Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista mexicana de ciencias agrícolas
versão impressa ISSN 2007-0934
Rev. Mex. Cienc. Agríc vol.5 spe 8 Texcoco 2014
Investigation notes
Humic and fulvic acid extraction method and characterization by infrared spectrophotometry
1CienciasAgrícolas- Subdirección de Estudios de Posgrado. UANL. Marín, Nuevo León, México. (rlsmorris@hotmail.com; r_vazquez_alvarado@yahoo.com.mx).
2CENID-RASPA-INIFAP. Margen derecha Canal de Sacramento, km. 6.5, C. P. 35140, Gómez Palacio Durango. (gonzalez.guillermo@inifap.gob.mx).
3Facultad de Agronomía-UANL. Marín, Nuevo León, México. (emolivares@gmail.com; juan.vidalescn@uanl.edu.mx; carranzaroberto5@yahoo.com.mx).
4Colegio de Posgraduados. Carretera México-Texcoco km 36.5, Montecillo. 56230, Texcoco, Estado de México. (manueloe@colpos.mx).
Organic extracts are now widely accepted by farmers mainly because of cost and success of their applications in crops, in order to obtain an organic-mineral complex or humate as an alternative for efficient delivery of nutrients to crops, an organic fossil mineral was collected from a mine, humic acid (HA) and fulvic (FA) fractions were extracted in the laboratory. The pH of these acids was adjusted with chemical fertilizers. For humic and fulvic acids pH 6, 7, 8 and 4, 6, 7 was handled respectively and finally solidified. Functional groups of these acids were characterized by an infrared spectrometer (IR). Results show that humic and fulvic acids have similar functional groups but different amount, the organo-mineral mixture of HA and FA showed a similar behavior in adsorption of the added element, however both HA and FA at pH 7 showed the highest Ca and Fe adsorption, since they are pH dependent compounds and complex formation is due to the cation exchange reaction by free radicals.
Keywords: organic extracts; functional groups; organic-mineral
Los extractos orgánicos en la actualidad tienen gran aceptación por los productores agrícolas fundamentalmente por su costo y al éxito de sus aplicaciones en los cultivos, con el fin de obtener un compuesto orgánico-mineral o humato como alternativa para eficientar los nutrientes a los cultivos, se recolectó un mineral fósil oigánico de una mina, en laboratorio se extrajeron las fracciones de ácido húmico (AH) y fúlvico (AF). Después se ajustó el pH de estos ácidos con fertilizantes químicos. Para los ácidos húmicos y fúlvicos se manejó un pH de 6, 7, 8 y 4, 6, 7 respectivamente y finalmente se solidificaron. Para la caracterización de los grupos funcionales de estos ácidos se utilizó un espectrómetro de luz infrarroja (IR). Los resultados muestran que ácidos húmicos y fúlvicos presentan grupos funcionales similares pero en cantidad diferente, la mezcla orgánico-mineral de AH y AF mostraron un comportamiento similar en adsorción del elemento incorporado, sin embargo el AH y AF ambos con pH 7 presentaron la mayor adsorción del Ca y Fe, esto se debe a que son compuestos pH dependientes y la capacidad de agrupamiento se debe a la reacción de intercambio catiónico dada por los radicales libres.
Palabras clave: extractos orgánicos; grupos funcionales; orgánico-mineral
Introduction
Terrestrial ecosystems represent the most interactive carbon (C). Up to 67% of the 1500 Gt of soil organic C is present between 20 and 100 cm deep, the soil organic C is called organic matter (OM), consists of a heterogeneous mixture of organic compounds of different origins. The subsoil OM is mostly associated with mineral phases forming organic-mineral complexes, which prevent MO degradation, however, the soils are not only made of mineral or organic substances, these two components interact with one another, therefore controlling the soil surface chemistry, these interactions are important, but especially soils receiving or accumulating large quantities of organic substances (Juneta et al., 2013).
