Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista mexicana de ciencias agrícolas
versão impressa ISSN 2007-0934
Rev. Mex. Cienc. Agríc vol.6 spe 12 Texcoco Nov./Dez. 2015
Articles
Germination and micropropagation of tetraploid husk tomato (Physalis ixocarpa)
1Universidad Autónoma Agraria Antonio Narro-Departamento de Horticultura. Calzada Antonio Narro 1923, Colonia Buenavista, C. P. 25315 Saltillo, Coahuila, México. Tel: 01 844 4 11 02 03. (abenmen@gmail.com; hgosuna@hotmail.com).
2Universidad Autónoma Agraria Antonio Narro-Departamento de Fitomejoramiento. Calzada Antonio Narro 1923, C. P. 25315. Saltillo, Coahuila, México. (godramf@gmail.com; bocardo_lety@hotmail.com).
The tetraploid variety of tomatillo (Phisalis ixocarpa) has adaptive characteristics both at morphological and physiological level. One of the limitations of the seed is low viability, germination and seedling emergence, as well as limited vegetative propagation capacity, which is why the objective of this study was to evaluate the response in germination of tetraploid seeds of tomatillo applying regulators and organic acids. Imbibition with regulators and organic acid treatments were applied to seeds, which were: gibberellic acid (AG), organic acids: benzoic acid (AB), salicylic acid (AS), sulfosalicylic (ASS), at concentrations of 10-2 M, 10-4 M and 10-6 M. Emergence began on the fourth day after planting with germination percentage for AG1, AG2 and AG3 of 73.33, 56.66, 63.33% and an IVG of 20.26, 10.44 and 11.59 respectively. For micropropagation the combination of regulators benzyladenine (BAP), kinetin and naphthaleneacetic acid (ANA) were evaluated at different concentrations. The best treatment was 3 mg L-1 BAP with 9.5 buds per explant.
Keywords: benzoic acid; gibberellic acid; salicylic acid; sulfosalicylic acid
La variedad tetraploide de Phisalis ixocarpa presenta caraterísticas adaptativas tanto a nivel morfológico como fisiológico. Una de las limitantes de la semilla es la baja viabilidad, germinación y emergencia de la plántula, así como la poca capacidad de propagación vegetativa, es por ello que el objetivo del presente trabajo fue evaluar la respuesta en la germinación de semillas tetraploides de tomatillo con la aplicación de reguladores y ácidos orgánicos. Se realizó la aplicación de tratamientos de imbibición con reguladores y ácidos orgánicos a las semillas, los cuales fueron: ácido giiberélico (AG), los ácidos orgánicos: benzoico (AB), salicílico (AS), sulfosalicílico (ASS), a concentraciones de 10-2 M, 10-4 M y 10-6 M. La emergencia inició en el cuarto día después de la siembra con un porciento de germinación para AG1, AG2 y AG3· de 73.33, 56.66, 63.33% y un IVG de 20.26, 10.44 y 11.59 respectivamente. Para la micropropagación se evaluó la combinación de los reguladores benciladenina (BAP), Kinetina y ácido naftalenácetico (ANA) a diferentes concentraciones. El mejor tratamiento fue con 3 mg L-1 de BAP con 9.5 brotes por explante.
Palabras clave: ácido benzoico; ácido giberélico; ácido salicílico; ácido sulfosalicílico
Introduction
The fruit of the species Physalis ixocarpa (Brot, former Hornem) is known as tomatilllo, husk tomato, mil tomate, fresadilla tomato, leaf tomato, milpero tomato; it is solanum native from Mexico. Mexico is recognized as the center of origin, diversity and domestication of genus Physalis as it is represented by 50 species (D'Arcy, 1991; Menzel, 1951; Peña and Márquez, 1990; Santiaguillo et al., 1994). The species P. ixocarpa has been a food source since pre-Hispanic times and its distribution is from United States to Nicaragua (Sánchez et al., 2006). In 2014 the cultivated area was 46,524 hectares and yields of 14.94 t ha-1 (SIAP-SAGARPA, 2014), which place it among the 10 most important horticultural species nationwide.
