Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista mexicana de ciencias agrícolas
versão impressa ISSN 2007-0934
Rev. Mex. Cienc. Agríc vol.6 no.8 Texcoco Nov./Dez. 2015
Articles
Organic substrates in the production of basil (Ocimum basilicum L.) and its phytochemical quality
1Facultad de Agricultura y Zootecnia-Universidad Juárez del estado de Durango (UJED). Gómez Palacio, Durango. Tel: 871 7118918. (civaz60@hotmai.com).
2Facultad de Ciencias Forestales-Universidad Juárez del estado de Durango (UJED). Durango, Dgo. Tel: 871 7118918. (gina_ojeda592003@yahoo.com.mx).
3Instituto Tecnológico de Torreón. Carretera Torreón-San Pedro. A. P. 42, km 7.5, Torreón, Coahuila, México. C. P. 27070. Tel. 8717507199. (ppreciador@yahoo.com.mx).
4Dirección General de Educación Tecnológica Agropecuaria. (DGETA-BEDR 122). Nezahualcóyotl Núm. 110. Palacio Municipal, Colonia Centro. Texcoco, Estado de México. Tel: 595 1065738. (Jacob_antonio@yahoo.com).
The objective of this study was to evaluate the yield and quality of phytochemical of basil (Ocimum basilicum L.) in different organic substrates under conditions of shade cloth. Organic fertilizers were evaluated; vermicompost (VER), compost (COM) and solarized manure (ES), mixed in different proportions with sand (A), pumice (P) and agricultural land (S). Six treatments with four replications were used in a completely randomized experimental design. The treatments were: T1, sand + solarized dung (80:20); T2 sand + solarized dung + soil (80: 15: 5); T3, sand + vermicompost (80:20); T4, sand + mineralized compost (80:20); T5, sand + vermicompost + soil (80: 15: 5) and; T6 (control) sand + pumice (80:20), with Steiner solution. Four cuts were made at 30, 71, 139 and 179 days after transplantation, the leaf area index was determined (LAI), fresh leaf weight (PFH), dry leafweight (PSH), fresh stem weight ( PFT) and dry stem weight (PST) as well as the total phenols and antioxidant capacity. Substrates in physical and chemical parameters were determined. The results showed significant differences (Tukey, p≤ 0.05) for yield, IAF, PSH, total phenols and antioxidant capacity. The phenolic content of the basil leaves were significantly different, with values of 62.42 and 41.55 mg equiv. Ac. Gallic 100 g in dry weight for T6 and T2, respectively. For the antioxidant capacity method ABTS+ significant difference was found with values of 156.43 and 101.86 (μM equiv Trolox / 100 g PF) obtaining the highest value T3 and the lowest T2. These organic treatments obtained the highest phenolic content and antioxidant capacity, which were similar to the phytochemical quality of the product obtained under traditional fertilization (Steiner solution), indicating the feasibility of organic treatments for their low cost for implementing of the basil organic production in shade cloth.
Keywords: Ocimum basilicum L.; antioxidants; phenols; solarization; vermicompost
El objetivo del presente estudio fue evaluar el rendimiento y la calidad fitoquimica de albahaca (Ocimum basilicum L.) en diferentes sustratos orgánicos bajo condiciones de malla sombra. Se evaluaron los abonos orgánicos; vermicompost (VER), compost (COM) y estiércol solarizado (ES), mezclados en diferentes proporciones con arena (A), piedra pómez (P) y suelo agrícola (S). Se utilizaron seis tratamientos con cuatro repeticiones en un diseño experimental completamente al azar. Los tratamientos fueron: T1, arena+estiércol solarizado (80:20); T2, arena + estiércol solarizado + suelo (80:15:5); T3, arena + vermicompost (80:20); T4, arena + compost mineralizada (80:20); T5, arena + vermicompost + suelo (80:15:5) y T6 (testigo), arena + piedra pómez (80:20), con solución Steiner. Se evaluaron cuatro cortes a los 30, 71, 139 y 179 días después del trasplante, se determinó el índice de área foliar (IAF), peso fresco de hoja (PFH), peso seco de hoja (PSH), peso fresco de tallo (PFT) y peso seco de tallo (PST), así como fenoles totales y capacidad antioxidante. En los sustratos se determinaron parámetros físicos y químicos. Los resultados mostraron diferencias significativas (Tukey; p≤ 0.05) para rendimiento, IAF, PSH, fenoles totales y capacidad antioxidante. El contenido fenólico de las hojas de albahaca presentaron diferencias significativas, con valores de 62.42 y 41.55 mg equiv. de Ac. Gálico 100 g en peso seco para T6 y T2, respectivamente. Para la capacidad antioxidante método ABTS+ se encontró diferencia significativa con valores de 156.43 y 101.86 (μM equiv Trolox / 100 g PF) obteniendo el mayor valor el T3 y el menor T2, respectivamente. Estos tratamientos orgánicos obtuvieron el mayor contenido fenólico y capacidad antioxidante, los cuales resultaron similares a la calidad fitoquímica del producto obtenido bajo fertilización tradicional (solución Steiner), lo cual indica la factibilidad de los tratamientos orgánicos por su bajo costo en la implementación de la producción orgánica de albahaca en malla sombra.