Humic substances (HS) comprise humic acids (HA), fulvic acids (FA) and residual humin (RH) defined as organic macromolecules, with a complex, distinct and stable chemical structure derived from plant and animal degradation by the enzymatic activity of microorganisms and organic metamorphism (Schnitzer, 2000 and Sutton and Sposito, 2005), the term humus, formerly used referring to the entire soil, has subsequently been used as OM synonym, while currently, and as already mentioned, refers to an OM fraction comprising a group of substances (Berthelin et al., 2006), the most important organic fraction in soil based on its physical, chemical and biological activity (MacCarthy, 2001), The HA are soluble in alkaline media, but not in strong acid conditions (pH < 2).
The FA are soluble in alkaline and acidic conditions unlike humins which are insoluble. The classification in three fractions do not represent three different types of organic molecules (Hayes et al, 1989), since humic substances contain various types of functional groups with different metal binding capacity since usually include an alkyl aromatic skeleton with functional groups like carboxylic acids, phenolic hydroxyls and quinone groups attached to them (Flores-Cespedes et al., 2006; Chen et al., 2009). The evidence suggests that metal complexation with inorganic and organic ligands in soil may influence metal mobility and bioavailability to soil organisms and plants.
An alternative for efficient nutrient delivery to crops consists of a combination with inorganic compounds, using HA as an organic amendment for soil in combination with other materials, resulting in a significant increase in plant growth and crop yield, by improving the hydrophysical properties and nutrient availability in soil. Organic-mineral complexes allow plants to overcome the adverse effects of salinity, improves soil aggregation, aeration, permeability, water holding capacity, micronutrient absorption and availability and decreased absorption of some toxic elements (Ryabova, 2010).
Spectroscopy methods quantify the chemical composition of the HA and FA layers, near infrared is the more accurate method (Moors et al, 2008). Infrared spectroscopy (IR) is a precise technique developed to characterize HS and their fractions: HA and FA. The IR spectra of SH are simple, compared to the spectra of defined structure from pure compounds. This simplicity results from the complex mixture of functional groups present in a wide variety of chemical environments, each characterized by different force constants, which broaden the IR bands (MacCarthy and Rice, 1985). Infrared spectroscopy provides information on the fiinctional groups contained in the HA and FA structure (Gostishcheva et al., 2009). This study aims to extract and characterize an organo-mineral, humic and fulvic complex and characterize its functional groups by infrared spectrophotometry.
Materials and methods
An organic mineral compound called leonardite was used, which was obtained from a mine located at 29° 30' 22.18'' North latitude, 103° 33' 57.13'' West longitude, at a 1 096 m altitude, 96.5 km north from Presidio, Texas, U.S.
The harvested leonardite was transported to the laboratory where it was ground and sieved with a mesh (1.0 mm diameter), 5 g of leonardite were weighed, placed in a 250 ml flask and were added 100 ml of potassium hydroxide (1N KOH, pH 13.5), heated to 60 °C in a water bath for two hours and allowed to cool, 25 ml of solution were taken, weighed and the density was determined. Then the pH was adjusted to 4 with acetic acid (pH 2.4), calibrating the potentiometer with standard buffer solutions at pH 4, 7 and 10. The solution was heated to 70°C and centrifuged at 8000 rpm for 30 min. Humic acids precipitate, while fulvic acids remain in solution. The FA extraction was identified based on the yellow to yellow-brown color remaining in solution after HA removal due to its acidification.
Infrared Spectrometer characteristics and how to use
The functional groups were determined by the generation of spectrograms using an IR spectrometer (Nicolet IR Series, FTIR Spectrometer, USA). The solid sample extracted from the compounds and mixed with potassium bromide (KBr) to a fine powder in an agate mortar. The sample: KBr ratio was 1:100 and a portion of these compounds was separated and placed on a sample holder to prepare subsamples as pellets using a press, the pellets should be homogeneous and thin, placed on a steel plate for samples to read in the IR. Sample contamination by dust must be avoided and instructions followed on how to use the press to make pellets (subsamples).
Subsamples were placed in the spectrometer and analyzed in the same band for 32 scans for the best spectrum which should be in the fine and intense band according to its transmittance percentage. The optimal spectrum is based on sample/solvent ratio (KBr), gain value and chart rate. Once the optimal spectrum for each sample was obtained, the spectrum scale was calibrated on paper, obtaining the wave number of the different components.
