Introduction
Physiologic Hyperprolactinemia (HP) is associated with reproductive processes, like breastfeeding and pregnancy. However, at pathological conditions, it participates in the development of galactorrhea, amenorrhea, and erectile dysfunction (ED). This latter is understood as the inability to keep an erection during the sexual intercourse, which is associated with a decrease of both sexual arousal and libido (Bernichtein, Touraine, & Goin, 2010; Ben-Jonathan, LaPensee, & LaPensee, 2008; Molitch, & Reichlin, 1982).
Sexual behavior is regulated by hormonal and dopaminergic mechanisms (Kruger, Hartmann, & Schedlowski, 2005). In this sense, some hormones, e.g., Progesterone (P), Testosterone (T), Estradiol (E), Luteinizing Hormone (LH) and Prolactin (PRL), work together in order to keep sexual behavior regulation. Accordingly, the endocrine system plays an essential role in the control and adequate execution of this activity; for that reason, a chronic decrease in T levels observed in patients with hypogonadism is associated with ED (Veronesi et al., 2011; Martinez-Jabaloyas, Queipo-Zaragoza, Pastor-Hernandez, Gil-Salom, & Chuan-Nuez, 2006). Moreover, the chronic HP (>100ng/ml) has been observed in most cases of ED, suggesting a negative effect of PRL on sexual behavior, in rats and humans (Abram et al., 1998; Doherty, Bartke, & Smith, 1985; Katovich, Cameron, Murray, & Gunsalus, 1985).
Previous studies from our group showed that hormonal levels of PRL and T increased significantly in sexually experienced male rats subjected to consecutive ejaculations (Hernandez et al., 2006; Hernandez et al., 2007). Likewise, in humans, PRL levels increased after orgasm, remaining high for a while, suggesting that temporal increases in PRL play an important role in facilitative effects and in the control of sexual arousal after orgasm (Hernandez et al., 2007; Kruger, Haake, Hartmann, Schedlowski, & Exton, 2002).
However, the chronic HP is associated with a significant reduction in the gonadal function in men and women, suggesting that PRL is a peripheral regulatory factor essential in reproductive functions (Kruger et al., 2002). What is more, PRL function is conditioned to dopamine (DA) in a dose-dependent manner (Manna, El Hefnawy, Kero, & Huhtaniemi, 2001). When the DA antagonist domperidone was injected (4 mg/kg) to male rats, the HP induced (>60-70 ng/ml), correlated with facilitative effects within 5 days, related to shorter ejaculation latencies when compared with controls
Conversely, when domperidone was injected for 30 days, opposite efects were observed, related to longer ejaculation and intromissions periods (Nasello, Vanzeler, Madureira, & Felicio, 1997). In this context, the induction of a chronic HP (>250 ng/ml) for 18 days, is enough to produce a negative effect on sexual behavior (Doherty, Bartke, Smith, & Davis, 1985). Moreover, when PRL is injected directly into the brain of the rats, facilitative effects were observed in only five hours, while the inhibitory effects were observed in five days, suggesting a direct effect of PRL when is released on the Central Nervous System (CNS) (Cruz-Casallas, Nasello, Hucke, & Felicio, 1999; Nasello et al., 1997).
In accordance with these results, previous studies developed by Nasello et al., showed that PRL is able to induce changes in the dopaminergic activity. Thus, the regulation of sexual behavior may be occurring through a negative feedback of PRL to dopaminergic neurons in the CNS (Kruger et al., 2005; Nasello et al., 1997).
The most of patients with sexual dysfunction, showed a strong hypogonadism (Martinez-Jabaloyas, et al., 2006), however, the studies described above suggested that PRL and its inhibitor (DA), are essentials to produce a dysregulation of sexual behavior, in which, PRL could be decreasing DA levels in specific brain areas, like the nucleus accumbens, paraventricular nucleus, medial preoptic area (MPOA), and the striatum (Martinez-Jabaloyas et al., 2006; Giuliano & Allard, 2001 a ).
