of AB 10-4 M decreased 7.85 g compared to the control from PFR and increased PFT and PFH, 5.01 and 6.46 g respectively. These results suggest that exogenous application of AB (10-4, 10-5 and 10-6 M) in this vegetable does not increase the root system from the seedlings. Instead, Ramírez et al. (2006) state that the application of AB 10-6 M increases total cabbage seedling fresh weight.
Table 3 shows the results of mineral content from tomato, tomatillo and pimento seedlings sprinkled with AB and AS where significant differences between some of the minerals were found. Overall results showed differences (p≤ 0.05) among the analyzed minerals (K, Ca, Mg, Na, Fe, Zn, Mn, Cu) and species studied. In tomato, the seedlings had a significant increase in Ca content by treatments AB 10-5 and AB 10-4 M; Mg increased by treatment AS 10-5 M; Na increased by treatments AB 10-6 and AS 10-5 M; Zn content was higher across all treatments compared to control; Mn increased by treatment AB 10-5 and the three concentrations of AS; finally Cu increased by treatments AB 10-5 M, AS 10-6 and AS 10-5 M.
Table 3 Effect of different concentrations of AB and AS in the mineral content of tomato seedlings, tomatillo and pepper.
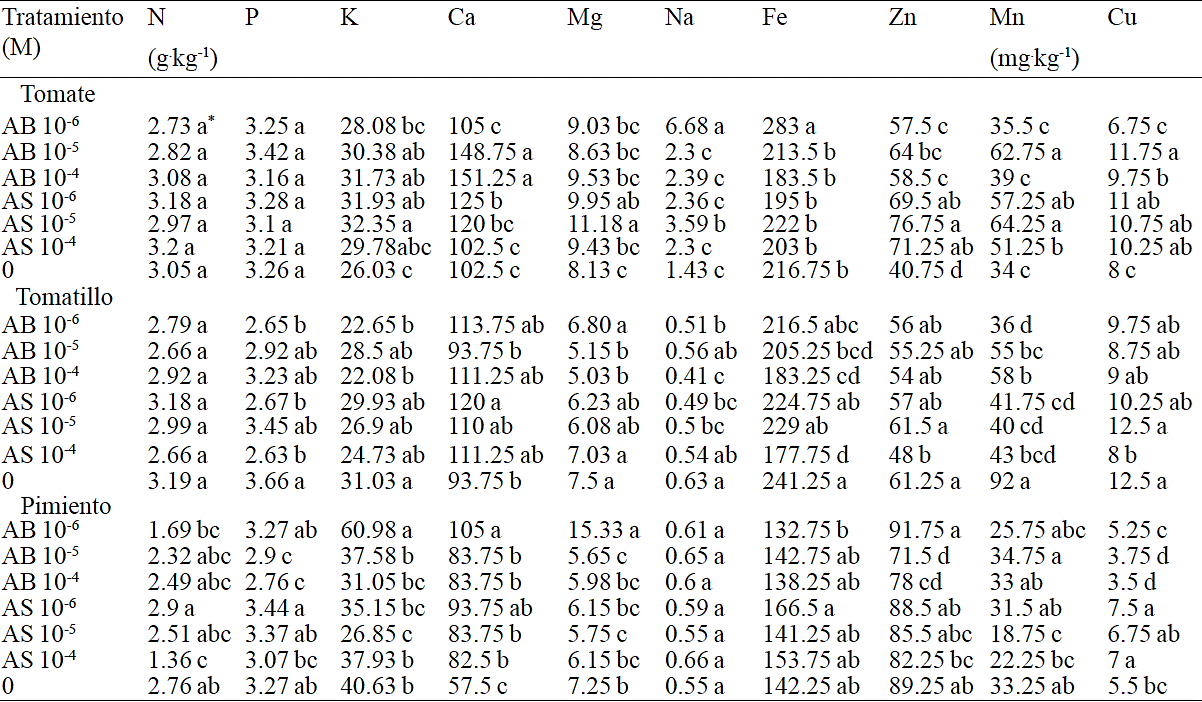
*Valores con la misma literal dentro de columnas para cada especie son iguales de acuerdo a la prueba LSD (p≤0.05). Cada valor representa la media de cuatro plantas.