Results and discussion
Measurement of humic and fulvic acids percentage and functional groups
From the solution, 50 ml were taken, the pH adjusted to 4 with acetic acid, separating humic from fulvic acids. Percentages of humic and fulvic acids were measured by reverse titration with potassium permanganate (K4MnO7) (Table 1).
They were measured for the total acidity (TA), i.e., the content of oxygenated functional groups, such as the carboxyls (-COOH) and hydroxyls (- OH), the methods described here are based on measurements of total and carboxylic acidity from Wright and Schnitzer (1959), and Schnitzer and Gupta (1965).
For total acidity measurement, the sample is treated with 2N barium hydroxide for 24 h. The Ba(OH)2 remaining in solution after reaction, is titrated with a standard acid solution.
For carboxylic group titration, humic materials are stirred for 24 h with a calcium acetate solution in excess causing the release of acetic acid according to this type of reaction:
The acetic acid released is then titrated with a standard bicarbonate solution.
The proportion of phenolic groups was calculated by the difference between total and carboxylic acidity.
Generally, fulvic acids are more oxidized than humic ones, i.e. they are more polymerized. In particular, fulvic acids are the most oxidized, since they show higher value of total acidity.
The above indicates that fulvic acids contain more negative electric charges.
Canellas et al. (2008) defined the HS as relatively small supramolecular associations basically grouped by hydrophobic interactions and hydrogen bonds. Schulten and Schnitzer (1993) developed a two-dimensional (2D) molecule, the HA model structure with alkyl aromatic rings which play an important role. Oxygen is present as carboxyls, phenolic and alcoholic hydroxyls, esters and ketones, while nitrogen is produced in nitriles and heterocyclic structures. The resulting carbon skeleton exhibits high microporosity with pores of various sizes, which can trap and bind other organic and inorganic soil components, as well as water.
Calcium humate and iron fulvate extraction by regulating pH in the solution.
Following the criteria established by Iakimenko, 2005 and Rady, 2012, who comment that a humic acid binding a mineral, is called here calcium humate and iron fulvate where addition of these minerals in our study was as follows:
Calcium humate: to 100 mL of HA, originally at pH 8, 125 g of Ca(NO3)2 were added, reaching pH 7 then 20 g were added i.e. overall 145 g of Ca(NO3)2 reaching pH 6.
Iron fulvate: to 100 mL of FA originally at pH 4, 0.5 mL of KOH were added, increasing pH to 8, then 10 g of FeSO4 were added, reaching pH 7, then 1.75 g more were added to a final pH 6.
Solidification of humic and fulvic acids, calcium humates and iron fulvates
The solidification was performed by a rotary evaporator (Yamato, SE 500 Model, Japan), placing 500 mL of humic acid (pH 8) and its fractions prepared at pH 6 and 7, also for fulvic acid (pH 4) and its derivatives at pH 6 and 7, overall six treatments, which were deposited in a 1 L flask, heated on water bath at 60 oC, rotating at 80 rpm until water is removed, the solidification required 8 h. The solvent was not completely removed so it was transferred to a porcelain dish and heated at 40 °C for 2 h on an electric grill to remove the remaining water and thereby obtaining the solid.
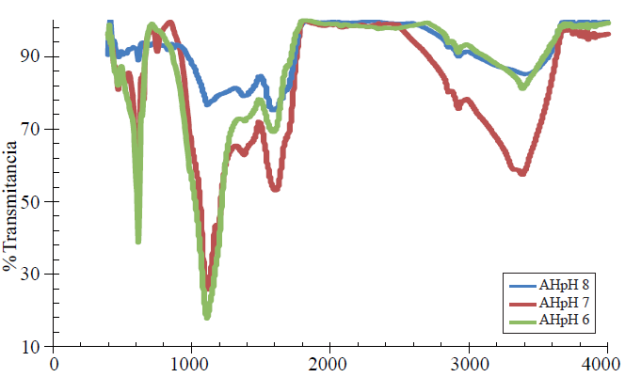
The Figure 1 shows the spectrogram of humic acid enriched with calcium nitrate (Ca (NO3)2) at pH 6, 7 and the original pH 8 after extraction.