Despite the wide genetic variability, both wild tomato and domesticated, the national average yield is considered low, according to the yield potential of the species 40 t ha-1 this due to limited agricultural activity by the use of native varieties of low yield and inefficient production systems, water shortages of agricultural inputs and poor seed quality (Peña and Santiaguillo, 1999). There have been numerous efforts to improve yield, functional and nutritional quality of this fruit as diallel crosses, stratified visual mass selection, plant x plant crosses and more recently induction of polyploidy among others (Peña et al., 1998; Peña-Lomelí et al., 2002; Santiaguillo et al., 2004; Robledo et al., 2011; Ramírez et al., 2012).
Genetic improvement by chromosome duplication has achieved tetraploid populations in which is observed an increase in life cycle, plant height, fruit weight and equatorial diameter of fruit, fruits per plant and soluble solids and vitamin C (Robledo- Torres et al., 2011; Jiménez-Santana et al., 2012). In polyploid seed shows greater size and amount of mass, which can affect vigor or dispersibility, it also presents reduced viability, germination and seedling emergence (Bretagnolle et al., 1995; Al et al., 1998; Beaulieu et al., 2007; Zhang et al., 2010).
Seeds are the conventional method for propagation; however, the great genetic variability does not guarantee constant yields. Furthermore, seed propagation is limited for its low viability (Ramírez et al., 2013) and low germination rate. In this regard it is necessary, to increase seed emergence of tetraploids for which there are different techniques that favor and increase emergency synchronization, speed and percentage of seed germination, also generates resistance to biotic and abiotic factors. These techniques include seed imbibition in osmoregulators, saline solutions and growth regulators (Dahal et al., 1990). Arroyo-Medina et al. (2008) mention that the application of organic acids in seeds of horticultural interest had a positive effect on germination, dry weight and stems length and radicle.
However, to maintain and multiply tetraploid selected materials, vegetative reproduction methods are required, allowing to perpetuate the genotype through generations (Van and Kroon, 1990), which is not possible through seed, so a good alternative to keep the qualities in improving biomass production, functional properties in tetraploids would be micropropagation, which has been widely reported in Physalis ixocarpa, the primer was made by Bapat and Schieder (1981) by protoplast culture, then other researchers have reported from direct organogenesis, somatic embryogenesis and anther culture (Ramírez-Malagón and Ochoa-Alejo, 1991; Ortuño et al, 1998; Manzo-González et al., 1998; Contreras and Almeida, 2003; Yousry 2013; Yoursdy, 2014) propagation techniques used was somatic embryogenesis, adventitious stems, however there is no information on mass propagation of tetraploid varieties.
The aim of this study was to evaluate the response of tetraploid seeds in germination percentage and germination rate index with the application of regulators and organic acids and also micropropagate the tetraploid variety with the combination of different growth regulators.
Materials and methods
Germination
The work was conducted at the Universidad Autonoma Agraria Antonio Narro in the Laboratory of Plant Tissue Culture from the Plant Breeding Department.
Hydration stage
Hydration was evaluated by weighing the seed every 4 h until seed weight was constant.
Viability
This variable was evaluated determining initial seed viability by the germination capacity test. Where the methodology to assess viability was conducted in accordance with international rules ISTA (2009), determining viable and non-viable seeds, with three replications of 20 seeds, subjected to imbibition in water for 24 hours in a culture tube 15 X100 mm. The seeds were dissected in half, immersed in tetrazolium in dark and incubated at 30 °C for 2 h; subsequently were observed in stereo microscope to evaluate percentage viability.
Emergency rate index
IVE (emergancey rate index) was obtained by counting the seeds emerged daily for 21 days after planting. Considering testa rupture as seed emerged. To calculate its value the following formula was used Maguire (1962).
Where: IVE= emergency rate index; Núm. P= number of emerged plants; D= days after sowing.