All these data suggest that the effect of PRL on sexual behavior is mediated by high and sustained PRL levels. Conversely, in order to observe changes in a short time, is necessary the administration of PRL directly in the CNS to modify the sexual behavior parameters (Cruz-Casallas et al., 1999).
However, data obtained in a parallel study in our lab, showed that moderate increases in PRL levels were enough to induce changes in the morphology of the prostatic tissue and, hence, could be able to modify the sexual behavior in sexually experienced male rats (Hernandez et al., 2006). For that reason, the present study aims to analyze the acute increments in prolactin levels on sexual behavior of male rats subjected to fifteen treatment days.
Materials and Methods
Animals and housing
Three-month old Wistar male rats were used for this study. Animals were subjected to an inverted lightdark cycle (12-12 h, lights of at 8:00 AM) within plastic cages (50 × 30 × 20 cm) containing wood chip bedding. Water and food (Harlan Mexico, rodent chow) were available ad libitum. Animal care during experimentation was conducted according to the Mexican official standard NOM-062-ZOO-1999, and under the Society for Neuroscience policy on the uses of animals in neuroscience research.
Sexual training
Sixty-four sexually experienced male rats were used in this experiment (Garcia-Martinez et al., 2010). Previously, these animals were subjected to a sexual behavior training every tree days. Males were placed in a plexiglas arena (60 centimeters of diameter × 60 centimeters of high), five minutes for habituation. Immediately, a receptive female (OVX with previous injection of benzoate de estradiol [10 μg; SIGMA] and progesterone [2 mg; SIGMA], 48 and 4 hours before the test), was introduced in the arena until the male achieved the ejaculation (20 minutes maximum). After four sessions, subjects who got an ejaculation between five to ten minutes were randomly assigned to four groups of sixteen rats each one: 1) Ctrl (saline solution injection); 2) oPRL (ovine PRL injection, SIGMA, Cat. L6520, 50 μg each 12 hours); 3) Graft (pituitary transplantation) and; 4) Sham (false surgery).
HP induction .
Moderate Hyperprolactinemia (HP) was induced by oPRL injection (IP, 100 μg dairy) for the oPRL group, and pituitary transplantation to the kidney for the graft group (Sato et al., 1997). Transplantation was carried out obtaining the pituitary from female donors by quickly decapitation and keeping them in sterile culture dishes containing saline solution. Simultaneously, males were anesthetized with sodium pentobarbital (Smith Kline, Mexico; 30 mg/kg b.w, IP), and the left kidney was exposed through a dorsal skin incision. Pituitary was introduced carefully under the renal capsule. Animals were kept during one week in recuperation before the beginning of sexual behavior test. In the sham group, the same procedure was performed, without introducing the pituitary (false surgery). The main difference between treatments is the plasmatic PRL level, since with oPRL administration we observed significant pulses (~40ng/ml) in PRL levels after administration in the morning and night, returning to the physiological state (~12ng/ml) after a while, whereas with the transplant, HP was maintained during the entire treatment (Abram et al., 1998).
Sexual behavior assessment
The stereotyped pattern of male copulatory activity in rats led to development of parameters that are useful in the analysis of male sexual behavior. This parameters involve the implementation of behavioral responses without penile insertion, called mounts (M); characteristic movements with intravaginal penile insertion, called intromissions (I) and, characteristic movements of penile insertion with ejaculation (E) (Manzo et al., 2002 [a;b]).
