Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista mexicana de ciencias agrícolas
versão impressa ISSN 2007-0934
Rev. Mex. Cienc. Agríc vol.6 spe 12 Texcoco Nov./Dez. 2015
Articles
Assessment and morphological characterization from isolates of native mycorrhizal associated with tomatillo
1Universidad Autónoma Agraria Antonio Narro-Ciencias en Horticultura.
2Universidad Autónoma Agraria Antonio Narro- Departamento de Horticultura. Calzada Antonio Narro 1923. Buenavista. Saltillo. Coahuila C. P. 25315. México.
3Centro Nacional de Referencia de Control Biológico. Laboratorio de biología molecular. Carretera Tecomán-Estación FFCC. A. P. 67, Col. Tepeyac. C. P. 28110 Tecomán, Colima, México.
4Centro de Investigación de Química Aplicada. Blvd. Enrique Reyna Hermosillo No. 140 C. P. 25294. Saltillo, Coahuila México.
The diversity of arbuscular mycorrhizal fungi (HMA) is an alternative for biofertilization in agriculture, which makes necessary to perform studies to assess the relationship of HMA communities regarding soil physicochemical conditions and host. In 2014, at the Universidad Autonoma Agraria Antonio Narro evaluated and morphologically characterized native spores of HMA associated with tomatillo, trap crop (wheat) under controlled conditions. A soil sampling was conducted (profile: 0-30 cm) in Arteaga Coahuila to perform a physicochemical analysis of soil (MO, N, P, K, pH and texture), HMA spores associated to the rhizosphere from tomatillo cultivated and wild were extracted through wet sieving and decanting (sieve opening: 50 µm , 325 µm , 400 µm); grouping by morphology (shape, color and size), a monosporic crop (wheat) was established in pots of 1 L, with sand as substrate (sterile), and Steiner solution modified in phosphorus at 20%. Morphological characterization of mycorrhizal isolates (INVAM, 2014) was performed. 21 morphotypes (12 wild and 9 cultivated) were obtained, of which 9 from the wild system established symbiosis and two from the cultivated system. N, P, K and MO content was higher in the soil from the agricultural system compared to wild; both soils are classified as alkaline. The amount of N, P, K and MO in the two systems where mycorrhizae develops, were factors that influenced on the establishment of symbiosis in wheat and morphological characters of HMA under controlled conditions.
Keywords: morphology; potential inoculum; tomatillo; wheat
La diversidad de hongos micorrìcicos arbusculares (HMA) son una alternativa de biofertilización en la agricultura, lo que hace necesario estudios que permitan evaluar la relación de comunidades de HMA respecto a condiciones fisicoquímicas del suelo y hospedero. En 2014, en la Universidad Autónoma Agraria Antonio Narro se evaluaron y caracterizaron morfológicamente esporas nativas de HMA asociadas a tomatillo, cultivo trampa (trigo) bajo condiciones controladas. Se llevó a cabo un muestreo de suelo (perfil: 0-30 cm) en Arteaga Coahuila del cual se realizó análisis fisicoquímico de suelo (MO, N, P, K, pH y textura), se extrajeron esporas de HMA asociadas en la rizósfera de tomatillo cultivado y silvestre mediante tamizado húmedo y decantación (tamiz abertura de: 50 µm, 325 µm, 400 µm); se realizó agrupación morfológica (forma, color y tamaño), se estableció cultivo monospórico ( trigo), en macetas de 1 L, con arena como sustrato (estéril), y fertilización Steiner modificada en fósforo al 20%. Se realizó caracterización morfológica de aislados micorrìcicos (INVAM, 2014). Se obtuvieron 21 morfotipos (12 de silvestre y 9 de cultivado), de los cuales 9 del sistema silvestre establecieron simbiosis y dos del sistema cultivado. El contenido de N, P, K y MO fue mayor en el suelo del sistema agrícola en comparación al silvestre, ambos suelos son clasificados como alcalinos. La cantidad de N, P, K y MO en los dos diferentes sistemas donde se desarrollaron las micorrizas fueron factores que influyeron sobre el establecimiento de simbiosis en trigo y caracteres morfológicos de HMA bajo condiciones controladas.
Palabras clave: morfología; potencial de inóculo; tomatillo; trigo
Introduction
The importance of biodiversity functions have been increasing over time Cardinale et al. (2007), a reason to understand and improve the functionality and production of agricultural systems Tilman et al. (1996); (Moonen and Bàrberi, 2008). The concern comes from the global demand for food, as it has been projected that world population will be eleven billion by the end of this century (UN, 2011). Consequently the demand for resources such as land, energy, biomass, and phosphorus reserves will grow. The challenge is to feed the entire population without causing damage with irreparable consequences to the environment (Rockström et al., 2009a; Rockström et al., 2009b).
Flows of reactive nitrogen and phosphorus represent a great danger to the ecosystem, in general the flow of excess nutrients causes eutrophication, consequently biodiversity decreases, this is one of many problems caused by agriculture trying to meet the demand for food. Soil microbial diversity has taken too much importance until now because this have the ability to improve the efficiency in nutrients use, increase soil aggregate stability, improve the formation of organic matter and water efficiency (Mäder et al., 2002; Brussaard et al., 2007).