Palabras clave: Ocimum basilicum L.; antioxidantes; fenoles; solarización; vermicompost
Introduction
In recent years, interest in aromatic herbs has increased due to their aromatic, therapeutic and conservation characteristics that these plants have (Sangwan et al., 2001). Recently, they have also been identified as a valuable source of many phytochemicals, many of which have significant antioxidant activity. A species of these plants is basil (Ocimum basilicum L.), for its essential oil is widely used in food, perfumery and medical industries. It is also considered as a source of aroma compounds and has a range of biological activities as well as antioxidant properties (Lee, 2005).
Basil is grown in many countries for its medicinal, aromatic, ornamental and honey qualities. Its essence is used in perfumery industry and cosmetics and as flavouring for vinegar, canned vegetables and mustard (Cheping, 1993). In Mexico, during the last years has increased the consumption of this herb in both flavouring meals, and infusions with therapeutic purposes.
Basil is grown in climates with temperatures between 7 and 27 °C, with soil pH ranging from 4.3 to 8.2. The species does best in long days in the sun. It can be sown directly or transplanted into the field (Kintzios and Makri, 2007). Since the boom of this crop has meant that it has to face different climatic and soil conditions. However, information about their nutritional requirements according to each area of production and cultivation is scarce. It is well known that fertilization of a plant affects its production, in the case of Basil deserves more attention because the parts used are generally the leaves and stems. The quality and production will then depend on the environmental conditions where they occur. Fertilization must have a NPK ratio of1-1-1. In order to meet the nutritional requirements of the plant, an application is recommended of250 - 500 kg ha-1 of nitrogen (Kintzios and Makri, 2007).
An alternative to conventional fertilizers and organic fertilizers to replace the use of nonrenewable resources and transform organic waste into useful soil that eventually pollute the environment. Thus the plant growth is promoted by a contribution of micro and macronutrients which otherwise should be incorporated by chemical fertilization (Atiyeh et al., 2000b). Among the organic sources used is compost, manure vermicompost and solarized in mixtures of organic substrates (Rippy et al., 2004; Vázquez et al., 2012; Márquez-Hernández et al., 2013; Fortis et al., 2014). The use of these organic fertilizers leads to an improvement in the physical and chemical properties of the substrates, which is reflected in a better crop development.
Furthermore, the use of organic materials previously treated as solarized manure or vermicompost would be an excellent alternative for the production of organic vegetables with more phytochemical content. Bansal and Kapoor (2000) show that using the manufacture of organic fertilizers, bovine manure through Eisenia foetida helps maintain biodiversity of organisms and can find a good carbon/nitrogen ratio.
Vermicompost is the product of a series of biochemical and microbiological transformations occurring in the organic matter to pass through the digestive tract of earthworms (De la Cruz-Lázaro et al., 2009). The substrate can meet the nutritional demand of horticultural crops in greenhouses and significantly reduces the use of synthetic fertilizers. Furthermore, vermicompost contains active substances that act as growth regulators, raise the cation exchange capacity (CEC), have high humic acid content, and increases the moisture holding capacity and porosity, which facilitates aeration, drainage soil and growth medium (Atiyeh et al., 2000a, 2000b; Moreno et al., 2005).
For more detailed information on the use of organic substrates and give proper value to the large amount of organic waste, such as cattle manure produced in the Laguna region, this study aims to evaluate the production and phytochemical basil crop quality produced with mixtures of organic fertilizers under conditions of shade cloth.
Materials and methods
The work was conducted in 2014 at the Technological Institute of Torreon (ITT), located on the old road Torreon - San Pedro, km. 7.5 the of the municipality of Torreon, Coahuila.