Sexual behavior was recorded with the Sexual Behavior Recorder Software (SBR), during the dark period (12:00 and 16:00 h) (Larsson, 1956). Males were placed in the arena with a receptive female, and the index of mount and intromissions was measured during the sexual intercourse (Paredes, & Agmo, 2004). The experiment was performed every three days (sessions 1, 2, 3, 4 and 5). The parameters recorded were: I) mount frequency (total number of mounts made by a male, measured by the number of mounts during the sexual intercourse); II) intromission frequency (total number of intromissions made by the male, measured by the number of intromissions during the sexual intercourse); III) first mount latency (is the time from the onset of sexual behavior until the first mount occurs, measured in seconds); IV) first intromission-ejaculation latency (time since the first intromission until the male ejaculates; measured in seconds), and; V) hit rate (sum of the number of mounts and intromissions, divided by the sum of the number of mounts, intromissions and ejaculations [I+E/I+M+E]). Hit rate is a measure of sexual behavior performance, with values above 0.5 indicating a good copulatory activity (Manzo et al., 2002 [a;b]).
PRL quantification
Blood samples were obtained at the end of last session by heart puncture. Previously, males were anesthetized with sodium pentobarbital (30 mg/kg BW, IP), and blood was obtained using sterile syringes. Ten samples were collected from each group, and they were centrifuged, separated into aliquots and stored at -20°C. PRL levels were determined by the immunoassay PRL ELISA kit ALPCO (Cat. 12-MKVRP1) (Beach, Miles, Lukes, & Vigersky, 1985). First, a standard curve of 5, 10, 20, 40 and 80 ng/ml PRL concentrations was prepared. Afterwards, 20 μl of unknown serum samples were added to the microplate, previously prepared with Anti-PRL antibody. Samples were analyzed with a microplate spectrophotometer (BIORAD 550) and absorbance was read at 450 nm wavelength. PRL concentrations were computed by linear regression fitting using Sigma Plot software (data were reported in ng/ml).
Statistical analysis
PRL levels between groups were analyzed with a one way ANOVA followed by the Student-Neuman-Keuls test. Sexual behavior parameters were analyzed with repeated measures ANOVA, followed by the Student-Newman-Keuls test when the F value and statistical tests were at a 0.05 significance level, validating the assumptions of normality and homogeneity of variances.
Results
PRL levels in serum
Blood samples were obtained by heart puncture, after last session and PRL levels were analyzed with the ELISA kit. Plasmatic PRL levels were presented in all subjects, however, the graft group showed a significant increase (40.46±1.18 ng/ml), compared with the baseline levels obtained from intact rats (~12 ng/ml) (Hernandez et al., 2006). Similarly, the control group showed a significant increase (19.90±1.18 ng/ml). In contrast, in the oPRL group, the increase was not significant (14.60±1.18 ng/ml), with similar results in the sham group (13.13±1.39 ng/ml)(Fig. 1) (F=8.36, p<0.01).
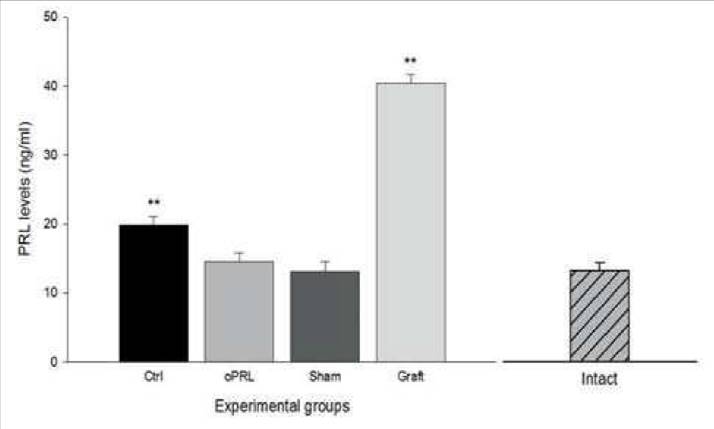
Figure 1 PRL levels in serum. The figure shows the PRL levels obtained in the four groups. In the ctrl and Graft group, the increase was significant compared with data obtained from an intact rat (not used for this experiment). Data are expressed as means ±SEM. Asterisks denote statistical differences.
