1. Introduction
Recently, there is interest in jackfruit culture because of its unique organoleptic characteristics and nutritive properties. Nayarit is the main producer region in Mexico and exports most of the production (Medina-Tiznado et al., 2018). It has a high success at regional, national and international level due to its production yield, high commercial value (Luna-Esquivel et al., 2013), and a great commercial demand in many countries (Ragazzo-Sánchez et al., 2011). Jackfruit research represent an opportunity for studies because the information about growing methods or pest and rot management is scarce (Luna-Esquivel et al., 2013).
Jackfruit (Artocarpus heterophyllus Lam.) is a climacteric fruit with a high respiration rate, and during the ripening, there are many changes in their composition as a change in acidity and sugar content. The main problem in the quality of jackfruit is the over-ripening, malformation of fruit or pedunculi and rots that cause rapid decay of fruit due to high temperatures and relative humidity, conditions highly encountered in their production area (Ragazzo-Sánchez et al., 2011; Luna-Esquivel et al., 2013). Concerning postharvest disease of jackfruit, Rhizopus artocarpi and other species of Rhizopus have been reported as a cause of rots (Ghosh et al., 2015; García-Estrada et al., 2019), as well as Lasiodiplodia theobromae (Medina-Tiznado et al., 2018), Aspergillus niger (Ragazzo-Sánchez et al., 2011) and Colletotrichum gloeosporioides (Bhunjun et al., 2019).
Fruit surface is normally colonized by a mixture of microorganisms as epiphytes. Normally, most of these microorganisms are not pathogenic, but they role in fruit health, quality and disease resistance, before and after harvest is largely unknown (Droby & Wisniewski, 2018). Regarding postharvest disease, the impact of interactions of the pathogenic fungi is little known. Interspecific interactions can occur between fungi including mutual intermingling, mutual inhibition, and dominance by one species over another. The main types of interaction between filamentous fungi are competition for space, consequently, competition for resources in the substratum. However, biotic and abiotic factors affect the spatial competition such as temperature, pH, humidity, type of substratum, species identity, etcetera (Kolesidis et al., 2019). It might give them an advantage to outcompete other fungal colonizers. Understanding their interactions and their dominance will result in a prediction of their growth with the aim of avoiding damage and loss of quality in storage or transport conditions of jackfruit. The objectives of this study were to in vitro examine the effect of temperature on the growth of three fungal species isolated from jackfruit epicarp and their interaction.
2. Methods, techniques, and instruments
Fungal strains
Rhizopus stolonifer AhRs-01 isolated from jackfruit (Artocarpus heterophyllus Lam.) held in the Laboratory of Food Microbiology of the Technological Institute of Tepic, Nayarit Mexico, and two native molds isolated directly from different rots of jackfruit were included in this research. Macro and microscopical characterization of the two isolates were carried out according to Barnett & Hunter (1998) and Carrillo (2003).
Medium
Jackfruit pericarp agar (AJ) was specifically designed for this study, in which composition contains 0.6 % of pectin (Xu et al., 2018). The AJ medium contains 28 g of finely ground jackfruit pericarp and 15 g of agar per 1,000 mL of distilled water at pH 5.5 ± 0.2. The medium was autoclaved and poured into 90-mm sterile petri dishes. The water activity (wa) was measured at the beginning and at the end of the experiments (AquaLab Pre Water Activity Meter, Decagon Devices, Pullman, WA), and must remain stable during the experiment and near 1.
Inoculation
All the strains were cultured on PDA at 25 °C. From the margin of a 5-day old growing colony, a disc of 5 mm diameter agar made with a core borer was taken for all strains, except for one faster growing-strain, which a one-day old colony was taken. A disc of the paired isolate was taken and transferred to the AJ plate and placed 4.0 cm apart from the other in the dish. Petri dishes of AJ medium were inoculated centrally with agar the disc of each isolate as a control. They were incubated at 13, 25, and 35 °C. Once the growth started, radial measurements were periodically made of each colony, one on a line joining the two inoculation points, the other at the opposite side of the colony. All isolates were cultures alone at the same temperature conditions as a control.