The factors under study were five mixtures of organic substrates made from solarized dung (ES), vermicompost (VER), mineralized compost (COM), pumice (P), sand (A) and agricultural land (S). Mixtures of substrates are made on a volume basis (v/v), being as follows: T1= sand 80% + 20% solarized manure; T2= sand 80% + solarized dung 15% + dirt 5%; T3= sand 80% + 20% vermicompost; T4 = sand 80% + 20% mineralized compost; T5= sand 80% + 15% vermicompost + dirt 5% and T6= sand 80% + 20% pumice (control with Steiner nutrient solution). The treatment of chemical fertilizer applied was the Steiner solution (1984), prepared using highly soluble commercial fertilizers, adjusted to pH 5.5 with sulphuric acid (Capulín-Grande et al., 2011), and the electrical conductivity (EC) of 2 dS m-1 measuring with water (Carballo et al., 2009).
Table 1 Chemical composition of solarized dung and vermicompost.
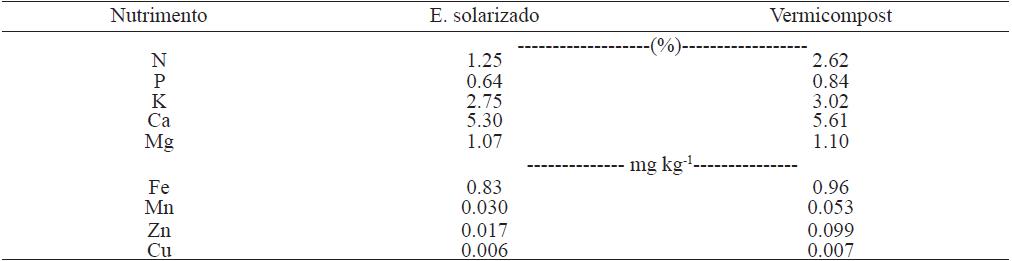
Fuente: análisis realizados en la Cooperativa de la Comarca Lagunera (2014).
The evaluated material was the Genovese basil Variety "Nuffar". It was planted in a baking seedbed using peat moss. Transplantation to substrates was performed at 20 days after planting (DAP) when the seedling tray had developed 2 to 3 true leaves.
Table 2 Formulation of the nutrient solution used in the experiment.
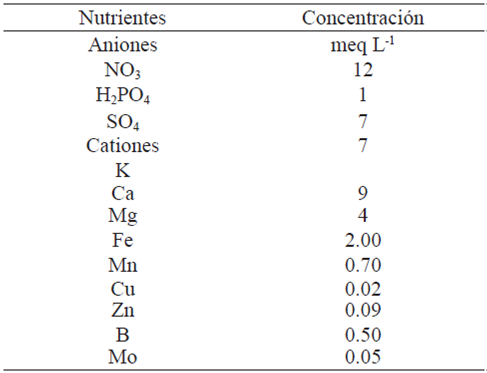
Fuente: elaboración basada en metodología de Steiner (1984).
Basil seedlings were placed in black plastic bags gauge 800 mad of 10 k capacity; placed a seedling/pot; distributed in two rows with a spacing of 30 cm at a density of population of 4 plants m-2. The watering of plants was made twice a day, with a volume of 0.500 litters-pot day-1 from transplanting to harvest. For the control treatment, the irrigation criterion was to apply the nutrient solution to water while controlling pH of 5 and an electrical conductivity of 2 dS m-1. Organic products were used for whitefly control, as Neem vegetal oil and garlic extract at 5 cm3 per litter of water.
Physical and chemical analysis were performed to determine the cation exchange capacity (CEC), field capacity (CC), bulk density (Da), hydrogen potential (pH), organic matter (OM), nitrate (N-NO3-), calcium carbonate (CaCO3), electrical conductivity (EC), sodium adsorption ratio (SAR) and exchangeable sodium percentage (PSI), these determinations were performed on samples obtained at the beginning and end of the study.
Variables evaluated on the plant. Yield (kg m-2). At each cutting date (30, 71.139 and 179 days of transplantation) fresh samples of leaves and stems were weighed, were dried and weighing was repeated to obtain the data of fresh leaf weight (PFH), dry weight of the leaf (PSH), fresh weight of stem (PFT), dry weight of stem (PST) and the leaf area index (IAF). Nutraceutical quality was also evaluated (phenolic content and antioxidant capacity by the ABTS+).