It is important to note that each species demands nutrient requirements that allow optimal growth and vigor, these requirements are not constant and change according to the species and the environment in which they grow and develop (Timmer and Armstrong, 1987). The use of sprinkled AS on leafs has a benefit on growth of the aerial parts of the plants in situations where some stresses are present (Khodary, 2004; Najafian et al., 2009; Azooz and Youseef, 2010; Wang et al., 2010); however, in this study the seedlings were not subjected to any stress.
In contrast, on tomatillo seedlings can be seen a negative effect by effect of treatment. Na content decreased significantly with treatment AB 10-4 M; this treatment significantly decreased Fe content. Similarly, Mn content was adversely affected by all treatments. Treatments application modified the profile of mineral nutrients accumulation in the tissues. Possibly through interactive effects between the same mineral elements in the plant resulted on an overall decrease in their concentration.
For pepper seedlings, K and Mg content increased with treatment AB 10-6 M; Ca content significantly increased by effect of all treatments; finally Zn content was lower when applying AB 10-5 M. Reports indicate that the application of AS 10-8M in habanero pepper (Capsicum chinense Jacq) increases K, Fe, Zn and Cu content (Guzmán-Antonio et al., 2012). Similarly, for jalapeno pepper seedlings (Capsicum annuum L.), Preciado et al. (2007) reported contents between 12.6 and 28.7 mg per plant of N; between 0.93 and 1.11 mg of P and between 9.9 and 14 mg of K, it is worth noting that these differences are attributed to the biomass produced. In the present study, applications of AB and AS showed no clear trend regarding mineral content in vegetables evaluated, so in the future should explore a range of various concentrations and increase the number of applications.
Conclusions
The application of AS and AB in tomato and pepper seedlings resulted in positive responses in some growth variables and mineral composition. In tomatillo almost all variables responded negatively to AS and AB.
Tomato seedlings increased height when sprayed with AS 10-5 M; in addition leaf area was greater when applying AS 10-5 and AB 10-4 M. Tomatillo seedlings reduced height with treatment AS 10-5 M, however AB 10-6 M increased stem diameter on this vegetable. Furthermore, pepper seedlings were higher with treatments AB 10-6, AS 10-5 AS 10-4 M; also, leaf area was higher with treatment AS 10-6 M. The response pattern of aerial and root biomass of the three species was not systematic or predictable, so if a variable increased the other could decrease.
Zn content in tomato seedlings was significantly higher compared to control in all treatments. The application of AS and AB decreased mineral content in tomatillo seedlings. Finally Ca content in pepper seedlings was significantly higher than control in all treatments.
Literatura citada
Abreu, M. E. and Munne, B. S. 2009. Salicylic acid deficiency NahG transgenic lines and sid2 mutants increases seed yield in the annual plant Arabidopsis thaliana. J. of Experimental Botany. 60(4):1261-1271. [ Links ]
Ahmad, I.; Basra, S. M. A.; Afzal, I.; Farooq, M. and Wahid, A. 2013. Growth improvement in spring maize through exogenous application of ascorbic acid, salicylic acid and hydrogen peroxide. International J. of Agriculture and Biology. 15: 95-100. [ Links ]
AOAC (Association of official analytical chemiste). 1980a. Official Methods of Analysis 13th edition. Association of Official Analytical Chemists. Washington, DC., USA. 547-562 pp. [ Links ]