The radius of the colony was plotted versus time to calculate the growth rate. Nonlinear regression was applied to estimate the maximum growth rate (µ max , mm/day), by fitting the experimental data to the Baranyi & Roberts model (Baranyi & Roberts, 1994) (equations 1 and 2).
Where: R is the colony radius (mm); R0 is the colony radius at the time t = 0, Rmax is the maximum colony radius in Petri dishes, and A is an integral variable running from 0 to t, in function of the curvature of the plot. The curve fitting procedure used Marquardt's algorithm of the StatGraphics Centurion XV.II (Maryland, USA) with 95 % of confidence.
Effect of temperature
The effect of temperature on µmax for each unpaired fungus was estimated using the cardinal model with inflexion (CMI) developed by Rosso et al. (1993) (equation 3). Cardinal temperatures (Tmin , Tmax , and Topt ) values were estimated using this model. Temperatures in this work were chosen to simulate the optimum and the extremes conditions that may surround the jackfruit supply chain.
Where μmax is the growth rate (mm/d); µopt is the growth rate at optimal temperature; T is the temperature (° C); and Tmin , Tmax , and Topt are cardinal temperatures. Marquardt’s algorithm was used for model fitting with Statgraphics Centurion XV.II (Maryland, USA) with 95 % of confidence.
Types of interaction and ID calculation
Fungal paired interaction was observed as a function of the growth rate of each fungus alone or paired to another. Data were statistically analyzed by the HSD test. When significant differences existed in the growth rate of fungus in the line face of the other fungi, and when it grew at the opposite side and/or alone, implied that fungus was affected by the other. Then, a numerical score assigned based on the type of interaction according to Magan & Lacey method: [1] when mutual intermingling exist; [2] mutual antagonism on contact or with free space between fungus colonies < 2 mm; [3] mutual antagonism at a distance; dominance on contact [4 for the dominant species, 0 for the inhibited species] and dominance at a distance [5 for the dominant species, 0 for the inhibited species] (Magan & Lacey, 1985; Sempere & Santamarina, 2010).
Microscopic observation
A microscopic study of the fungi interactions according to Li et al. (2013). was performed. Brief, glass microscope slides with PDA were placed into sterile Petri dishes whit a humidified medical tissue. Discs (5 mm) were cut from the edge of each colony as previously described. One disc of each isolate was transferred to one end of each agar-covered slide and one of the other isolates to the other end, then the two discs were 2 cm apart. Slides containing the isolate growing alone served as controls. Slides into the Petri dishes were incubated at 25 °C in the dark until an interaction zone appeared. Interactions between each paired isolate were observed under a microscope Motic BA310 and results were photographed.
Validation
An experiment was performed on jackfruit to compare the growth of mold on the surface of the fruit and validate the predictions of the models. Jackfruit was disinfected with sodium hypochlorite (1 % v/v) for 2 min and rinsed with sterile water (Arias & Toledo, 2000), then, fruit was inoculated by triplicate with either single or mixed isolates of the fungal species into an artificial wound (3 mm diameter by 3mm deep) with 10 µl of suspensions adjusted to 10 6 spores/ml (Iñiguez-Moreno et al., 2020). The fruit was incubated at 25 °C at a RH > 85 %. The diameter of infection and lesion was daily measured. The radius of each infection against time was plotted and the Baranyi & Roberts model was used to calculate the growth rate. Comparison between the predicted growth rate and the observed growth rate was assessed by using the accuracy, A f and the bias B f factors (Ross, 1996).
The fungal genera could be differentiated from each other on the jackfruit peel based on their color and morphology, thus a microscopical observation of rots was performed (Steel et al., 2011).
3. Results and discussion
3.1 Fungal isolates
The two isolates taken from different rot lesions from jackfruits were morphologically different at the naked eye. The microscopic structural characters were recorded with a digital camera (data not shown). Both isolates were identified following standard literature (Barnett & Hunter, 1998; Carrillo, 2003) as Colletotrichum sp., named AhCx-02 and AhCx-03.
The experiments were performed at three different temperatures and the wa remains stable during the incubation time at all conditions (aw = 0.97 ± 0.03). The data of each isolate individually cultured on AJ medium at 13, 25, and 35 °C were plotted against time to individually obtain the growth curves (Figure 1).