Obtaining extract. Five grams of the sample (fresh basil leaves) were mixed with 10 ml of methanol in a plastic screw cap tube, that were placed on a shaker (ATR Inc., USA) during 6 h (20 rpm ) at 5 °C. The tubes were centrifuged at 3000 rpm for 10 min, and the supernatant was removed for analytical testing.
Total phenolic content. The total phenolic content was measured using a modified version of the method Folin-Ciocalteu (Esparza-Rivera et al., 2006). 300 μl of sample were mixed and were added with 1 080 ml of distilled water and 120 μl of Folin-Ciocalteu (Sigma-Aldrich, St. Louis MO, USA), shaking a vortex for 10 s. After 10 min we added 0.9 ml of sodium carbonate (7.5% w/v) shaking it for10 s. The solution was left standing at room temperature for 30 min, and then its absorbance was read at 765 nm on a HACH 4000 spectrophotometer. The phenolic content was calculated using a standard curve using acid Gallic (Sigma, St. Louis, Missouri, USA) as a standard, and the results are reported in mg of Gallic acid equivalent per g of sample on dry base (Mg equiv AG g-1 BS). The analyses were performed in triplicate.
Antioxidant capacity equivalent in Trolox (Method ABTS+). The antioxidant capacity equivalent in Trolox was evaluated according to the method in vitro ABTS+ published by Esparza-Rivera et al. (2006). We prepared a ABTS+ solution with 40 mg of ABTS (Aldrich, St. Louis, Missouri, USA) and 1.5 g of manganese dioxide (Fermont, Nuevo Leon, Mexico) in 15 ml of distilled water. The mixture was shaken vigorously and allowed to stand still covered for 20 min. Then, the solution was filtered on a Whatman 40 paper (GE Healthcare UK Limited, Little Chalfont, Buckinghamshire, UK) and the absorbance was adjusted to 0.700 ± 0.010 at a wavelength of 734 nm using 5 mM of phosphate buffer solution. For the determination of antioxidant capacity we mixed 100 ul of sample and 1 ml of ABTS+ solution and after 60 and 90 seconds of reaction the sample absorbance was read at 734 nm. A standard curve with Trolox was prepared (Aldrich, St. Louis, Missouri, USA), and the results were reported as equivalent antioxidant capacity in μM also equivalent in Trolox per g on dry basis (μM equiv Troloxgm-1 BS). Analyses were performed in triplicate.
The experimental design was completely random; considering four replications. ANOVA and mean comparison test were performed using SAS (Statistical Analysis System) version 9.1, using Tukey test at 5% significance (p≤ 0.05).
Results and discussion
In the statistical analysis for the variable leaf area index (LAI) of Ocimum basilicum L., there is a significant difference (p≤ 0.05) between the control (T6) and the other treatments, with an average of the four cuttings of4 981.6 g (Figure 1). The IAF decreases in all treatments in the final cut but the trend remains. The growth rate of the leaves depends on the mass and irreversible expansion of young cells, which are produced by cell division in the meristem tissue (Carranza et al., 2009). The IAF, was directly related to the amount of chlorophyll, it is an important parameter to predict the ability of the plant to synthesize dry matter (Warnock et al., 2006).
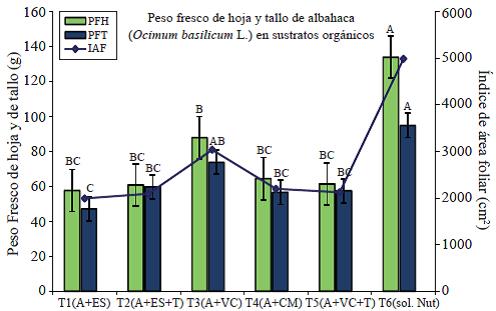
Figure 1 Determination of the leaf area index (LAI), leaf fresh weight (PFH) and fresh weight of the stem (PFT) in the cultivation of basil produced in organic substrates with shade cloths.
In the determination of the fresh weight of the leaf (PFH), is included the water found in plant tissues and serves as a reference parameter to establish the development of foliage and thus the development of the plant. The fresh leaf weight (PFH), also known as biomass of the leaf shows an increase in all treatments at 71 days of the transplant (date of the second cut) but gradually decreases in all treatments except the control (Steiner solution). There is a significant difference between treatments; being T3 and T6 (control) those with more biomass production (Figure 2).