Arbuscular mycorrhizal fungi (HMA; Glomeromycota; Schüßler et al. (2001) are microorganisms that establish symbiosis with more than 80% of terrestrial plants (Read et al., 2008). This symbiosis that arises from more than 450 million years is undoubtedly the most abundant symbiosis from the world (van der Heijden et al., 2008). HMA have the ability to provide important ecosystem services (Philippot et al., 2013) such as tolerance to biotic and abiotic stress, improve water and nutrients absorption (Hodge and Storer, 2015) and plant phenotypic plasticity. This symbiosis represents an important advantage in heterogeneous environments where resource allocation is limited between growth and resistance to various stresses leading the plant to adapt and survive (Pozo et al., 2015). Despite the large number of ecological studies that are focused on diversity in function of eco and agro-ecosystem, the information is limited (Shennan 2008; Toljander et al., 2008).
The actual evidence on the number of species on the edge of Glomeromycota Schüßler et al. (2001) is close to 200 (Redecker et al., 2000). However, the number of species may actually be of greater magnitude (Vandenkoornhuyse et al., 2002). One reason why a small number of species are reported is for technical reasons since taxonomic delimitation is one of the major problems.
The current taxonomy is still the determination of morphological characters of asexual spores at rest Vandenkoornhuyse et al. (2003); however, this does not discard the usefulness of determining morphological characters and molecular techniques to confirm the types of HMA present in the soil and the amount. Their isolation allows to study more deeply the organism to which they belong and the relationship between genetic structure of mycorrhizal fungi communities, efficiency and functional diversity are related to environmental conditions and plant species. Agricultural systems are subjected to selection pressures of both pathogens and beneficial microorganisms due to short crop cycles of agricultural interest, feature not shared by natural systems (Burdon and Thrall, 2008). That is why in this research aimed to evaluate and characterize at morphological level monosporic isolates of native HMA associated with tomatillo in wheat plants under controlled conditions.
Materials and methods
Soil sampling
In December 2012, were taken soil samples of 1 kg in a natural area (not cultivated) and soil for agricultural use. The sample was taken from the rhizosphere of cultivated tomatillo (rendidora variety) and wild tomatillo in the profile of 0-30 cm deep. In two locations of Arteaga, Coahuila, Mexico 25° 20' 56.35" north latitude - 100° 40' 45.84" west longitude and 25° 23' 23.63' north latitude - 100° 37' 33.97 west longitude respectively. Note that tomatillo has been grown for 10 consecutive years and regarding tomatillo from the natural system it has remained the same time without cultural practices.
Physicochemical analysis of soil
Two soil analysis were performed at the Universidad Autonoma Agraria Antonio Narro (25° 23' north latitude - 101° 02' west longitude) in the soil department, in order to determine the physicochemical conditions where tomatillo and wheat plants developed, organic matter was determined using the method of Walkley and Black Bornemisza (1979), nitrogen (N) with Kjeldahl Bremner (1960), phosphorus (P) Olsen (1954), potassium (K) (atomic absorption spectrometry), pH (soil: water 2: 4) and texture (Bouyoucos, 1936).
Monosporic culture
The spores from arbuscular mycorrhizal fungi were extracted from the soil samples through wet sieving (sieve. 50, 325 and 400µm) and decanting Gerdemann et al. (1963) followed by a gradient of 2M sucrose (Furlan et al., 1980; Horn et al., 1992). A morphological clustering was performed considering shape, color and size (INVAM, 2014). The obtained morphotypes were inoculated in Triticum aestivum L. (1 spore per plant / 10 reps) seedlings. The monosporic culture was established in pots of 1 liter capacity, and as substrate sterile sand, also to avoid contamination the pots were covered with plastic. The plants were kept in a greenhouse at an average temperature of 40 °C, Steiner et al., (1973) nutrient solution was applied, modified in phosphorus 20%, pH 7.5 - 8, watering daily. Soil samplings were performed monthly to evaluate the propagation of the inoculated spore with different morphotypes; in the fourth month, one wheat seed was placed per pot for a second crop cycle in order for the spores to multiply and establish symbiosis. In addition, the plants were subjected to water stress, completely suspending the irrigation in the last two months in order to accelerate this symbiosis.
Root colonization (Triticum aestivum L.)
In order to determine the degree of root colonization, an analysis was carried out in the eighth month of each of pot by morphotype. To assess successful symbiosis from morphotypes of arbuscular mycorrhizal fungi on wheat were clarified and stained with trypan blue wheat root fragments of 1cm length from the different morphotypes following technique Clarification-staining roots and estimating root colonization by mycorrhizal -arbuscular fungi (Walker et al., 2005). Finally, the root fragments were mounted vertically on slides (3 slides per plant / 50 observations) adding a drop of fresh acidified glycerol for observation under microscope to estimate colonization (McGonigle et al., 1990).
Morphological analysis
The morphological characteristics of spores were described from spores mounted in a mixture (1: 1) reagent PVLG and Melzer (Brundrett et al., 1994). For color and shape was taken as reference the international collection of HMA (INVAM, 2014). The pictures were taken in light microscope (Axio Scope A1, Carl Zeiss Microscopy GmbH, Gottingen, Germany) with integrated camera Axio Cam Icc 1 10X objective, measurements were performed using the software Axion Vision Release 4.8.2.
Results and discussion
Physicochemical analysis of soil
Table 1 shows in detail the results of physicochemical analysis where tomatillo plants were grown under agricultural systems (TC) and natural system (TS). It is noted that tomatillo plants of both systems grew in alkaline soils, soil texture clay and sandy clay loam respectively; organic matter content (MO), nitrogen (N), phosphorus (P) and potassium is higher in soil for agricultural use (TC) compared to a natural soil system (TS). Long-term studies have shown that fertilization cause significant impact on arbuscular mycorrhizal fungi communities both in species diversity and its functioning (Thomson et al., 1992; Kahiluoto et al., 2009).