Effect of HP on sexual behavior
First mount latency
During the experiment, subjects were tested every three days (sessions 3, 6, 9, 12 and 15). The statistical analysis did not showed differences between sessions (data not shown), but the differences between groups were significative. The first mount latency increased significantly in the oPRL group compared with the control group. Similarly, the Graft group showed a significant increase in the first mount latency compared with the Sham group (Table 1) (F=3.99, p<0.05, Fig. 2).
Table 1 Sexual behavior parameters
| Mean (X) | Ctrl | oPRL | Sham | Graft |
| First Mount Latency (seconds) | 5,8 ±0.40 | 9,4 ±1.20* | 6,3 ±1.40 | 8,5 ±0.74* |
| Intromission Frecuency (l) | 9,0 ±0.08 | 8,9 ±0.08 | 9,7±0.08 | 8,5 ±0.20 |
| Mount Frecuency (M) | 6,4 ±0.19 | 6,7 ±0.18 | 5,6 ±0.66 | 11,9±0.22** |
| First Intromission-Ejaculation (E) Latency (seconds) | 237 ±}0.10 | 239 ±0.10 | 217 ±0.35 | 358 ±0.14** |
| Hit rate [I+E/I+M+E] | 0,64±0.03 | 0.62 ±0.03 | 0,74 ±0.06 | 0,52 ±0.04** |
Data are presented as Means ± SEM. *p<0.05, ** p<0.01
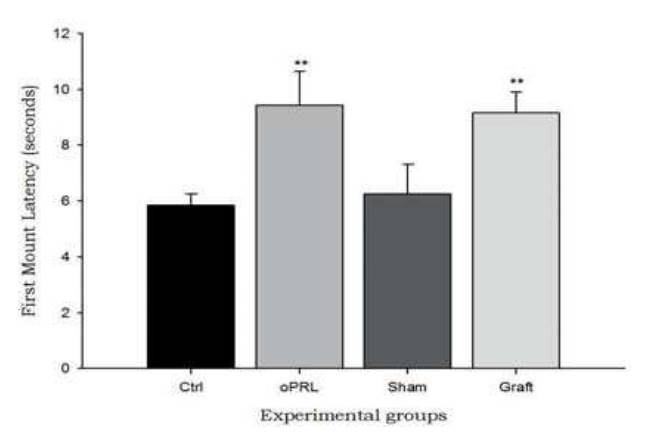
Fig. 2 The first mount latency. Figure shows that both treatments (ovine prolactin administration and pituitary transplant), increase the first mount latency when compared with the control groups (saline injection and sham-operated animals) (F=3.99, P<0.05). Data are expressed as means ±SEM. Asterisks denote statistical differences.
Mount and Intromission Frequency
In order to demonstrate the effect of HP induction in the mount and intromission frequency, there were analyzed all sessions using a repeated measure ANOVA. The analysis showed differences between groups but not between sessions (data not shown). The mount frequency did not increase in the oPRL group, but, in the graft group had a significant increase (F=13, p<0.01, Fig. 3 A , Table 1).Conversely, no treatment affected the intromission frequency (F=1.4, p=0.24, Fig. 3 B , Table 1).

Figure 3 Graph shows results of the Mount and Intromission frequency between the different groups. We can observe that HP obtained by the pituitary transplant is enough to produce an increase in the mount frequency in 15 days (F=12.68, p<0.001) (panel A). Panel B shows results of HP on the intromission frequency. We did not observe differences between groups (F=1.41, p=0.24). Bars show mean ± SEM. Asterisks denote statistical differences.
The Intromission-ejaculation latency and Hit rate. The oPRL administration did not produce any change in the intromission-ejaculation latency, however, the graft treatment increased this parameter when compared with the other groups (F=19, p<0.01, Fig. 4 A ). Similarly, the oPRL administration did not show differences in the hit rate, but the Graft group showed a significant reduction compared with the other groups (F=13.46, p<0.01, Fig. 4 B )(Table 1).