Pretreatment
The following pretreatments were formed: gibberellic acid: G1= 10-2 M, G2= 10-4 M, G3= 10-6M; sulfosalicylic acid: ASS1= 10-2 M, ASS2= 10-4 M, ASS3= 10-6 M; salicylic acid: AS1= 10-2 M, AS2= 10-4 M, AS3= 10-6 M; benzoic acid: AB1= 10-2 M, AB2= 10-4 M, AB3= 10-6 M and control (T). Each treatment consisted of three replications with 10 seeds per jar. It was determined in a preliminary study the time in which the seeds reached the maximum absorption of water which was 4 h. With this data the seeds were imbibed in solutions with different regulators and organic acids.
Once waterlogging time has passed, the pretreated seeds are disinfected in alcohol solution 70%for one minute and rinsed with sterile distilled water, then placed in sodium hypochlorite 20% and rinsed with sterile distilled water three times under a laminar flow hood, then planted on Murashige and Skoog (MS) to half its concentration supplemented with 100 mg L-1 of myo-inositol (SIGMA®, I-3011), 1 mg L-1 thiamine-HCl (SIGMA®, T-3906), 1 mg L-1 of pyridoxine-HCL (SIGMA®, P-8 666), 1 mg L-1 Kinetin, 30 g L-1 sucrose (SIGMA®, K-0753 ), 8 g L-1 of agar (SIGMA®, A-1 296) adjusting pH to 5.7 and sterilized at 120 °C for 15 min and transferred to an incubation room at temperature of 25±1 °C, with 16 h light and 8 of darkness at 2500 lux.
Establishment
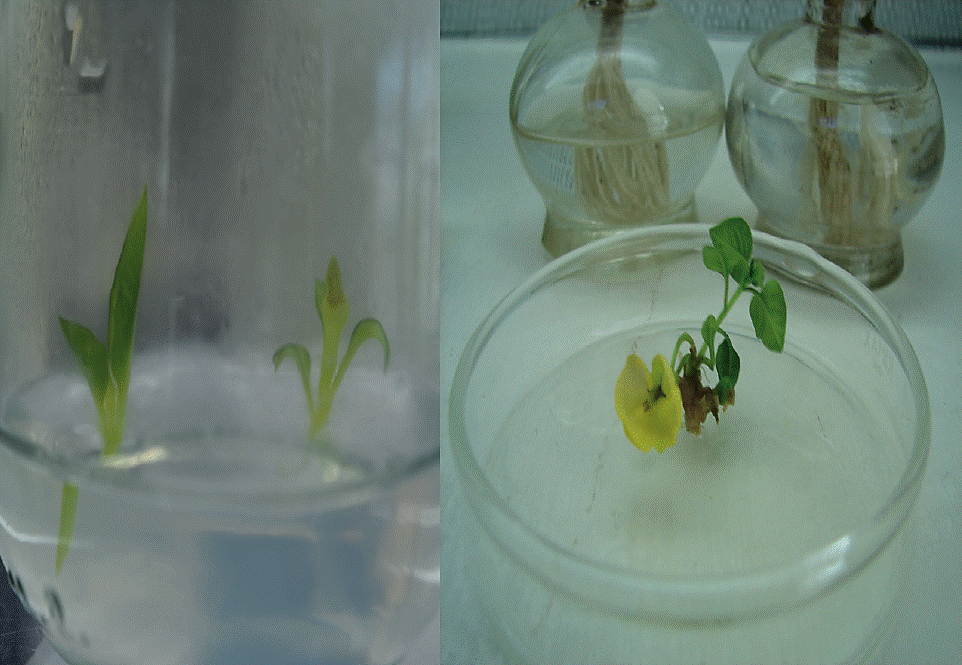
For this stage germinated seedlings in vitro on MS basal medium at half concentration added with 100 mg L-1 myo-inositol (SIGMA®, I-3011), 1 mg L-1 thiamine-HCL (SIGMA®, T-3906), 1 mg L-1 pyridoxine-HCL (SIGMA®, P-8666), 80 mg L-1 of adenine, 30 g L-1 of sucrose (SIGMA®, K-0753), 4 g L-1 of phytagel (SIGMA®, A-1296), adjusting pH to 5.7, were used. Seedlings generated after 15 d were sectioned to obtain explants.