Figure 1 Radii of growth versus time describing the growth of «R. stolonifer» and «Colletotrichum» sp. strains AhCx.02 and AhX-03 isolated from jackfruit rots.
Growth of the unpaired isolates followed a biphasic model at extremes temperatures. Rhizopus stolonifer showed a linear growth at 35 °C, then a linear regression by Excel was used to evaluate µmax (Table 1) with a determination coefficient R2 > 0.82. For all the other strains and conditions, the Baranyi & Roberts model was used to estimate µmax (R2 > 0.95) (data not shown). All sets of data were statistically examined.
Table 1 Radial growth rate (µ max ) estimated by linear regression or the Baranyi-Roberts model for «Rhizopus» AhRs-01 and «Colletotrichum» AhC-02 and AhC-03 isolates from jackfruit epicarp.
| *µ max (mm/d) at 13 °C | *µ max (mm/d) at 25 °C | *µ max (mm/d) at 35 °C | |
|---|---|---|---|
| AhRs-01a | 8 ± 0.1 | 18.9 ± 2.3 | 0.6 ± 0.1 |
| AhCx-02 b | 1.9 ± 0.05 | 5.9 ± 0.1 | 4.4 ± 0.1 |
| AhCx-03b | 1.8 ± 0.05 | 6.1 ± 0.1 | 3.4 ± 0.1 |
Note: * Values are means ± standard errors. Different letters indicate significant differences (P < 0.05).
In general, growth rates increased with temperatures near to de optimum and decreased at extreme conditions. Significant differences were observed among the unpaired isolates. Colletotrichum AhCx-02 and 03 formed a homogeneous group (P > 0.05) with a very similar value in µmax at the same temperature. On the other hand, R. stolonifer grew faster than the others at 13 and 25 °C occupying all the area in the Petri dish in a few days (P < 0.05). However, at 35 °C this isolate had poor growth.
By the CMI model (equation 3), the effect of temperature on µmax was determined and the cardinal values were estimated. The fitted curves showing the influence of temperature on µmax are presented in Figure 2.
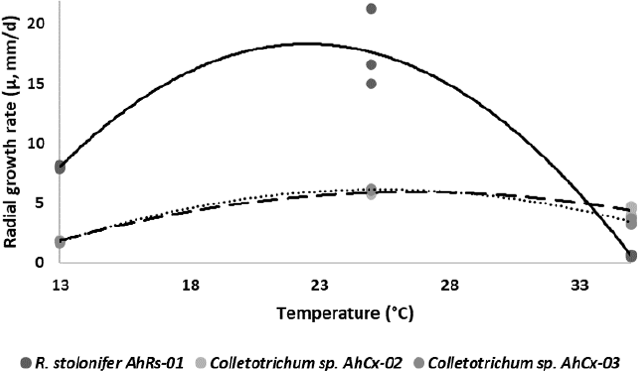
Figure 2 Radial growth rate (µmax , mm/day) versus temperature (T, °C) for «R. stolonifer» (continuous line), «Colletotrichum» ps. AhCx-02 (hatched line) and «Colletotrichum» ps AhCx-03 (dotted line) isolated from jackfruit. Points are observed data, and lines indicate the fit of the data to the cardinal model with inflexion (equation 3).
Coefficient estimations are in Table 2 for all strains. The calculated optimal µmax was higher for the strain AhRs-01, value three times higher than the other strains. The theoretical Tmax was different depending on the isolate, varying between 35 - 43 °C and the theoretical Topt was 22 to 27 °C. All parameters obtained are in the 95 % confidence interval.