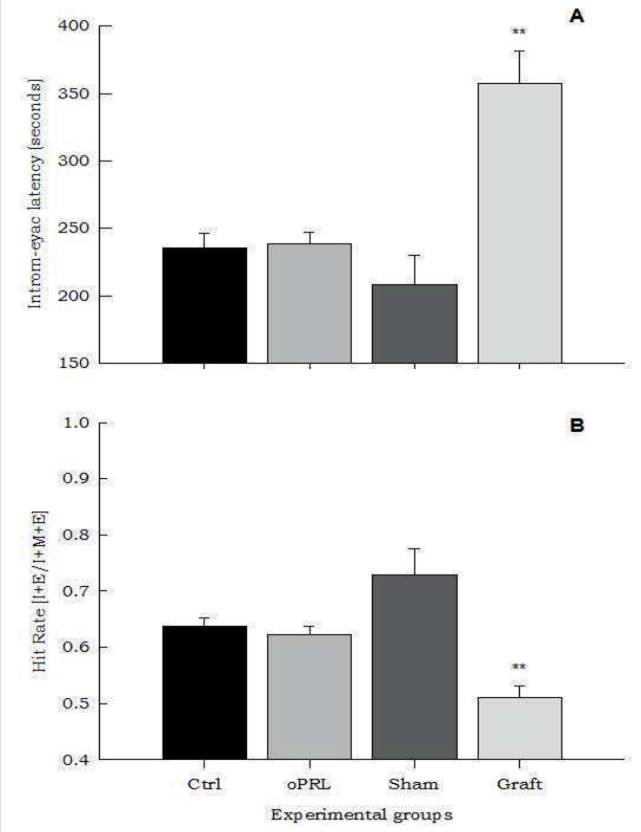
Figure 4 Panel A shows that in the transplanted group, the First Intromission-Ejaculation Latency was increased (F= 19.6, p<0.01). This increase was associated with a significant decrease in the Hit Rate, compared with the others groups (F=13.46, p<0.01) (panel B). Data are presented as means ±SEM. Asterisks denote statistical differences.
Discussion
Several clinical studies sustained that erectile dysfunction is the main symptom of chronic hyperprolactinemia (Martinez-Jalaboyas, 2010), however, this study was designed to compare if the induction of an acute HP to sexual experienced male rats, it was able to induce an impairment of the anticipatory/consummatory phases of sexual behavior.
The results obtained showed that in both groups (oPRL and the pituitary graft), it was observed a significant increase in the first mount latency. What is more, in the graft group was showed a significant increase the mount frequency and the first intromission-ejaculation latency with a significant reduction in the hit rate. Conversely, no treatment affected the intromission frequency.
Interestingly, this effect was not observed in subjects with oPRL administration. The probable explanation, is due to the used dose (100μg/d), since with this concentration, PRL levels increase only temporarily (40 ng/ml), returning to physiological levels after a while (12-14 ng/ml). This is consistent with studies reporting negative effects on sexual behavior with the induction of a sustained Hyperprolactinemia (>100 ng/ml/d) (Martinez-Jalaboyas, 2010; Abram et al., 1998; Doherty et al., 1985; Katovich et al., 1985). For that reason, the HP induced in the graft group (40ng/ml), was enough to modify the sexual function, with significant effects on the sexual behavior parameters tested (Hernandez et al., 2007).
The plasmatic PRL levels were slightly increased in the control group, however, are not related with a pathological condition, since some studies have shown that the acute increases in prolactin levels after coitus, are related with sexual satiety, suggesting the participation of PRL in different contexts of sexual behavior, like has been reported previously (Hernandez et al., 2006; Hernandez et al., 2007).