Table 1 Physicochemical characteristics of soil where plants tomatillo developed under two different systems.

* TC= tomatillo cultivado; TS= tomatillo silvestre.
Characteristics from arbuscular mycorrhizal fungi differ, because studies have shown that some species form more than one spore morphotype Stockinger et al. (2009); however, the current characterization performed in this study, indicates the types of arbuscular mycorrhizal fungi present in soil and their abundance. This likewise allows isolation to study more deeply the body belongs.
Morphological analysis
The morphological diversity of spores from arbuscular mycorrhizal fungi that were associated to tomatillo rhizosphere from wild soil (TS) was higher compared to cultivated tomatillo (TC) (Figure 1). It has been shown in several studies that agricultural systems are exposed to intense microbial selection pressures due to short cycles and crop management, therefore sporulation of arbuscular mycorrhizal fungi is affected by agriculture, being the opposite in natural systems Burdon and Thrall (2008); Oehl et al. (2003), which is consistent with the results obtained in this study.
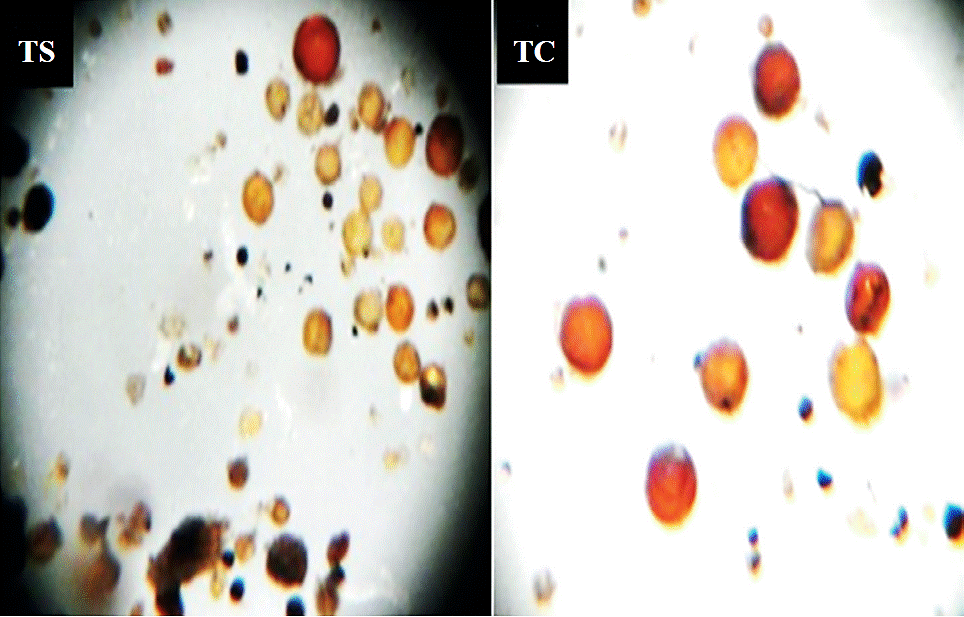
Figure 1 Morphological diversity of arbuscular mycorrhizal fungi associated with rhizosphere of wild tomatillo (TS) and tomatillo cultivated (TC) (objective 3 X).
According to the morphological characteristics of the spores associated to the rhizosphere of cultivated tomatillo (rendidora variety) nine morphotypes (Table 2) were grouped.
Table 2 Morphological characteristics of spores of arbuscular mycorrhizal fungi associated to rhizosphere grown tomatillo (Physalis ixocarpa cv. Rendidora).
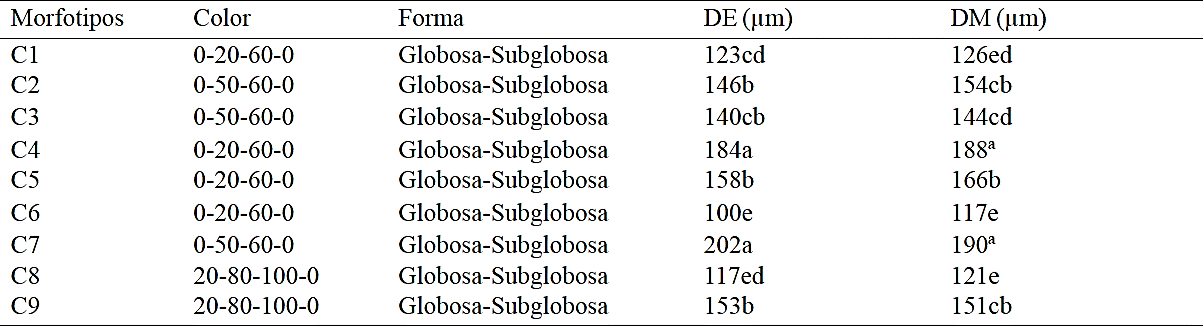
*0-20-60-0= red- brown, 0-50-60-0=reddish-orange, 20-80-100-0= red- brown (INVAM, 2014). DE= diámetro ecuatorial; DM= Diámetro meridional. Promedios con la misma letra indican diferencia no significativa (Tukey´s 0.05).
Means comparison test Tukey (≤ 0.05) indicates that the C7 and C4 morphotypes have greater equatorial and meridional diameter with 202 µm - 190 µm; 184 µm - 188μm respectively; while morphotype C6 has smaller diameter 100 µm - 117 µm, certain spore colors are 0-50-60-0 (reddish-orange), 20-80-100-0 (red- brown) and 0- 20-60-0 (red- brown) being this the most frequently observed, most morphotypes showed a globular - subglobular shape.