The blue line represents the HA at pH 8 in which major signals or functional groups involved in ion exchange are noticeable: the -OH in the extractant or extraction vehicle appears in the 1 375 cm-1 band, NH amines or amides at 1 620 cm-1, the -CH3 and -CH2 at 2 922cm-1 and -OH free functional groups at 3 400 cm-1, the HA at pH 7 and 6, red and green lines, respectively, show additional peaks after addition of the mineral element, thus the signal description is in greater proportion, favoring its description, differentiating peaks by purity and concentration, in the functional group at the lowest wavelength range an aromatic-N-R group was found, this represents a tertiary amine at the 760 and 631 cm-1 bands for pH 7 and 6, the secondary -OH for HA at both pH were found at 1 130 and 1 120 cm-1, the CH-OH, -OH groups in the extraction vehicle were found at 1 390 cm-1, amines or amides N-H or with carboxyl-C= O at 1 620 and 1 600 cm-1, respectively, the -CH3 showed a similar value of 1 930 cm-1, the CH2-CH3 was located at2 870 and 2 840 cm-1, the free -OH appeared at 3 390 cm-1 in both organic-mineral complexes.
The HA at pH 6 shows a higher secondary -OH content followed by the HA at pH 7, however the latter shows more N-H, C= O and free -OH, this indicates less light absorption or higher individual frequency of this type of compounds, and thus higher Ca (NO3)2 absorption in HA at pH 7.
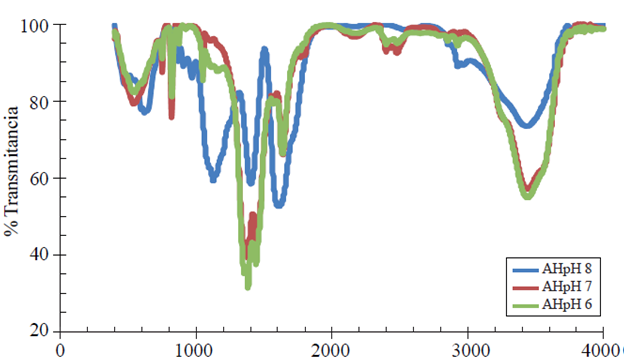
The fulvic acid spectrogram (Figure 2) at the original pH, plus the two levels generated after mixing with ferrous sulfate (FeSO4) yielded the following results.
The FA at pH 4 (blue line) represents the original pH at the time of extraction, in this spectrum, the reading shows the following functional groups, methylene -CH2-CH3 has the best signal at 1 162 cm-1, for the -OH as extraction vehicle 1 439cm-1, N-H amines or amides group at 1 620cm-1 and free -OH at 3 445cm-1, the FA at pH 7 and 6, red and green lines respectively, showed different peaks after mixing with FeSO4, for both pH the primary -OH is located at 1 050 cm-1, just as the -CH2-CH3 at 1 360 cm-1, the -CH2-(C=O)- , -CH2-(C=N) groups at 1 380 and 1 390 cm-1 respectively, for the extraction -OH 1 440 and 1 450 cm-1, -N-H amines or amides at 1640 cm-1 for both mixtures, an overtone caused by an -NH- amines or amides overlap at 1 810 and 1 830 cm-1 for pH 6 and 7, the -CH3 group is at 1 860 cm -1 for both pH, just as the -CH2- CH3 for both pH is at 2 930 cm-1, and free -OH at 3 570 cm-1 and 3 430 cm-1 for pH 6 and 7 respectively. The FA at pH 6 and 7 show many similarities, however FA at pH 7 shows higher frequency for the (-OH) free functional group and thus higher iron adsorption.
These results agree with those obtained by Chassapis et al. (2010), regarding the use of a typical infrared spectrum showing interactions and differences in humic substances, absorption, adsorption, or associations of different relevant phenolic groups distributed in the regions 1 050, 1 650 and 3 400 cm-1, this coincides with Sivakova et al. (2010) who showed IR spectra with characteristic absorption bands for humic acids due to the presence of carboxyl and carbonyl groups (1 720-2 700 cm-1), hydroxyl groups (3 400-1 256 cm-1), oxygen-ether (1650 cm-1), -CH3 -CH2 (2 930 cm-1).