Micropropagation
For micropropagation MS medium and PCL2 were used, added with 100 mg L-1 of myo-inositol (SIGMA®, I-3011), 1 mg L-1 thiamine-HCL (SIGMA®, T-3906), 1 mg L -1 pyridoxine-HCL (SIGMA®, P-8666), 40 mg L-1 of adenine, 30 g L-1 of sucrose (SIGMA®, K-0753), 4 g L-1 of phytagel (SIGMA®, A -1 296), adjusting pH to 5.7 and sterilized at 121 °C for 15 min. The seed was transferred to the incubation room at a temperature of 25+1 °C with 16 hours light and 8 of darkness at 2 500 lux. The medium was renewed every 15 days. The combination of plant growth regulators used: BAP (benzylaminopurine) and kinetin at 0, 0.5, 1, 2 and 3 mg L-1 with naphthalene acetic acid (ANA) at 0 and 0.5 mg L-1.
As explants the hypocotyls, cotyledons and nodal segments were used. Variables assessed were number of buds per explant and explant length, number of roots and root length.
Results and Discussion
Viability
75% of tomato husk seeds were viable and 25% non-viable, indicating that the seed is out of the range of physiological quality for seed marketing or for germination studies, SNICS recommends a minimum of 85%. These data are consistent with a previous study to determine the pollen viability on tetraploid varieties of tomatillo where a decrease in viability was observed compared with pollen from diploid plant (Ramírez et al., 2013), this data confirms the aforementioned by some authors on the decrease in viability and fertility of polyploid seed (Bretagnolle et al., 1995).
The comparison of various treatments (Figure 1 and 2) indicated that control had the lowest values for germination with 13.33% and an IVG of 0.19, initiating emergency on day 21. Treatment with AG showed a significant increase in germination at all concentrations (X= 64.44). The emergence began on the fourth day after planting with a germination percentage for AG1, AG2 and AG3 of 73.33, 56.66, and 63.33% and an IVG of 20.26, 10.44 and 11.59 respectively. It has been reported that AG participates in regulatory processes of plant growth and development.

Figure 2 Performance index of emergency speed tomatillo seeds in tetraploid (Physalis ixocarpa), applying regulators and organic acids.
Treatment with gibberellic acid is associated with rapid use of amino acids and amides sythesis, which increases the germination rate (Gupta and Mukherjee 1982). It is also related with the increase of phenols by modifying the biosynthetic pathway of flavonoids (Gosch et al., 2003; Halbwirth et al., 2003). In the seed phenols are produced in the testa and are related to the embryo morphogenetic processes (Dixon et al., 2005; Zhao et al., 2010; Dean et al., 2011).
Regarding organic acids the ASS treatment showed the highest average in germination (X= 28.88), followed by AB (X= 26.66), and the lowest average AS (X= 18.88). Note that although IVG (Figure 3) were lower, germination with organic acids initiated the first day after sowing. Organic acids derived from the metabolic pathway of phenylpropanoid compounds such as plant hormones involved in the intracellular signaling cascade and control the production of secondary compounds that work as plant defenses (Álvarez and Espinosa, 2004).
Seed imbibition pretreatment and planting the same day increase germination rate and seed emergence.
The results confirm that germination pretreatments are a tool that increases germination rate and percentage.