Table 3 shows the grouping of 12 morphotypes associated with rhizosphere of wild tomatillo according to the morphological characteristics of the spore. Means comparison test Tukey (≤ 0.05) indicates that morphotype S9 presents higher equatorial and meridional diameter with 249 μm and 238 μm- while morphotype S7 presents diameters of 95 μm - 104 μm considered as morphotype with the smallest size; determined spore colors are: 20-80-100-0 (red-brown), 0-1090-5 (orange), 0-60-100-10 (orange brown) and 0-50-60-0 (reddish-orange) observed more frequently at 0-60-100-10 (orange brown), the shape of all morphotypes is globular or subglobular.
Table 3 Morphological arbuscular mycorrhizal fungi spores associated with rhizosphere of wild tomatillo Features.
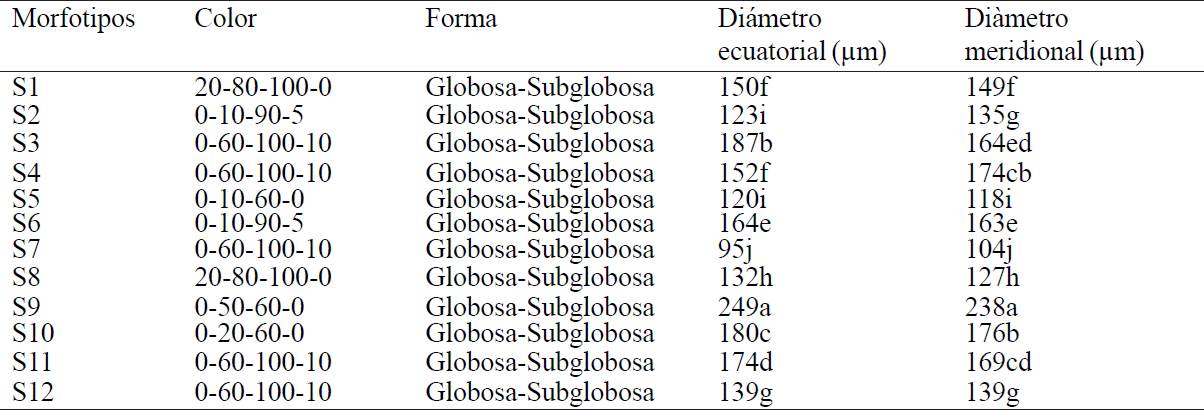
*20-80-100-0=red-brown, 0-10-90-5=orange, 0-60-100-10=orange brown, 0-10-60-0=pale orange-yellow, 0-50-60-0=reddish-orange, 0-20-60-0=red brown (INVAM, 2014). Promedios con la misma letra indican diferencia no significativa (Tukey p ≤ 0.05).
In this study it was determined that there is greater morphological diversity of arbuscular mycorrhizal fungi spores associated with the rhizosphere of wild tomatillo with 12 morphotypes compared to nine morphotypes of cultivated tomatillo, it is important to note that tomatillo plants from the two systems have been grown on this site during 10 years. There have been recent publications Oehl et al. (2009) showing that in cultivated land with crop rotation and presence of weeds or perennial grasses (clover) were more rich in species of arbuscular mycorrhizal fungi, contrary to that happening in crops consecutively planted (monoculture) and even richer than natural grasslands, so it is clear that the results of morphological diversity of spores of arbuscular mycorrhizal fungi are lower in cultivated tomatillo because it is an intensive agricultural system, therefore the presence of other hosts (weeds) is zero or minimum for very short periods that there is no opportunity for more mycorrhizal morphotypes to express and vice versa in the natural wild tomatillo system.
Long-term studies have shown the impact of fertilization on arbuscular mycorrhizal fungi communities, reporting negative impacts on the composition and functionality of the different species (Thomson et al., 1992; Kahiluoto et al., 2009). It has been found that the addition of nitrogen fertilizers can alter the functional diversity of arbuscular mycorrhizae, besides, performing high nitrogen fertilization in soils rich in phosphorus decrease the richness and diversity of the communities of arbuscular mycorrhizal fungi (Egerton and Warburton et al., 2007). From the latter can be understood that wild tomatillo had higher spore diversity of mycorrhizal due to nitrogen limitations presenting 0.16% rated according to the physicochemical analysis as mid-soil compared to cultivated tomatillo (rendidora variety) with 0.22% considered moderately rich.
Organic matter and pH are parameters that influence on the composition of arbuscular mycorrhizal fungi; studies made in Colombia found high diversity and abundance of arbuscular mycorrhizal fungi in acid soils (Seralde and Ramirez, 2004).The real relationship whether strong or weak (Hijri et al., 2006) between soil properties and distribution of arbuscular mycorrhizal fungi species under field conditions is little known (Carvalho et al., 2003).
Morphological analysis of mycorrhizal isolated in wheat (Triticum aestivum L.)
The resulting spore from isolates have a globular- sub globular shape, the equatorial diameter (DE) of isolates from wild and cultivated system range from 108 μm - 170 μm and meridional diameter (DM) from 112 μm- 196 μm, the thickness of its spore wall (EPE) is 7 μm - 12.8 μm, the spore colors of the different isolates were: 0-60-100-10 (orange-brown), 0-10-60-0 (pale orange-yellow), 0-10-90-5 (orange), 0-0-40-0 (pale yellow), 0-50-60-0 (reddish-orange), 0-20-60-0 (red-brown), the number of layers of the spore is 1 on its majority except morphotype S1 which had two spore layers, all morphotypes has gallbladder and arbuscule (Table 4).