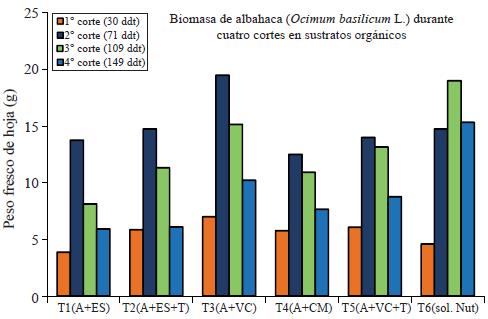
Figure 2 Determination of biomass of basil (Ocimum basilicum L.) during four cuts evaluated in organic substrates.
T3 and T6 (Control) showed a statistically significant difference (p≤ 0.05) compared to other treatments for leaf dry weight, averaging 15.62 g and 13.60 g, respectively; however, during the first and second cut T3 has the largest PSH including the control treatment. All treatments decrease PSH at the fourth cut except for the control that retains high the PSH.
With respect to the fresh weight of the stem (PFT) in the treatments T1 (47.1 g) and T2 (59.7 g) containing solarized dung, significant differences were observed with respect to the control (94.8 g) and T3 (73.7 g). The PFT is between 47.1 g at T1 and 94.8 g in the control (T6).
In the dry weight of the stem (PST) there is no significant difference between the treatments T1, T2, T4 and T5, the T3 has a value of 12.96 g and the control of 13.42 g.
No statistically significant difference was found in the bulk density (Da= 1.4 g cm-3), nor in the field capacity (CC= 21.5%), nor in the cation exchange capacity (CIC= 8.4 meq/100 g) according to González et al. (2009), the organic materials have an elevated CIC (200-400 meq/100 g), which is a reservoir for nutrients; however, the mixture of the organic substrate with sand decreases CIC.
In all treatments is more alkaline the pH at the beginning of the study, this is explained by the basic character of the organic materials that have undergone a degradation process, the pH is basic due to the effect of evolution of ammonia from the decomposition of proteins (Fortis et al., 2012).
Regarding the organic matter (OM) of the substrates, there is significant difference of T3 with respect to other treatments. M.O loss in the substrates is attributed to water soluble organic compounds.
The little loss of MO in T2 allows considering the mixed solarized dung with soil as a biostable material (initial MO= 1.52 and final MO= 1.58). Biostability is the property of an organic material to lose some weight and maintain their physical and chemical characteristics for several months (Figure 3), especially when growing plants (Lemaire, 1997).
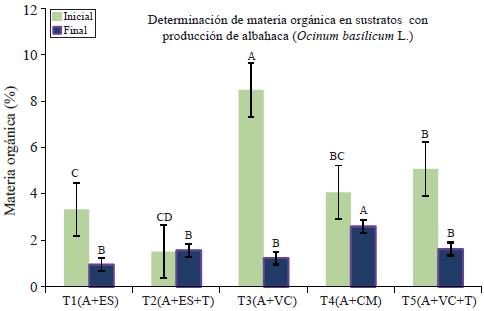
Figure 3 Determination of organic matter (M.O.) in organic substrates for production of basil in shade clothing.
Electrical conductivity (EC) is high (average of 4.7 dS m-1) in all substrates (Figure 4); salt content is higher (7.9 dS m-1) at the beginning of the study due to the decomposition of organic materials, which resemble the behaviour of saline soils; that is, the yield is affected on most of the crops; however, it decreases considerably during the study, at the end taking readings of CE= 2.2 for T1 and CE= 2.3 for T3, so we got the behaviour of a regular soil.
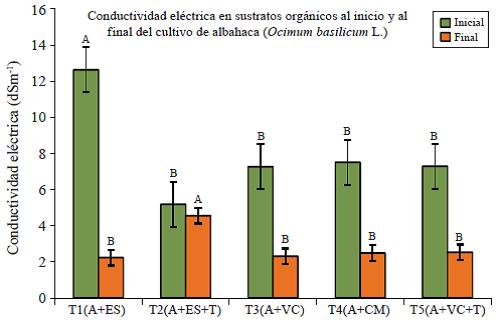
Figure 4 Electrical conductivity (initial and final) in organic substrates for production of basil in shade clothing.
The exchangeable sodium percentage (PSI) found in the T3 (4.19%) and T5 (4.5%) indicates that organic substrates have a sodification problem, that happens if the PSI >15%, the highest value PSI is presented at the end of the study in T2. (PSI= 5.05%).