Micropropagation
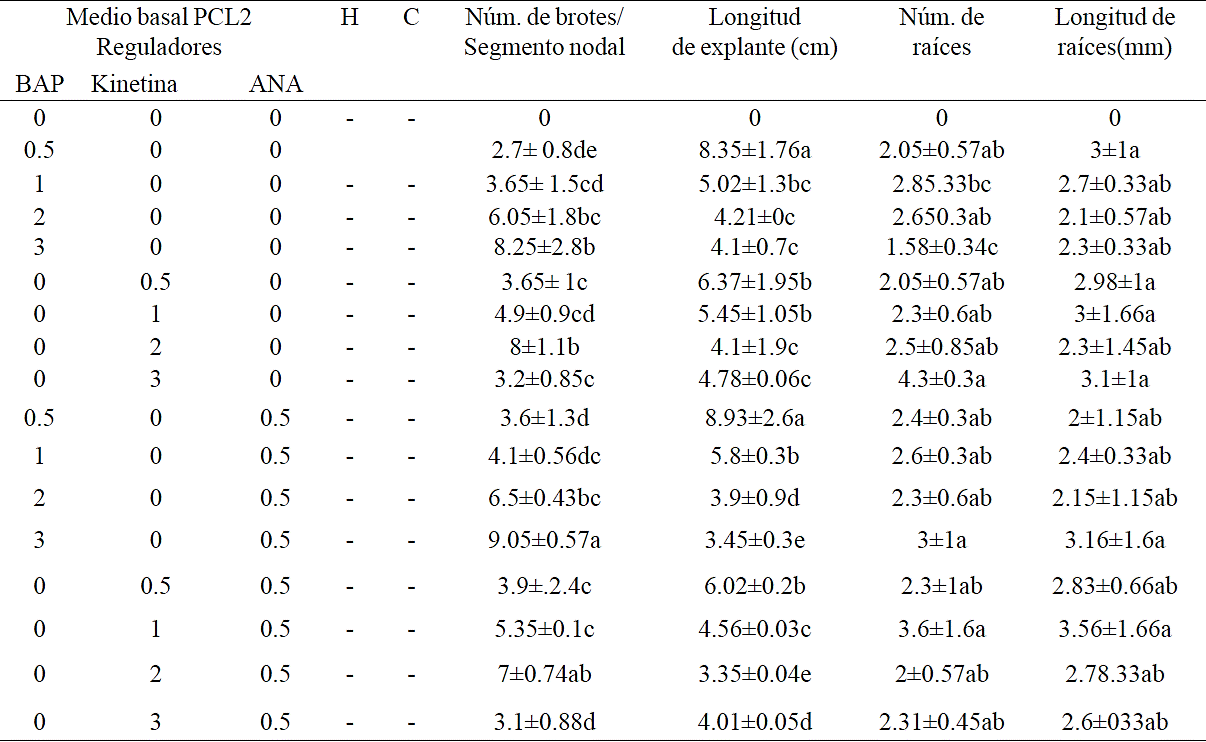
This stage aimed to establish a protocol for clonal propagation of the tetraploid cultivar P. ixocarpa. For this, two basal mediums and different combinations and concentrations of regulators were tested. All treatments resulted in regeneration of shoots (Table 1 and 2), but the induction was greater in the treatment with 3 mg L-1 BAP in combination with ANA with 9.5 shoots per explant, from nodal segments. The highest bud length (8.93 cm) was obtained with 0.5 mg L-1 BAP with 0.5 ANA (8.02 cm) from PLC2, this medium also showed the highest root number (4.3) and highest length of the same (4.6 mm), was achieved in treatment MS medium with 1 mg L-1 BAP. The lowest response (2.7 shoots / explant) was recorded in the BAP treatment (0.5 mg L-1) alone, in the PCL2 medium.
Table 1 Comparison of means of the effect of different concentrations of BAP, kinetin and NAA in the number of shoots, shoot length, number and length of roots in MS basal medium. (H= hypocotyls, cotyledons = C).

Table 2 Comparison of means of the effect of different concentrations of BAP, kinetin and NAA in the number of shoots, shoot length, number and length of roots in PCL2 basal medium (H= hypocotyl C= cotyledons).
A differential response between the various explants both cotyledons and hypocotyl did not generate a morphogenetic response contrary to that observed by Contreras and Almeida (2003), who mention a greater number of buds (66) with hypocotyl on MS medium with BAP ( 2 mg L-1), while the cotyledons generated lower response (54) with zeatin. Also Ramírez-Malagón and Ochoa-Alejo (1991) mention a higher proliferation of shoots from hypocotyl with 2.5 mg L-1 BAP and with 1.0 mg L-1 ANA.
An interesting observation was the ability of flower formation in vitro system (Figure 3). Between the two cytokinins, BAP was the most effective in inducing greater number of shoots per explant compared to kinetin. In this regard, other researchers agree that BAP was the cytokinin inducing higher formation of buds (29-32) in the genus Physalis (Afroz et al., 2009).