Accumulating data suggest that the neurotransmitter DA, plays an important role in the sexual behavior regulation, results presented here, supported this hypothesis and proposed strongly that PRL is able to induce alterations in DA levels in specific brain areas associated with the male copulatory activity (Giuliano & Allard, 2001b). The precise mechanism of how DA modifies the sexual behavior has been discussed. It has been shown that some neural nuclei are associated with specific phases of sexual behavior, for example, the nucleus accumbens and medial preoptic area, are associated with anticipatory and consummatory phases of sexual behavior, for that reason, the first intromissionejaculation latency was increased in the pituitary graft group (Giuliano & Allard, 2001[a;b]). Moreover, nucleus accumbens plays a central role in the reward circuit, in which, dopamine and serotonine are regulators of desire and saciety, so, when this nucleus is impaired, the frequency of intromissions and ejaculations are decreased together with the mount latency. On the other hand, the impairment of the paraventricular nucleus only modifies the mount frequency, suggesting that, in our model, prolactin could be affecting DA levels in specific brain areas (Paredes & Agmo, 2004).
According to many studies, the maintaining of high PRL levels, is directly related with an impairment of the sexual activity (Hernandez et al., 2006; Kruger et al., 2002; Manna et al., 2001; Svare et al., 1979; Ambrosi et al., 1976), but, in our model, the effects were observed in only 15 days, suggesting that the acute increase of PRL levels in the graft group was enough to impair the sexual behavior. In the oPRL group, fluctuations in prolactin levels did not have a significant effect in the sexual parameters, which means that increases have to be constants to observe negative effects on sexual behavior (Abram et al., 1998; Doherty, Bartke, Hogan, Klemcke, & Smith, 1982).
The increase observed in the first mount latency and the first intromission-ejaculation latency in the transplanted group, support the idea that PRL could down-regulate DA levels in the CNS. In this context, it has been demonstrated that the hyperprolactinemia induced by hypothalamic tumors or antipsychotics drugs, are associated with negative effects on sexual behavior (Martinez-Jabaloyas, 2010; Cavieres, 2008).
Moreover, few studies showing negative effects in the same time used in this experiment, but the serum prolactin levels obtained were above 1000 ng/ ml (Doherty, Bartke, & Smith, 1985; Doherty, Bartke, Smith, & Davis, 1985), while in our model, there were obtained PRL levels of 40ng/ml (Hernandez et al., 2006). However, our model shown clearly that with the levels obtained, the sexual behavior was affected.
Several studies have shown that the negative effects on sexual behavior (decrease of mounts, intromissions and ejaculations) (Veronesi et al., 2011), are related with a decrease in the levels of T (Paick, Yang, Kim, & Ku, 2006; Sato et al., 1997) and in DA receptors in specific brain areas (Bernichtein et al., 2010; Calarge et al., 2009; Fitzgerald & Dinan, 2008; Paredes & Agmo, 2004). The proposed mechanisms that explain this regulation is not clearly understood. The first suggested a downregulation in the hypothalamic-pituitary-gonad axis, decreasing T levels by a decrease in LH levels. The other is through a negative feedback of PRL to the dopaminergic receptors in areas related with the sexual function like the nucleus accumbens, the striatum or the preoptic area, and, in consequence, the decrease in DA release from the hypothalamus (Buvart, 2003; Kalra, Simpkins, Luttge, & Kalra, 1983), but, in our model, we do not know which areas could be affected since the hormone has to cross the hematoencephalic barrier to achieve significant concentrations in the brain. This means also that, with a intra-cerebral infusion the effects on sexual behavior are produced in a shorter time. For that reason, we believe that in the transplanted group, were observed more effects because of the constant levels obtained in this model (Giuliano F, & Allard J 2001a; Cruz-Casallas et al., 1999). Based on these studies, our results strongly suggested that T levels and the DA receptors are decreased in these animals; however we not analyzed this, being one of the limitations of the study.
Lastly, this results are interesting since a clinical view point, because the presented data demonstrated that a moderated HP is able to produce alterations on sexual behavior, reason why, PRL levels need to be analyzed in men with problems related with sexual dysfunction (Venetikou et al., 2008), and even more, in those with a sexual active life, since the moderate increase in PRL levels during the reproductive years could be related with a negative response on the sexual behavior.











 nueva página del texto (beta)
nueva página del texto (beta)