Table 4 Morphological characteristics of isolates of arbuscular mycorrhizal fungi associated with rhizosphere of wheat (Triticum aestivum L.).

*0-50-60-0= reddish-orange, 0-20-60-0= red-brown, 0-10-90-5= orange, 0-60-100-10= orange- brown, 0-10-60-0= pale orange-yellow, 0-040-0= pale yellow (INVAM, 2014). Promedios con la misma letra indican diferencia no significativa (Tukey p≤ 0.05).
According to statistical analysis in equatorial diameter the S9 isolate is significantly different with a mean 183a according to the mean group (Tukey p≤ 0.05) followed by S10 with a mean of 170ba; regarding to the meridional diameter isolated S4 showed significant difference wotj a mean of 196a followed by S10 with a mean of 173ba. Isolate S7 showed the lowest diameter both equatorial and meridional averaging 108d-112e respectively. The thickness of the spore wall according to statistical analysis and means comparison test (Tukey p≤ 0.05) isolate S4 and S10 are not statically different but numerically their diameter differs being larger S10 with an average of 12.8a followed by S4 with 12.6a, isolate S7 shows less thickness in spore wall averaging 7c.
It is appreciated that morphological characteristics from isolates differ from its natural system both cultivated and wild, as previously mentioned some species form more than one spore morphotype Stockinger et al. (2009). Recently Ehinger et al. (2009) studied individuals genetically different of G. intraradices isolated from the same field and found that isolates showed different growth strategies depending on the host and different levels of phosphorus, this means that the availability of phosphate affects the genotype of a forming arbuscular micorrhizal fungi isolated through multiple generations.
Similar dynamics have been found by Oliveira et al. (2010) working with isolates of Glomus geosporum during a year in two soils with different pH (acid-alkaline), these studies have proven that lineages resulting from arbuscular mycorrhizal fungi share a third of their genetic markers which means that when grown under the same conditions, mycelium density and spores density differ significantly. Situation that could be different in morphological characters from isolates of native mycorrhizal since its natural environment was modified subjecting them to Steiner nutrient solution modified to 20% in phosphorus and pH of 7.5- 8 and in substrate sand type under controlled conditions.
Inoculum potential of isolated mycorrhizal on wheat (Triticum aestivum L.)
From the 21 morphotypes that were obtained from wild system and 12 from cultivated, not all established successfull simbiosis in wheat plants (Figure 2). Of which 10 morphotypes belong to the wild system (S1, S2, S3, S4, S5, S6, S7, S9, S10 and S11) and 2 (C2 and C5) to cultivated system.
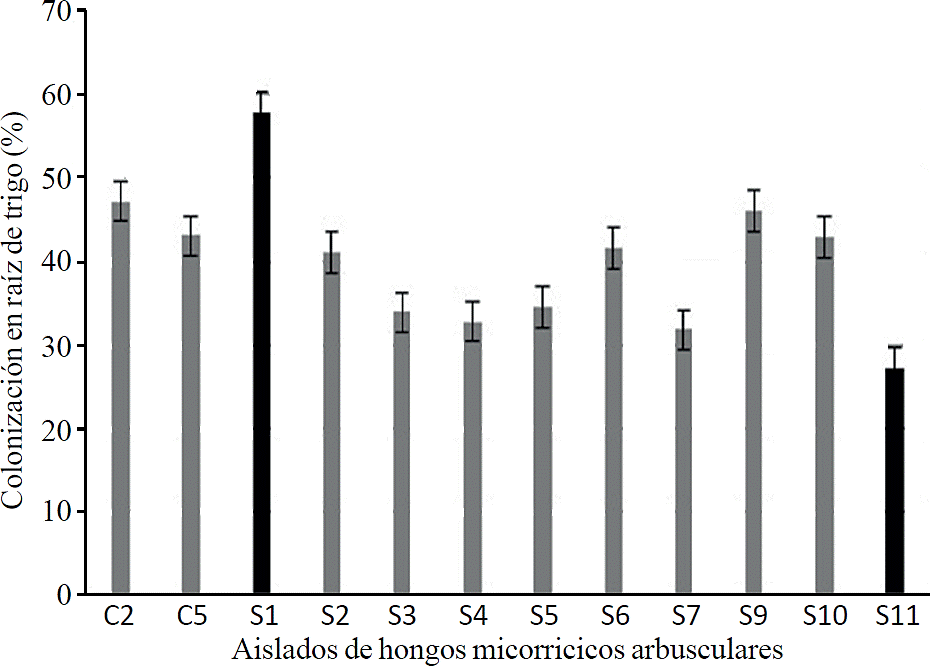
Figure 2 Colonization of mycorrhizal fungi isolated in root wheat (Triticum aestivum L.) 8 months after inoculation.