AOAC (Association of official analytical chemiste). 1980b. Official Methods of Analysis of the Association of Official Analytical Chemists. 30th edition. Association of Official Analytical Chemist.Washington, D.C. USA. 39 pp. [ Links ]
Arfan, M.; Athar H. R. and Ashraf, M. 2007. Does exogenous application of salicylic acid through the rooting medium modulate growth and photosynthetic capacity in two differently adapted spring wheat cultivars under salt stress? J. Plant Physiol. 6(4):685-694. [ Links ]
Ashraf, M.; Akram, N. A.; Arteca, R. N. and Foolad, M. R. 2010. The physiological, biochemical and molecular roles of brassinosteroids and salicylic acid in plant processes and salt tolerance. Cri. Rev. Plant Sci. 29(3):162-190. [ Links ]
Avanci, N. C.; Luche, D. D.; Goldman, G. H. and Goldman, M. H. S. 2010. Jasmonates are phytohormones with multiple functions, including plant defense and reproduction. Genet. Mol. Res. 9(1):484-505. [ Links ]
Azooz, M. and Youseef, M. 2010. Evaluation of heat shock and salicylic acid treatments as inducers of drought stress tolerance in wheat. American J. of Plant Physiol. 5:56-70. [ Links ]
Benavides-Mendoza, A.; Salazar-Torres, A. M.; Ramírez-Godina, F.; Robledo-Torres, V.; Ramírez-Rodríguez, H. y Maiti, R. 2004. Tratamiento de semilla de chile con ácidos salicílico y sulfosalicílico y respuesta de las plántulas al frío. Terra Latinoamericana. 22(1):41-47. [ Links ]
Chipley, J. 2005. Sodium benzoate and benzoic acid. In: Antimicrobials in foods. Davidson, P. M.; Sofos, J. N. and Branen, A. L. (eds.). Third edition. CRC Press. Boca Raton, FL, USA. 11-48 pp. [ Links ]
Echeverría-Machado, I.; Escobedo, G. M. R. M. and Larqué-Saavedra, A. 2007. Responses of transformed Catharanthus roseus roots to femtomolar concentrat ions of salicylic acid. Plant Physiology and Biochemestry. 45:501-507. [ Links ]
Farooq, M.; Aziz, T.; Basra, S. M. A.; Cheema, M. A. and Rehman, H. 2008. Chilling tolerance in hybrid maize induced by seed priming with salicylic acid. J. Agron. Crop. Sci. 194(2):161-168. [ Links ]
Fick, K. R.; Miller, S. M.; Funk, J. D.; McDowell, L. R. and Houser, R. H. 1976. Methods of mineral analysis for plant and animal tissues. University of Florida institute of food and agriculture. Sciences, Departament of Animal Sciences, Gainesville, F L. USA. 81 p. [ Links ]
Gallego, G. L.; Escamilla, T. L.; Jackson, L. A.and Dixon, R. A. 2011. Salicylic acid mediates the reduced growth of lignin downregulated plants. Plant Biology. 108(51):20814-20819. [ Links ]
Gemes, K., Poor, P., Sulyok, Z., Szepesi, A., Szabo, M. and Tari, I. 2008. Role of salicylic acid pre-treatment on the photosynthetic performance of tomato plants (Lycopersicum sculentum Mill. L. cv. Rio Fuego) under salt stress. Acta Biologica Szegediensi. 52:161-162. [ Links ]
Gunes, A.; Inal, A.; Alpaslan, M.; Eraslan, F.; Guneri Bagci E. and Cicek, N. 2007. Salicylic acid induded changes on some physiological parameters symptomatic for oxidative stress and mineral nutrition in maize (Zea mays L.) grown under salinity. J. of Plant Physiol. 164:728-736. [ Links ]
Gutiérrez-Coronado, M. A.; Trejo-López, C. and Larqué-Saavedra, A. 1998. Effect of salicylic acid on the growth of roots and shoots in soybean. Plant Physiol. and Biochem. 36(8):563-565. [ Links ]
Guzmán-Antonio, A.; Borges-Gómez, L.; Pinzón-López, L.; RuizSánchez, E. y Zúñiga-Aguilar, J. 2012. Efecto del ácido salicílico y la nutrición mineral sobre la calidad de plántulas de chile habanero. Agronomía mesoamericana. 23(2):247-257. [ Links ]
Hayat, S.; Ali, B. and Ahmad, A. 2007. Salicylic acid: biosynthesis, metabolism and physiological role in plants. In: Salicylic acid a plant hormone. Hayat, S. and Ahmad, A. (eds). Springer. Dordrecht, The Netherlands.1-14 pp. [ Links ]