There are other works on this genus where BAP and ANA combination (Alejo, 1991; Yousry, 2013) achieved an optimal morphogenetic response.
An interesting observation was the ability of flower formation in vitro system (Figure 3). Also in this case, the root system developed spontaneously eliminating the need of a medium with auxin before acclimatization. According to Zhao et al. (2008) a high ratio between cytokinin and auxin promotes root regeneration.
Andrade-Rodríguez et al. (2005) observed a wide variability in obtaining shoots in different varieties of Physalis ixocarpa from 1.9 in wild varieties to 21.3 in domesticated. These authors mention that the organogenic capacity is given by the genotype of the variety in use. The organogenic ability of this cultivar is below that reported for domesticated cultivars.
Plant response depends largely from the genotype and some modifications and adjustments must be made when new species or cultivars are considered for this technique. The tetraploid cultivar showed need for high concentration of exogenous cytokinin in the propagation step.
Literatura citada
Al, H. A.; Monneveaux, P. and Nachit, M. M. 1998. Direct and indirect selection for drought tolerance in alien tetraploid wheat durum wheat crosses. Euphytica. 100: 287-294. [ Links ]
Afroz, F.; Hassan, A. K. M. S.; Bari, L. S.; Sultana, R.; Begum, N.; Jahan, M. A. A. and Khatun, R. 2009. In vitro shoot proliferation and plant regeneration of Physalis minima L a perennial medicinal Herb. Bangladesh J. Sci. Ind. Res. 44(4):453-456. [ Links ]
Andrade-Rodríguez, M.; López-Peralta, M. C.; González-Hernández V. A.; García-Velázquez A. y Peña-Lomelí A. 2005. Efecto del genotipo en la micropropagación de tomate de cáscara. Rev. Chapingo Serie Horticultura. 11(1):31-37. [ Links ]
Arroyo-Medina, C.; Benavides-Mendoza, A.; Ramírez, R. H. y RuizTorres, N. A. 2008. Efecto de ácidos orgánicos sobre la germinación de semillas de hortalizas. Libro Científico Anual de Ganadería y Ciencia Forestal, UAAAN. 107-115. [ Links ]
Álvarez, T. M. C. y Espinosa, F. B. 2004. Jasmonatos y Salicilatos: Fitohormonas clave en las reacciones de defensa de las plantas y de comunicación en el ecosistema. In: la ecofisiología vegetal: una ciencia de síntesis. (Ed.). Reigosa, J. M.; Pedrol, N. y Sánchez, A. Ed. Thomson. Madrid España. 633-723. [ Links ]
Beaulieu, J. M.; Moles, A. T.; Leitch, I. J.; Bennett, M. D.; Dickie, J. B. and Knight, C. A. 2007. Correlated evolution of genome size and seed mass. New Phytologist. 173:422- 437. [ Links ]
Bretagnolle, F.; Thomson, J. D. and Lumart, R. 1995. The influence of seed size variation on seed germination and seedling vigor in diploid and tetraploid Dactylis glomerat L. Ann. Bot. 76:607-615. [ Links ]
Contreras, I. y Almeida, J. 2003. Micropropagación de Tomatillo (Physalis ixocarpa L). Revista de la Facultad de Farmacia. 45(1):61-64. [ Links ]
Dahal, P. and Bradford, K. J. 1990. Effects of priming and endosperm integrity on seed-germination rates of tomato genotypes. 2. Germination at reduced water potential. J. Exp. Bot. 41(11):1441-1453. [ Links ]