According to statistical analysis, isolate S1 is significantly different with 57.81 according to grouping mean (Tukey p≤ 0.05); followed by C2 with an average of 47.2ba, isolate S11 was who set lower symbiosis in wheat root with an average of 27.16c. Isolates C1, C2, C3, C4, C6, C7, C8 and C9 from the cultivated system and S8, S12 from the wild system did not establish symbiosis in wheat plants so they are not considered as potential inoculum in this research. In this study it can be said that shape, diameter and wall thickness characteristics are not indicators of mycorrhizal fungi with inoculum potential. It has been shown that there is a strict specificity between host and arbuscular mycorrhizal fungi, the result of the interactions dependent on its host and environmental conditions (Walder et al., 2012; Smith and Smith, 2015). Wheat is a crop that does not depend heavily on the association with arbuscular mycorrhizal fungi to obtain nutrients, but there are studies showing that wheat plants benefit in establishing symbiosis (Manske, 1990; Tawaraya, 2003).
Figure 3 shows morphological characteristics from isolates that established successful symbiosis in wheat (Triticum aestivum L.), which are considered as potential inoculum.
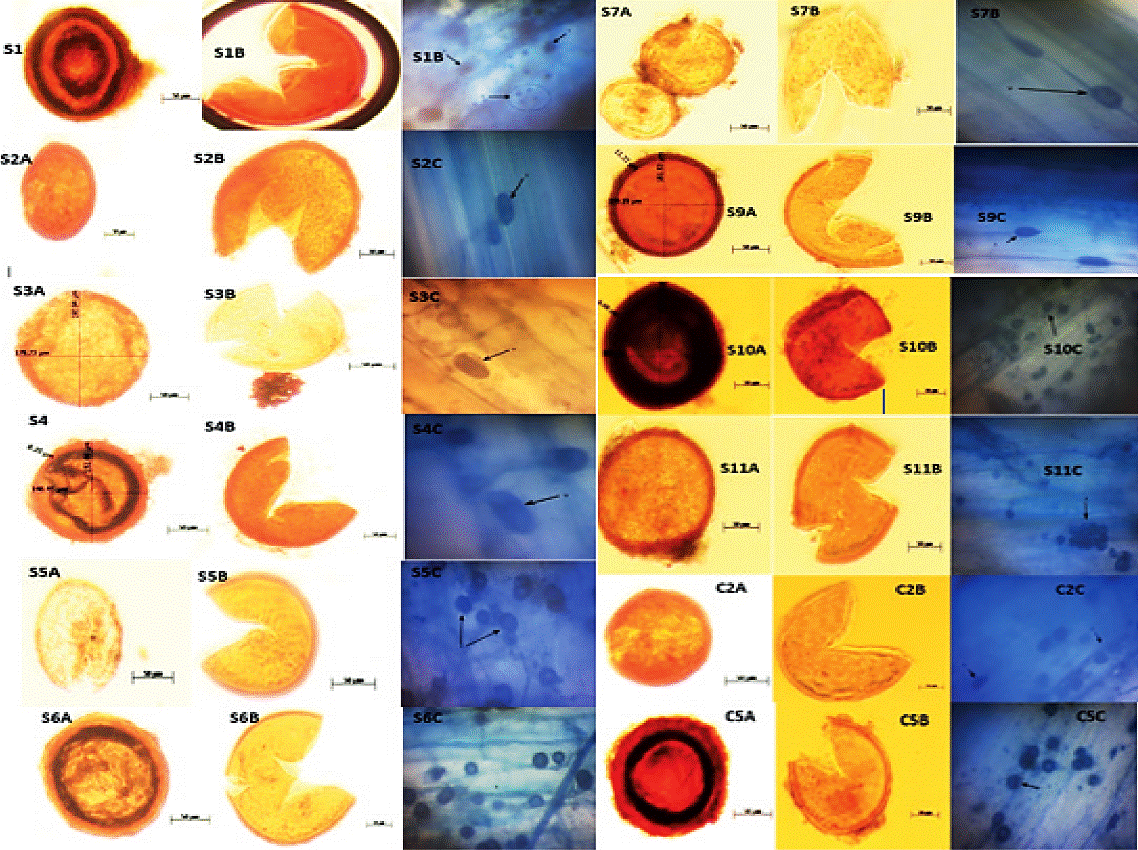
Figure 3 Morphological characters isolated arbuscular mycorrhizal fungi inoculum potential in wheat. S1A, S1B, S1C, S2A, S2B, S2C, S3A, S3B, S3C,S4A, S4B, S4C, S5A, S5B, S5C, S6A, S6B, S6C, S7A, S7B, S7C, S9A, S9B, S9C, S10A, S10B, S10C, S11A, S11B and S11C are wild morphotypes system; C2A, C2B, C2C and C5a, C5B, C5C are cultivated morphotypes of the system where (A) spore complete; (B) fractional spore; and (C) in root colonization respectively.
Conclusions
The morphological diversity of arbuscular mycorrhizal fungi associated in the rhizosphere of tomatillo of a wild system is higher compared to the rhizosphere of tomatillo (rendidora variety) from an intensive agricultural system; this was due to the availability of nutrients, null cultural practices in the wild system and intensive management in cultivated system.
Spore morphology of arbuscular mycorrhizal fungi differs according to the conditions in which these are established.
Morphotypes isolated in wheat plants from wild system had higher inoculum potential because the 12 morphotypes inoculated, 10 established successful symbiosis excelling isolate S1 with the highest percentage of colonization being the morphotypes isolated from cultivated system of nine inoculated morphotypes just two established successful symbiosis.