Horváth, E.; Pál, M.; Szalai, G.; Páldi, E. and Janda T. 2007. Exogenous 4-hydroxybenzoic acid and salicylic acid modulate the effect of short-term drought and freezing stress on wheat plants. Biol. Plant. 51(3):480-487. [ Links ]
Janda, T.; Szalai, G.; Tari, I. and Paldi, E. 1999. Hydroponic treatment with salicylic acid decreases the effects of chilling injury in maize (Zea mays L.) plants. Planta. 208:175-180. [ Links ]
Kaur, H.; Inderjit and Kaushik, S. 2005. Cellular evidence of allelopathic interference of benzoic acid to mustard (Brassica juncea L.) seedling growth. Plant Physiol. Biochem. 43(1):77-81. [ Links ]
Khodary, S. 2004. Effect of salicylic acid on growth, photosynthesis and carbohydrate metabolism in salt-Stressed maize plants. International J. of Agriculture and Biology. 6:5-8. [ Links ]
Khan, N.; Syeed, S.; Masood, A.; Nazar, R. and Iqbal, N. 2010. Application of salicylic acid increases contents of nutrients and antioxidative metabolism in mungbean and alleviates adverse effects of salinity stress. Int. J. Plant Biol. 1(e1): 1-9. [ Links ]
Kovácik, J.; Grúz, J.; Backor, M.; Strnad, M.and Repcák, M. 2009. Salicylic acid-induced changes to growth and phenolic metabolism in Matricaria chamomilla plants. Plant Cell Reports. 28:135-143. [ Links ]
Larqué-Saavedra, A.; Martín-Mex, R.; Nexticapan-Garcéz, A.; Vergara-Yoisura, S. y Gutiérrez-Rendón, M. 2010. Efect o del ácido salicílico en el crecimiento de plántulas de tomate (Lycopersicon esculentum Mill.). Revista Chapingo Serie Horticultura. 16(3):183-187. [ Links ]
López-Delgado, H.; Dat, J. F.; Foyer, C. H. and Scott, I. M. 1998. Induction of thermotolerance in potato microplants by acetylsalicylic acid and H2O2. J. of Experimental Botany. 49(321):713-720. [ Links ]
López-Tejeda, R.; Camacho-Rodríguez, V. y Gutiérrez-Coronado, M. A. 1998. Aplicación de ácido salicílico para incrementar el rendimiento agronómico en tres variedades de trigo. Terra Latinoamericana. 16(1):43-48. [ Links ]
Mateo, A.; Funck, D.; Mühlenbock, P.; Kular, B.; Mullineaux, P. M. and Karpinski, S. 2006. Controlled levels of salicylic acid are required for optimal photosynthesis and redox homeostasis. J. of Experimental Botany. 57:1795-1807. [ Links ]
Metwally, A.; Finkemeier, I; Georgi, M. and Dietz, K. J. 2003. Salicylic acid alleviates the cadmium toxicity in barley seedlings. Plant Physiol. 132(1):272-281. [ Links ]
Mora-Herrera, M. E. y López-Delgado, H. A. 2006. Tolerancia a baja temperatura inducida por ácido salicílico y peróxido de hidrógeno en microplantas de papa. Fitotecnia Mexicana. 29(2):81-85. [ Links ]
Najafian, S.; Khoshkhui, M.; Tavallali, V. and Saharkhiz, M. J. 2009. Effect of salicylic acid and salinity in thyme (Thymus vulgaris L.): Investigation on changes in gas exchange, water relations, and membrane stabilization and biomass accumulation. Australian Journal of Basic and Applied Sciences. 3:2620-2626. [ Links ]
Ortega-Ortiz, H.; Benavides-Mendoza, A.; Mendoza-Villarreal, R.; Ramírez-Rodríguez, H. and De Alba, R. K. 2007. Enzymatic activity in tomato fruits as a response to chemical elicitors. J. Mex. Chem. Soc. 51(3):141-144. [ Links ]
Preciado, R. P.; Lara-Herrera, A.; Segura, C. M. A.; Rueda, P. E. O.; Orozco, V. J. A.; Yescas, C. P. and Montemayor, T. J. A. 2007. Amonio y fosfato en el crecimiento de plántulas de chile jalapeño. Terra Latinoamericana. 26:37-42. [ Links ]
Purcărea, C. and Cachiţă-Cosma, D. 2010. Studies regarding the effects of salicylic acid on maize (Zea mays L.) seedling under salt stress. Studia Universitatis Vasile Goldiş Seria Ştiinţele Vieţii. 20(1):63-68. [ Links ]
Qualley, A. V.; Widhalm, J. R.; Adebesin, F.; Kish, C. M. and Dudareva, N. 2012. Completion of the core β-oxidative pathway of benzoic acid biosynthesis in plants. Proc. Natl. Acad. Sci. USA. 109(40):16383-16388. [ Links ]