Phytochemical quality of basil. The phenolic content of the basil leaves differ significantly, with values of 62.42 and 41.55 mg equiv. ac. gallic 100 g in dry weight for T6 and T2, respectively. For the antioxidant capacity, the method ABTS+ had significant difference with values of 156.43 and 101.86 (μM equiv Trolox / 100 g PF) obtaining the highest value T3 and the lower T2, respectively (Table 3).
Table 3 Total phenolic content and antioxidant capacity of basil produced with different organic substrates.
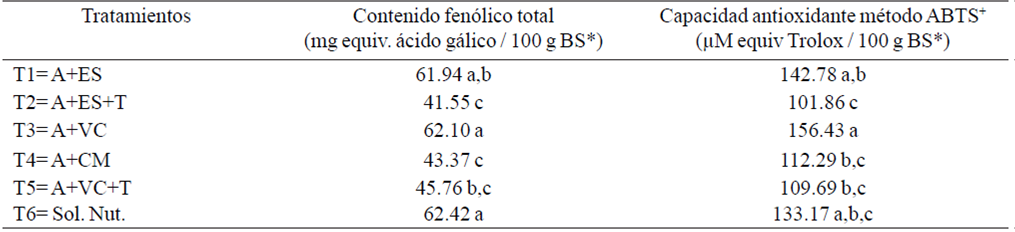
The total phenolic content and antioxidant capacity of basil were affected by the type of substrate used (Tukey, p≤ 0.05). López-Blanco (2014) reported values of total phenols between 4 and 5 mg kg-1 PFr (fresh weight). Kwee and Niemeyer (2011) reported differences in the concentrations of total phenols in different varieties of basil, with the case of 'Spice Blue' and 'Gecofure' which had the highest levels, 'than Lemon Sweet Dani' and 'Nufar'.
Regarding the antioxidant capacity, the values obtained in this experiment ranged from 101.86 and 156.43 μM equiv Trolox / 100 g BS, for the treatments T1 and T3, respectively. Being the organic treatments which had the highest oxidant activity (Table 3). López-Blancas (2014), obtaining maximum values of 79.9 mg VCEAC/g/PFr, lower than those found in this work.
It should be noted that various factors have a direct effect on the presence of individual antioxidants, such as cultivating, harvesting season, genetics or environmental factors (Oboh, 2004).
Kalt (2005) says that the levels of phenolic antioxidants appear to be more sensitive to environmental conditions pre and post-harvest; although the content of certain phenolic compounds may increase during suitable storage conditions. Therefore, preserving the phenolic content has great impact on the quality due to the contribution of not only phenols reactions enzymatic browning, but also the nutritional value of the product (Kevers et al., 2007).
Basil capacity as a natural antioxidant has been attributed to the high prevalence of phenolic compounds, being the rosmarinic acid the main active component found in O. basilicum, and it has been shown to have medicinal value and its antioxidant activity is superior to vitamin E (r-tocopherol) (Jayasinghe et al., 2003). In O. sanctum, its constituents interrupt the free radical chain of oxidation by hydrogen donation from the hydroxyl group of phenol, thereby forming stable free-radicals thus, avoiding subsequent browning (Tangkanakul et al., 2009; Tabassum et al., 2009).
The response of plants to stressful environments, and organic production systems, creates defence mechanisms including the production of antioxidants (Winter and Davis, 2006). In the present study we used organic materials as a nutritional source, plus conferring certain properties to substrates used due to the rates applied (10 to 20% in base on v/v). It is possible that the nutritional contribution of organic treatments used in the experiment has been a stressful for the plant in the early stages of growth factor, which resulted in the formation of phenolic compounds at a relatively high level.
In the present study, organic treatments that obtained the highest phenolic content and antioxidant capacity were T3 (A+VC) and T1 (A+ES), which were similar to the phytochemical quality of the product obtained under traditional fertilization (Steiner solution), indicating the feasibility of organic treatments for their low cost in implementing organic production of basil. Given the relative antioxidant activity of basil, it is suggested that ths may be a source of phenolic antioxidant compounds in the diet (Juliani and Simon, 2002).
Conclusions
The best organic substrate that reported the highest IAF (3031.94) and PFH (87.8 g) was the mixture of vermicompost with sand (80:20). Mixtures of organic substrates presented CE>4 and a PSI<15, are alkaline (pH>7). They did not show statistically significant differences in bulk density (Da= 1.4 g cm-3), field capacity (CC= 21.5%) and in the cation exchange capacity (CEC= 8.4 meq/100g). The use of vermicompost in mixtures of substrates could help to reduce chemical fertilization, without affecting the crop's yield. Phytochemical quality showed no significant differences between the treatments; however, higher values were found in the organic treatments, indicating the feasibility of its use and implementation in organic production of basil in shade cloths and promote dung recycling through the vermicomposting process.