D’Arcy, W. G. 1991. The Solanaceae since 1976, with a review of its biogeography. In: Solanaceae III: taxonomy, chemistry, evolution (Eds.). Hawkes, J. G.; Lester, R. N.; Nee, M. and Estrada, N. Royal Botanic Gardens, Kew. United Kingdom, 75-138 pp. [ Links ]
Dean, G.; Cao, Y. G.; Xiang, D.; Provart, N. J.; Ramsay, L. and Ahada, A. 2011. Analysis of gene expression patterns during seed coat development in Arabidopsis. Mol. Plant. 4:1074-1091. [ Links ]
Dixon, R. A.; Xie, D. Y. and Sharma, S. B. 2005. Proanthocyanidins - a final frontier in flavonoid research? New Phytol. 165:9-28. [ Links ]
Gosch, C.; Puhl, I.; Halbwirth, H.; Schlangen, K.; Roemmelt, S.; Andreotti, C.; Costa, G.; Fischer, T. C.; Treutter, D.; Stich, K. and Forkmann, G. 2003. Effects of prohexadione-Ca on various fruit crops: Flavonoid composition and substrate specificity of their dihydroflavonol 4-reductases. European J. Hortic. Sci. 68(3):144-151. [ Links ]
Gupta P. and Mukherjee, D. 1982. Influence of GA3 pre-soaking of seeds on biochemical changes in seedling parts of Pennisetum typhoides Rich. Proceedings of Indian National Science Academy B. 48(5):642-648. [ Links ]
Guzmán, R. E. E.; Godínez, F. H.; De La Vega, O. M. and Alejo, N. O. 2009. In vitro embryo formation and plant regeneration from anther culture of different cultivars of Mexican husk tomato (Physalis ixocarpa Brot.) Plant Cell Tissue Organ Culture. 96:181-189. [ Links ]
Halbwirth, H.; Fischer, T.; Roemmelt, S.; Spinelli, F.; Schlangen, K.; Peterek, S.; Sabatini, E.; Messina, C.; Speakman, J. B.; Andreotti, C.; Rademacher, W.; Bazzi, C.; Costa, G.; Treutter, D.; Forkmann, G. and Stich, K. 2003. Induction of antimicrobial 3-deoxyflavonoids in pome fruit trees controls fire blight. Zeitschrift fur Naturforschung. 58:765-770. [ Links ]
Hardy, O. J.; Vanderhoeven, S.; De Loose, M.; and Meerts P. 2000. Ecological, morphological, and allozymic differentiation between diploid and tetraploid knapweeds (Centaurea jacea) from a contact zone in the Belgian Ardennes. New Phytol. 146:281-290. [ Links ]
Izadpanah, M. and Khui, M. K. 1989. Comparisons of in vitro propagation of tomato cultivars. Iran Agric. Res. 8(1): 34-47. [ Links ]
Maguirre, J. D. 1962. Speed of germination-aid in selection and evaluation for seedling emergence and vigor. Crop Sci. 2:176-177. [ Links ]
Mandal, S. M.; Chakraborty, D. and Dey, S. 2010 Phenolics acid act as signaling molecules in plant-microbe symbioses. Plant Signal Behavior. 5: 359-368. [ Links ]
Menzel, Y. M. 1951. The citotaxonomy and genetics of Physalis. Proceedings of the American Philosophical Society. 95:132-183. [ Links ]
Nakamura, N.; Masuo, Y. and Ooyabu, E. 2007. Development of tetraploid chinese lantern (Physalis alkekengi L. var. franchetii Makino) and its characteristics. Hortic. Res. 6(3):341-345. [ Links ]
Ortuño, O.; Manzo, G. A. y Peña, A. 1997. Cultivo de anteras en tomate de cáscara (Physalis ixocarpa Brot.). Rev. Chapingo Serie Hortic. 4:39-43. [ Links ]
Peña, L. A. y Márquez, F. S. 1990. Mejoramiento genético de tomate de cáscara (Physalis ixocarpa Brot.). Revista Chapingo 71(72):85-88. [ Links ]
Peña, L. A. y Santiaguillo, H. J. F. 1999. Variabilidad genética de tomate de cáscara en México. Boletín Técnico #2. Departamento de Fitotecnia. Universidad Autónoma Chapingo. Chapingo (UACH). Texcoco, Estado de México. 26 p. [ Links ]