Raskin, I. 1992. Role of salicylic acid in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 43:439-463. [ Links ]
Ramírez, H.; Rancaño-Arrioja, J. H.; Benavides-Mendoza, A.; MendozaVillarreal, R. y Padrón-Corral, E. 2006. Influencia de promotores de oxidación controlada en hortalizas y su relación con antioxidantes. Revista Chapingo Serie Horticultura. 12(2):189-195. [ Links ]
Rivas-San Vicente, M. and Plasencia, J. 2011. Salicylic acid beyond defence: its role in plant growth and development. J. of Experimental Botany. 62(10):3321-3338. [ Links ]
Salisbury, F. B. and Ross, C. W. 1994. Fisiología vegetal. Tradu cido por González, V. V. Edit. Iberoamérica, México. 673 p. [ Links ]
San Miguel, R.; Gutiérrez, M. and Larqué-Saavedra, A. 2003. Salicylic acid increases the biomass accumulation of Pinus patula. Southern. J. of Applied Forestry 27:52-54. [ Links ]
Sánchez-Chávez, E.; Barrera-Tovar, R.; Muñoz-Márquez, E.; OjedaBarrios, D. L. and Anchondo-Nájera, Á. 2011. Efecto del ácido salicílico sobre biomasa, actividad fotosintética, contenido nutricional y productividad del chile jalapeño. Revista Chapingo Serie Horticultura. 17(1):63-68. [ Links ]
Statistical Analysis System Institute (SAS). 2001. PROC user’s manual. 6th ed. SAS Institute. Cary, NC, USA. 252 p. [ Links ]
Shakirova, F. M.; Sakhabutdinova, A. R.; Bezrukova, V.; Fatkhutdinova, R. A. and Fatkhutdinova, D. R. 2003. Changes in the hormonal status of wheat seedlings induced by salicylic acid and salinity. Plant Science. 164:317-322. [ Links ]
Srivastava, M. K. and Dwivedi, U. N. 2000. Delayed ripening of banana fruit by salicylic acid. Plant Sci. 158(1-2):87-96. [ Links ]
Steiner, B. 1961. A universal method for preparing nutrient solution of a certain desired composition. Plant and Soil. 16(2):134-154. [ Links ]
Timmer, V. R. and Armstrong, G. 1987. Growth and nutrition of containerized Pinus resinosa at exponentially increa sing nutrient additions. Canadian J. of Forest Research. 17:644-647. [ Links ]
Valdez-Sepúlveda, L.; González-Morales, S. and Benavides-Mendoza, A. 2015. Ácido benzoico: biosíntesis, modificación y función en plantas. Revista Mexicana de Ciencias Agrícolas. 6(7):1667-1678. [ Links ]
Villanueva-Couoh, E.; Alcántar-González, G.; Sánchez-Garc ía, P.; SoriaFregoso, M. y Larqué-Saavedra, A. 2009. Efecto del ácido salicílico y dimetilsulfóxido en la floración de [Chrysanthemum morifolium (Ramat) Kitamura] en Yucatán. Revista Chapingo Serie Horticultura. 15(2):25-31. [ Links ]
Wang, L. J.; Fan, L.; Loescher, W.; Duan, G. J.; Cheng, J. S.; Luo, H. B. and Li, S. H. 2010. Salicylic acid alleviates decreases in photosynthesis under heat stress and accelerates recovery in grapevine leaves. BMC Plant Biology. 10:34. [ Links ]
Waseem, M.; Athar H. U. R. and Ashraf, M. 2006. Effect of salicylic acid applied through rooting medium on drought tolerance of wheat. Pak. J. Bot. 38(4):1127-1136. [ Links ]
Wasternack, C. 2007. Jasmonates: an update on biosynthesis, signal transduction and action in plant stress response, growth and development. Ann. Bot. 100:681-697. [ Links ]
Xu, X. and Tian, S. 2008. Salicylic acid alleviated pathogen induced oxidative stress in harvested sweet cherry fruit. Postharvest biology and technology. 49:379-385. [ Links ]
Zhang, Z.; Li, Q.; Li, Z.; Staswick, P.; Wang, M.; Zhu, Y. and He, Z. 2009. Dual regulation role of GH3.5 in salicylic acid and auxin during Arabidopsis-Pseudomonas siringae interaction. Plant Physiology. 145:450-464. [ Links ]
Zhao, H. J.; Lin, X. W.; Shi, H. Z. and Chang, S. M. 1995. The regulating effects of phenolic compounds on the physio logical characteristics and yield of soybeans. Acta Agronómica Sinica. 21:351-355. [ Links ]
Received: August 2015; Accepted: November 2015











 texto en
texto en 