Literatura citada
Atiyeh, R. M.; Arancon, N.; Edwards, C. A. and Metzger. J. D. 2000a. Influence of earthworm-processed pig manure on the growth and yield of greenhouse tomatoes. Bioresour. Technol. 75:175-180. [ Links ]
Atiyeh, R. M.; Subler, S.; Edwards, C. A.; Bachman, G.; Metzger, J. D. and Shuster, W. 2000b. Effects of vermicomposts and composts on plant growth in horticultural container media and soil. Pedobiologia 44:579-590. [ Links ]
Bansal, S. and Kapoor, K. K. 2000. Vermicomposting of crop residues and cattle dung with Eisenia foetida. Bio. Technol. 73:95-98. [ Links ]
Carballo, T.; Gil, M. V.; Calvo, L. F. and Morán, A. 2009. The influence of aeration system, temperatura and compost origin on the phytotoxicity of compost tea. Compost Sci. Utiliz. 17:127-139. [ Links ]
Capulín-Grande, J.; Mohedano-Caballero, L.; Sandoval-Estrada, M. y Capulín-Valencia, J. C. 2011. Estiércol bovino líquido y fertilizantes inorgánicos en el rendimiento de jitomate en un sistema hidropónico. Rev. Chapingo Ser. Hortic. 17:105-114. [ Links ]
Carranza, C.; Lanchero, O.; Miranda, D. y Chaves, B. 2009. Análisis de crecimiento de lechuga (Lactuca sativa L.) "Batavia" cultivada en un suelo salino de la Sabana de Bogotá. Agr. Col. 27(1):41-48. [ Links ]
Cheping, N. 1993. Plantas medicinales. Serie Fitomed II. Ed. Ciencias médicas. Havana, Cuba. 117 p. [ Links ]
De la Cruz-Lázaro, E.; Estrada-Botello, M. A.; Robledo-Torres, V.; Osorio-Osorio, R.; Márquez-Hernández, C. y Sánchez-Hernández, R. 2009. Producción de tomate en invernadero con composta y vermicomposta como sustrato. Rev. Universidad y Ciencia. 25:59-67. [ Links ]
Esparza, R. J. R.; Stone, M. B.; Stushnoff, C.; Pilon, S. E. and Kendall, P. A. 2006. Effects of ascorbic acid applied by two hydrocooling methods on physical and chemical properties of green leaf lettuce stored at 5 °C. J. Food Sci. 71:270-276. [ Links ]
Fortis, H. M.; Preciado, R., P.; García, H. J. L.; Navarro-Bravo, A.; Antonio, G. J. y Omaña, S. J. M. 2012. Sustratos orgánicos en la producción de chile pimiento morrón. Rev. Mex. Cienc. Agríc. 3:1203-1216. [ Links ]
Galindo- Pardo, F. V.; Fortis- Hernández, M.; Preciado- Rangel, P.; Trejo- Valencia, R.; Segura- Castruita, M. A. y Orozco- Vidal, J. A. Caracterización físico-química de sustratos orgánicos para producción de pepino (Cucumis sativus L.) bajo sistema protegido. Rev. Mex. Cienc. Agríc. 5:1219-1232. [ Links ]
González, J. del Pardo, K. and Martín, S. 2009. Wood waste characterization for composting. Acta Hortic. 843:337-341. [ Links ]
Jayasinghe, C.; Gotoh, N.; Aoki, T. and Wada, S. 2003. Phenolics composition and antioxidant activity of sweet basil (Ocimum basilicum L.). J. Agric. Food Chem. 51:4442-4449. [ Links ]
Juliani, H. R. and Simon, J. E. 2002. Antioxidant activity of basil. Janick, J. and Whipkey, A. (Eds.). Trends in new crops and new uses. ASHS Press, Alexandria, VA. 575-579 p. [ Links ]
Kalt, W. 2005. Effects of production and processing factors on major fruit and vegetable antioxidants. J. Food Sci. 70(1):11-19. [ Links ]
Kevers, C.; Falkowski, M.; Tabart, J.; Defraigne, J. O.; Dommes, J. and Pincemail, J. 2007. Evolution of antioxidant capacity during storage of selected fruits and vegetables. J. Agric. Food Chem. 55(21):8596-8603. [ Links ]