It is also noted that the physicochemical properties of humic substances vary according to their origin (Fukushima et al., 2006), they are amorphous multifunctional biopolymers, comprising hundreds of organic compounds including carbohydrates and condensed aromatic rings, which may be substituted by phenol groups, carboxyls, hydroxyls and methyls, and these results are consistent with those obtained by Peuravuori et al. (2006) and Evangelou et al. (1999). These groups act like chelating agents by complexing and chelating cations in the soil solution, transferring them to the root cell wall, then these nutrients are translocated by the xylem stream to the growing points.
The metal-ion bond (Ca and Fe) has been shown to vary with the solute concentration of cations, pH, mineral and bond type (Puls and Bohn, 1988). Organo-mineral complexes allow plants to overcome adverse effects of salinity, improve soil aggregation, aeration, permeability, water holding capacity, micronutrients absorption, and decreased absorption of some toxic elements (Ryabova, 2010). Results from many authors suggest that the number and strength ofion groups are not related to atomic weight, atomic number, ionic radius, or hydrated radius of retained metal, since humic substances contain different types of functional groups with a wide range of ion complexing capacities.
One promising way to improve the quality of humic substances is their combination with inorganic products, conferring mechanical strength and resistance to acid and alkaline hydrolysis and improved sorption properties. This is a low-cost and accessible procedure to develop organic-mineral adsorbents since these raw materials are abundant, cheap, and can be chemically modified.
Conclusions
Using infrared spectrophotometry allowed us to characterize the functional groups of humic substances and their comparison.
Overall concentration of the chemical element, and the pH of humic substances, are the two major factors in the humic compound-chemical element interaction. Humic and fulvic acids, are more oxidized and thus have greater cation binding capacity.
Fulvic acids can be considered as associations of small hydrophilic molecules with acid functional groups sufficient to keep fulvic groups dispersed in solution at any pH. Humic acids are associations of predominantly hydrophobic compounds (polymethylene chains, fatty acids, steroid compounds), which are stabilized at a neutral pH by dispersion hydrophobic forces. Their conformations grow progressively as their intermolecular hydrogen bonds are formed at increasingly lower pH, until flocculation. Spaccini et al. (2008) suggested that, based on the concept of supramolecular association, the classical definitions of humic and fulvic acids should be reconsidered.
Humic and fulvic acids extracted from leonardite show a high adsorption capacity due to carboxyls, hydroxyls, phenols, amines, amides and methyls, where hydroxyls show the highest Ca and Fe exchange rate.
Hydroxyl free radicals are detected at high wavenumber intervals, differing from the hydroxyls in the extraction vehicle (KOH) which produce low wavenumber intervals.
The development of organic-mineral complexes was affected by pH management, the final mixture being affected by the type of element added, either from an acid or alkaline reaction.
Regardless of the original reaction of humic (pH 8) and fulvic (pH 4) acids, after modifying these to pH 7, lower infrared light absorbance was obtained, indicating greater Ca and Fe levels.
Literatura citada
Berthelin, J.; Babel, U. and Toutain, F. 2006. History of soil biology. In: Warkentin, B. P. (Ed.), Footprints in the Soil. People and ideas in soil history. Elsevier, Amsterdam. 279-306 pp. [ Links ]
Canellas, L. P.; Teixeira-Junior, L. R. L.; Dobbss, L. B.; Silva, C. A.; Médici, L. O.; Zandonadi, D. B. and Fafanha, R. A. 2008. Humic acids crossinteractions with root and organic acids. Ann. Appl. Biol. 153:157-166. [ Links ]