Literatura citada
Bornemisza, E. 1979. Organic carbon determination by the Walkley-Black and dry combustion in surface soils and andept profiles from Costa Rica. Soil Sci. 43:78-83. [ Links ]
Bouyoucos, G. S. 1936. Directions for making mechanical analysis of soil by hydrometer method. Soil Sci. 4:225-228. [ Links ]
Bremner, J. M. 1960. Determination of nitrogen in soil by the Kjeldahl method. J. Agric. Sci. 55:11-33. [ Links ]
Brundrett, MC. 1991. Mycorrhizas in natura ecosystems. In: Macfayden, A.; Begon, M.; Fitter, A. H. (Eds.). Advances in ecological research, London, UK: Academic Press. 21:171-313. [ Links ]
Brussaard, L; Ruiter, P. C. and Brown, G. G. 2007. Soil biodiversity for agricultural sustainability. Agric. Ecosyst. Environ. 121:233-244. [ Links ]
Burdon, J. J. and Thrall, P. H. 2008. Pathogen evolution across the agro-ecological interface: implications for disease management. Evol. Appl. 1:57-65. [ Links ]
Cardinale, B. J.; Wrigh, J. P.; Cadotte, M. W.; Carroll, I. T.; Hector, A.; Srivastava, D. S. and Loreau, M. 2007. Impacts of plant diversity on biomass production increase through time because of species complementarity.Proceedings of the National Academy of Sciences of the United States of America. 104:18123-18128. [ Links ]
Carvalho, L. M.; Correia, P. M.; Ryel, R. J. and Martins-Loução, M. A. 2003. Spatial variability of arbuscular mycorrhizal fungal spores in two natural plant communities. Plant Soil. 251:227-236. [ Links ]
Egerton - Warburton, L. M.; Johnson, and Allen, E. B. 2007. Mycorrhizal community dynamics following nitrogen fertilization: a crosssite test in five grasslands. Ecol. Monographs. 77:527-544. [ Links ]
Ehinger, M.; Koch, A. and Sanders, I. 2009. Changes in arbuscular mycorrhizal fungal phenotypes and genotypes in response to plant species identity and phosphorus concentration. New Phytologist. 184:412-423. [ Links ]
Furlan, V.; Bärtschi, H. and Fortin, J. A. 1980. Media for density gradient extraction of endomycorrhizal spores. Trans Br Mycol Soc. 75:336-338. [ Links ]
Gerderman, J. and Nicholson, T. 1963. Spores of mycorrhizal endogene species extracted from soil by wet sieving and decanting. J. BMS. 46:235-244. [ Links ]
Hijri, I; Sykorova, Z.; Oehl, F.; Ineichen, K.; Mäder, P.; Wiemken, A. and Redecker, D. 2006. Communities of arbuscular mycorrhizal fungi in arable soils are not necessarily low in diversity. Mol Ecol. 15:2277-2289. [ Links ]
Hodge, A. and Storer, K. 2015. Arbuscular mycorrhiza and nitrogen: implications for individual plants through to ecosystems. Plant Soil. 386:1-19. [ Links ]
Horn, K.; Hahn, A.; Pausch, P. and Hock, B. 1992. Isolation of pure spore and hyphal fractions from vesicular-arbuscular mycorrhizal fungi. J. Plant Physiol. 141:28-32. [ Links ]
INVAM (International culture collection of VA mycorrhizal fungi). 2014. http://invam.wvu.edu/the-fungi/species-descriptions. [ Links ]
Rockström, J.; Steffen, W.; Noone, K.; Persson, Å.; Stuart, F.; Chapin, III.; Lambin, E. F.; Lenton, T. M.; Scheffer, V.; Folke, C.; Schellnhuber, H. J.; Nykvist, B.; Wit, C. A.; Hughes, T.; Van-Deer, S.; Leeuw, F.; Rodhe, H.; Sörlin, S.; Snyder, P. K.; Costanza, R.; Svedin, U.; Falkenmark, M.; Karlberg, L.; Corell, R. W.; Fabry, W. J.; Hansen, J.; Walker; Liverman, D.; Richardson, K.; Crutzen, P. and Foley, J. A. 2009. A safe operating space for humanity. Nature. 461:472-475. [ Links ]
Kahiluoto, H.; Ketoja, E. and Vestberg, M. 2009. Contribution of arbuscular mycorrhiza to soil quality in contrasting cropping systems. Agric. Ecosyst. Environ. 134:36-45. [ Links ]
Mäder, P.; Fliessbach, A.; Dubois, D.; Gunst, L.; Fried, P. and Niggli, U. 2002. Soil fertility and biodiversity in organic farming. Science. 296:1694-1697. [ Links ]
Manske, G. G. B. 1990. Genetical analysis of the efficiency of V A mycorrhiza with spring wheat. Agric. Ecosyst. Environ. 29:273-280. [ Links ]
McGonigle, T. P.; Miller, M. H.; Evans, D. G.; Fairchild, G. L. and Swan, J. A. 1990. A new method that gives an objective measure of colonization of roots by vesicular-arbuscular mycorrhizal fungi. New Phytol. 115:495-501. [ Links ]
Moonen, A. C. and Bàrberi, P. 2008. Functional biodiversity: an agroecosystem approach. Agric. Ecosyst. Environ. 127:7-21. [ Links ]
Oehl, F; Sieverding, E.; Ineichen K.; Mader, P.; Wiemken, A. and Boller, T. 2009. Distinct sporulation dynamics of arbuscular mycorrhizal fungal communities from different agroecosystems in long-term microcosms. Agric. Ecosyst. Environ. 134:257-268. [ Links ]