Kwee, E. M. and Niemeyer, E. D. 2011. Variations in phenolic composition and antioxidant properties among fifteen basil (Ocimum basilicum L.) cultivars. Food Chem. 128:1044-1050. [ Links ]
Kintzios, S. and Makri, O. 2007. Ocimum sp. (Basil): botany, cultivation, pharmaceutical properties, and biotechnology. J. Herbs, Spices Medicinal Plants. 13(3):123-150. [ Links ]
Lee, S. J.; Umano, K.; Shibamoto, T. and Lee, K. G. 2005. Identification of volatile components in basil (Ocimum basilicum L.) and thyme leaves (Thymus vulgaris L.) and their antioxidant properties. Food Chem. 91:131-137. [ Links ]
Lemaire, F. 1997. The problem of bioestbility in organic substrates. Acta Hort. 450:63-69. [ Links ]
López-Blancas, E.; Martínez-Damián, M. T.; Colinas-León, M. T.; Solís, J. M. y Rodríguez-Pérez J. E. 2014. Calidad poscosecha de albahaca ʻNufarʼ (Ocimum basilicum L.) en condiciones de refrigeración. Rev. Chapingo Ser. Hortic. 20:187-200. [ Links ]
Márquez-Hernández, C.; Cano-Ríos, P. Figueroa- Viramontes, U.; Ávila- Díaz, J. A.; Rodríguez-Dimas, N. R. y García-Hernández, J. L. 2013. Rendimiento y calidad de tomate con fuentes orgánicas de fertilización en invernadero. Rev. Phyton. 82: 55-61. [ Links ]
Moreno, R. A.; Valdés, P. M. T. y Zárate, L. T. 2005. Desarrollo de tomate en sustratos de vermicompost/arena bajo condiciones de invernadero. Agric. Téc. Chile 65:26-34. [ Links ]
Oboh, A. 2004. Change in ascorbic acid, total phenol and antioxidant activity of sun dried commonly consumed green leafy vegetables in Nigeria. Nutr. Health. 18:29-36. [ Links ]
Rippy, J. F. M.; Peet, M. M.; Louis, F. J. and Nelson, P. V. 2004. Plant development and harvest yield of green house tomatoes in six organic growing systems. Hortscience. 39(2): 223-229. [ Links ]
Sangwan, N. S.; Farooqi, A. H. A.; Shabih, F. and Sangwan, R. S. 2001. Regulation of essential oil production in plants. Plant Growth Regul. 34:03-21. [ Links ]
StatisticalAnalysis System (SAS). 2002. SAS software version 9.1. SAS Institute, Inc. Cary, NC, USA. [ Links ]
Steiner, A. A. 1984. The universal nutrient solution. Proc. 6th Int. Cong. On Soilless Culture. ISOSC. Lunteren, Holanda. 633-649 pp. [ Links ]
Tangkanakul, P.; Auttaviboonkul, P.; Niyomwit, B.; Lowvitoon, N.; Charoenthamawat, P. and Trakoontivakorn, G. 2009. Antioxidant capacity, total phenolic content and nutritional composition of Asian foods after thermal processing. Int. Food Res. J. 16:571-580. [ Links ]
Tabassum, W.; Kullu, A. R. and Sinha, M. P. 2013. Effects of leaf extracts of Moringa oleifera on regulation of hypothyroidism and lipid profile. The BioscanSupplement on Medicinal Plants. 8:665-669. [ Links ]
Vázquez-Vázquez, C.; García Hernández, J. L.; Salazar-Sosa, E.; López-Martínez, J. D.; Valdez-Cepeda, R. D.; Orona-Castillo, I.; Gallegos-Robles, M. A. y Preciado-Rangel, P. 2011. Aplicación de estiércol solarizado al suelo y la producción de chile jalapeño (Capsicum annuum L.). Rev. Chapingo Ser. Hortic. 17:69-74. [ Links ]
Warnock, R.; Valenzuela, J.; Trujillo, A.; Madriz, P. y Gutiérrez, M. 2006. Área foliar, componentes del área foliar y rendimiento de seis genotipos de caraota. Agronomía Trop. 56:21-42. [ Links ]
Winter, C. K. and Davis, S. F. 2006. Organic Foods. J. Food Sci. 71:117-124. [ Links ]
Received: July 2015; Accepted: November 2015











 texto em
texto em 