Peña-Lomeli, A.; Molina-Galán, J. D.; Cervantes-Santana, T.; MárquezSánchez, F.; Sahagún-Castellanos, J. and Ortiz-Cereceres, J. 1998. Heterosis Intervarial en tomate de cáscara (Physalis ixocarpa Brot). Revista Chapingo Serie Horticultura 4 (1): 31-37. [ Links ]
Peña-Lomelí, A.; Molina-Galán, J. D.; Márquez-Sánchez, F.; SahagúnCastellanos, J.; Ortiz-Cereceres, J. y Cervantes-Santana, T. 2002. Respuestas estimadas y observadas de tres métodos de selección en tomate de cáscara (Physalis ixocarpa Brot.) Rev. Fitotec. Mex. 25:171-178. [ Links ]
Ramírez- Godina, F.; Robledo-Torres, V.; Foroughbakhch-Pournavab, R.; Benavides-Mendoza, A. y Alvarado-Vázquez, M. A. 2013. Viabilidad de polen, densidad estomática y tamaño de estomas en autotretraploides y diploides en Physalis ixocarpa. Bot. Sci. 91(1):11-18. [ Links ]
Ramírez, G. F. 2012. Caracterizacion de tetraploides y formación de híbridos triploides en tomate de cascara (Physalis ixocarpa Brot). Tésis de Doctorado. Universidad Autónoma de Nuevo León. 115 p. [ Links ]
Ramírez-Malagón, R. and Ochoa-Alejo, N. 1991. Adventitious shoot formation and plant regeneration from tissues of tomatillo (Physalis ixocarpa Brot.). Plant Cell Tissue and Organ Culture. 25(3):185-188. [ Links ]
Robledo, T. V.; Ramírez, G, F.; Foroughbakhch, P. R.; Benavides, M. A.; Hernández, G. G. and. Reyes, M. H. V. 2011. Development of tomatillo (Physalis ixocarpa Brot.) Autotetraploids and their chromosome and phenotypic characterization. Breed. Sci. 61:288-293. [ Links ]
Sánchez, M. J.; Padilla, G. J. M.; Bojórquez, M. B. A.; Arriaga, R. M. C.; Arellano, R. L. J.; Sandoval, I. E. y Sánchez, M. E. 2006. Tomate de cáscara cultivado y silvestre del occidente de México. Secretaria de Agricultura, Ganadería, Desarrollo Rural, Pesca y Alimentación (SAGARPA). Guadalajara, Jalisco, México. 176 p. [ Links ]
Santiaguillo, H. J. F.; López, R. M. Peña-Cuevas, A. L. y Sahagún, J. C. 1994. Distribución, colecta y conservación de germoplasma de tomate de cáscara (Physalis ixocarpa Brot.). Rev. Chapingo Serie Horticultura. 2: 125-129. [ Links ]
SAGARPA (Secretaría de Agricultura, Ganadería, Desarrollo Rural, Pesca y Alimentación) - SIAP (Servicio de Información y Estadística Agroalimentaria y Pesquera). 2015. www.siap.sagarpa.gob.mx. [ Links ]
Van, G. J. and Kroon, H. 1990. Clonal growth in plants: regulationand function. S. P. B. Academic Publishing. The Hague, The Netherlands. 196 p. [ Links ]
Yousry, M. M. 2013. In vitro propagation and somatic embryogenesis in Egyptian Husk tomato (Physalis pubescens L.). J. Appl. Sci. Res. 9(3):1415-1425. [ Links ]
Zhang, X. Y.; Hu, C. G. and Yao, J. L. 2010. Tetraploidization of diploid Dioscorea results in activation of the antioxidant defense system and increased heat tolerance. J. Plant Physiol. 167:88-94. [ Links ]
Zhao, J.; Pang, Y. and Dixon, R. A. 2010. The misteries of proanthocyanidin transport and polimerization. Plant Physiol. 153:437-443. [ Links ]
Zhao, X. Y.; Su, Y. H.; Cheng, Z. J. and Zhang, X. S. 2008. Cell fate switch during in vitro plant organogenesis. J. Int. Plant Biol. 50(7):816-824. [ Links ]
Received: April 2015; Accepted: July 2015











 texto em
texto em