Oehl, F.; Sieverding, V.; Ineichen, K.; Mäder, P.; Boller, T. and Wiemken, A. 2003. Impact of land use intensity on the species diversity of arbuscular mycorrhizal fungi in agroecosystems of Central Europe. Appl. Environ. Microbiol. 69:2816-2824. [ Links ]
Oliveira, R. S.; Boyer, L. R.; Carvalho, M. F.; Jeffries, P.; Vosatka, M.; Castro, P. M. L. and Dodd, J. C. 2010. Genetic, phenotypic and functional variation within a Glomus geosporum isolate cultivated with or without the stress of a highly alkaline anthropogenic sediment. Appl. Soil Ecol. 45:39-48. [ Links ]
Olsen, S. R.; Cole, C. V.; Watanabe, F. S. and Dean, L. A. 1954. Estimation of available phosphorus in soils by extraction with sodium bicarbonate. U. S. Department of Agriculture Circular 939. Banderis, A. D.; Barter, D. H. and Anderson, K. Agricultural and Advisor. 344-348. [ Links ]
Philippot, L.; Raijmakers, J. M.; Lemanceau, P.; and Van-Der, P. W. H. 2013. Going back to the roots: the microbial ecology of the rhizosphere. Nature Rev. Microbiol. 11:789-799. [ Links ]
Pozo, M. J.; Cordier, C.; Dumas-Gaudot, E.; Gianinazzi, S.; Barea, J. M. and Azcón-Aguilar, C. 2002. Localized versus systemic effect of arbuscular mycorrhizal fungi on defence responses to Phytophthora infection in tomato plants. J. Exp. Bot. 53:525-534. [ Links ]
Redecker, D.; Morton, J. B. and Bruns, T. D. 2000. Molecular phylogeny of the arbuscular mycorrhizal fungi Glomus sinuosum and Sclerocystis coremioides. Mycologia. 92:282-285. [ Links ]
Schüßler, A.; Schwarzott, D. and Walker, C. 2001. A new fungal phylum, the Glomeromycota: phylogeny and evolution. Mycological Res. 105:1413-1421. [ Links ]
Serralde, A. M. y Ramírez, M. 2004. Análisis de poblaciones de micorrizas en maíz (Zea mays) cultivado en suelos ácidos bajo diferentes tratamientos agronómicos. Revista Corpoica. Ciencia y Tecnología Agropecuaria. 5(1):31-40. [ Links ]
Shennan, C. 2008. Biotic interactions, ecological knowledge and agriculture Philosophical Transactions of the Royal Society B-Biological Sciences. 363:717-739. [ Links ]
Smith, F. A. and Smith, S. E. 2015. How harmonious are arbuscular mycorrhizal symbioses? Inconsistent concepts reflect different mindsets as well as results. New Phytol. 205:1381-1384. [ Links ]
Steiner, A. A. 1973. The selective capacity of tomato plants for ions in a nutrient solution. In: Proceedings 3rd International Congress on Soilles Culture. Wageningen. The Netherlands. 43-53 pp. [ Links ]
Stockinger, H.; Walker, C. and Schüßler, A. 2009. ‘Glomus intraradices DAOM197198’, a model fungus in arbuscular mycorrhiza research, is not Glomus intraradices. New Phytologist. (183):1176-118. [ Links ]
Tawaraya, K. 2003. Arbuscular mycorrhizal dependency of different plant species and cultivars. Soil Sci. Plant Nutrit. 49:655-668. [ Links ]
Thomson, B. D.; Robson, A. D. and Abbott, L. K. 1992. The effect of long-term applications of phosphorus-fertilizer on populations of vesicular-arbuscular mycorrhizal fungi in pastures. Aust. J. Agric. Res. 43:1131-1142. [ Links ]
Tilman, D.; Wedin, D. and Knops, J. 1996. Productivity and sustainability influenced by biodiversity in grassland ecosystems. Nature. 379:718-720. [ Links ]
Toljander, J. F.; Santos-Gonzalez, J. C.; Tehler, A. and Finlay, R. D. 2008. Community analysis of arbuscular mycorrhizal fungi and bacteria in the maize mycorrhizosphere in a long-term fertilization trial. FEMS Microbiology Ecology. 65:323-338. [ Links ]
UNU, 2011. World population prospects: the 2010 Revision, standard variants. 2011. http://esa.un.org/wpp/Excel-Data/population.htm. [ Links ]
Van-D.; Heijden, M. G. A.; Bardgett, R. D. and Van-Straalen, N. M. 2008. The unseen majority: soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems. Ecology letters. 11(3):296-310. [ Links ]
Vandenkoornhuyse, P.; Husband, R.; Daniell, T. J.; Watson, I. J.; Duck, J. M.; Fitter, A. H. and Young, J. P. W. 2002. Arbuscular mycorrhizal community composition associated with two plant species in a grassland ecosystem. Molecular Ecology. 11:1555-1564. [ Links ]
Vandenkoornhuyse, P.; Ridgway, K. P.; Watson, I. J.; Fitter, A. H. and Young, J. P. W. 2003. Co-existing grass species have distinctive arbuscular mycorrhizal communities. Mol. Ecol.129:3085-3095. [ Links ]
Walder, F.; Niemann, H.; Natarajan, M.; Lehmann, M. F.; Boller, T. and Wiemken, A. 2012. Mycorrhizal networks: common goods of plants shared under unequal terms of trade.Plant Physiol. 159:789-797. [ Links ]
Walker, C. 2005. A simple blue staining technique for arbuscular mycorrhizal and other root-inhabiting fungi. Inoculum. 56:68-69. [ Links ]
Received: June 2015; Accepted: September 2015











 texto em
texto em 