Chassapis, K.; Maria, R.; Vrettou, E. and Parassiris, A. 2010. Preparation of bioinorganic fertilizing media by adsorption of humates on lassy aluminosilicates. Colloids and surfaces B: Biointerfaces 81:115-122. [ Links ]
Chen, H.; Berndtsson, R.; Ma, M. and Zhu, K. 2009. Characterization of insolubilized humic acid and its sorption behaviors. Environ. Geol. 57:1847-1853. [ Links ]
Evangelou, V. P.; Marsi, M. and Vandiviere, M. M. 1999. Stability of Ca2+-, Cd2+-, Cu2+-[illite-humic] complexes and pH influence. Plant Soil 213:63-74. [ Links ]
Flores-Céspedes, F.; Fernández-Pérez, M.; Villafranca-Sánchez, M. and González-Pradas E. 2006. Cosorption study of organic pollutants and dissolved organic matter in a soil. Environ. Pollut 142:449-456. [ Links ]
Fukushima, M. and Tatsumi, K. 2006. Complex formation of water-soluble iron (III)-porphyrin with humic acids and their effects on the catalytic oxidation of pentachlorophenol. J. Mol. Catal. A: Chem. 245:178-184. [ Links ]
Gostishcheva, M. V.; Belousov, M. V.; Yusubov, M. S.; Ismatova, R. R. and Dmitruk, S. E. 2009. Comparative IR spectral characteristics of humic acids from peats of different origin in the Tomsk area. Khimiko-Farmatsevticheskii Zhurnal 43(7):44-47. [ Links ]
Hayes, M. H. B.; MacCarthy, P.; Malcolm, R. L. and Swift, R. S. 1989.The search for structure: setting the scene. In humic substances II: in search of structure. (Eds.) Wiley New York. 3-31 pp. [ Links ]
Iakimenko, O. S. 2005. Use of Humic Substances to remediate polluted environments: from theory to practice, Springer. Printed in the Netherlands. 365-378. [ Links ]
Juneta , A.; Isabelle, B. D.; Daniel, B.; Armand, M.; Samuel, L.; Christine, M.; Jerome, B.; Joelle, T. and Sylvie, D. 2013. Characterisation of organic matter from organo-mineral complexes in an Andosol from Reunion Island. J. Anal. Appl. Pyrol. 99:92-100. [ Links ]
Maccarthy, P. 2001. The principles of humic substances. Soil Sci. 166:738-751. [ Links ]
MacCarthy, P. and Rice, J. A. 1985. Spectroscopic methods (other than NMR) for determining functionality in humic substances. Wiley-Interscience, New York. 527- 559 pp. [ Links ]
Moros, J.; Herbello-Hermelo, P.; Moreda-Piñeiro, A.; Bermejo-Barrera, P.; Garrigues, S. and de la Guardia, M. 2008. Screening of humic and fulvic acids in estuarine sediments by near-infrared spectrometry. Anal. Bioanal. Chem. 392:541-549. [ Links ]
Rady, M. M. 2012.A novel organo-mineral fertilizer can mitigate salinity stress effects for tomato production on reclaimed saline soil. South Afr. J. Bot. 81:8-14. [ Links ]
Ryabova, I. N. 2010. Organomineral sorbent from shubarkol coal. Solid Fuel Chem. 44(5):335-338. [ Links ]
Peuravuori, J.; Zbankova P. and Pihlaja, K. 2006. Aspects of structural features in lignite and lignite humic acids. Fuel Process Technol. 87:829-839. [ Links ]
Puls, R. W. and Bohn, H. L. 1988. Sorption of cadmium, nickel, and zinc by kaolinite and montmorillonite suspension. Soil Sci. Soc. Am. J. 52:1289-1292. [ Links ]
Schnitzer, M. 2000. Life time perspective on the chemistry of soil organic matter. Sparks, D. L. (Ed.). Advances in Agronomy, Academic Press. 98:3-58. [ Links ]
Schnitzer, M. and Gupta, U. C. 1965. Determination of acidity in soil organic matter. Soil Sci. Soc. Am. Proc. 29:274-277. [ Links ]
Schulten, H-R. and Schnitzer, M. 1993.Astate of the art structural concept of humic substances. Naturwissenschaften. 80:29-3. [ Links ]
Sivakova, L. G.; Lesnikova, N. P.; Kim, N. M. and Rotova, G. M. 2010. Physicochemical properties of the humic substances of peat and brown coal. Solid Fuel Chem. 45(1):1-6. [ Links ]
Sutton, R. y Sposito, G. 2005. Molecular structure in soil humic substances: the new view. Environ. Sci. Technol. 39(23):9009-9015. [ Links ]
Wright, J. R. and Schnitzer, M. 1959. Oxygen-containing functional groups in the organic matter of a Podzol soil. Nature, London. 184:1462-1463. [ Links ]
Received: January 2014; Accepted: April 2014











 texto em
texto em