Table 2 Estimated cardinal coefficients for the Rosso et al. (1993) model fitted to growth rates for three unpaired fungal isolates from jackfruit on AJ medium*.
| Isolate | µopt | Topt | Tmax | Tmin | R2 | RMSE |
|---|---|---|---|---|---|---|
|
R. stolonifer AhRs-01 |
18.20 ± 1.2 | 22.76 ± 1E-7 | 35.20 ± 0.4 | 8.06 ± 2.4 | 0.95 | 4.3 |
|
Colletotrichum sp. AhCx-02 |
5.90 ± 0.1 | 27.00 ± 1E-8 | 43.00 ± 1.1 | 10.50 ± 3.7 | 0.98 | 0.1 |
|
Colletotrichum sp. AhCx-03 |
6.14 ± 0.1 | 25.34 ± 1E-8 | 39.90 ± 0.3 | 11.13 ± 0.9 | 0.99 | 0.03 |
Note: * Values are means ± standard deviations.
The plug of the mycelium of each species inoculated on Petri dishes in all experiments expands outwards in a radially symmetric way that competition was determined by observing the colony peripheries. Thus, a one-dimensional mathematical model sufficient to investigate such competition corresponding to the growth along the line connecting the centers of the inoculation sites, compared with the opposite side. The dual growth of each strain was affected by the temperature and the paired species. The effect of temperature on growth for each fungus alone or paired to the other species was showed in Figure 3. Initially, the growth of the paired colonies was not affected. Later, the interaction significantly influenced their development depending on the temperature.
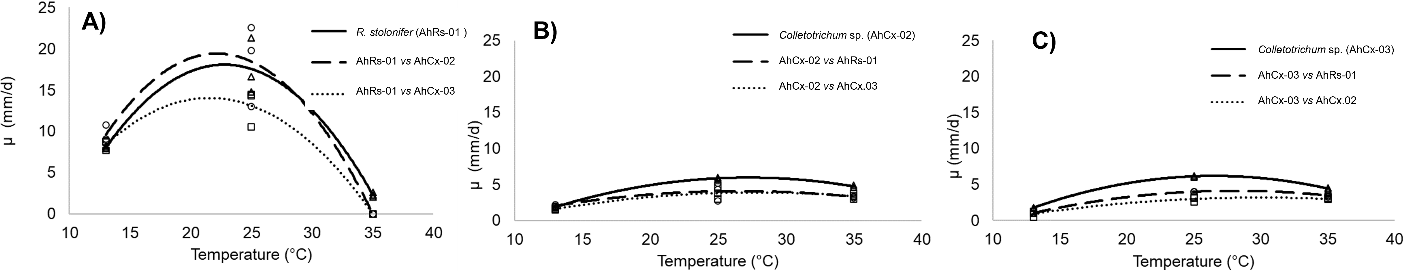
Figure 3 Effect of temperature on growth of A): «R. stolonifer», B): «Colletotrichum» sp. AhCx-02, and C) Colletotrichum sp. AhCx.03 in competence to the two other species. The different lines indicate the difference in growth rate as a function of temperature when fungus grows alone or paired.
At extremes temperatures, the interaction was no significant for all paired isolates while near the optimal conditions their interactions were more clearly observed.
Three distinct types of interactions have been observed: I) intermingling, when fungi overlap and both fungal colonies coexist such as pairwise AhRs-01 versus AhCx-02 at 13 and 25 °C; II) mutual antagonism with a significant reduction in the growth rate, even if they overlap like strains AhCx-02 versus AhCx-03 at 35 °C, and III): dominance on contact (strain AhCx-02 versus AhCx-03 at 13 °C) (Table 3). The isolates AhCx-02 and AhCx-03 cultured unpaired, their growth rate appeared to be similar (P > 0.05), but based on their interactions, they are not (P < 0.05).
Table 3 Index of dominance ID * given to different species when growing a paired with another species.
| Temperature | Isolate vs/ | AhRs-01 | AhCx-02 | AhCx-03 | ID |
|---|---|---|---|---|---|
| 13 °C | R. stolonifer AhRs-01 | _ | 1 | 1 | 2 |
| Colletotrichum sp. AhCx-02 | 1 | _ | 4 | 5 | |
| Colletotrichum sp. AhCx-03 | 1 | 0 | _ | 1 | |
| 25 °C | R. stolonifer AhRs-01 | _ | 1 | 0 | 2 |
| Colletotrichum sp. AhCx-02 | 1 | _ | 4 | 5 | |
| Colletotrichum sp. AhCx-03 | 4 | 0 | _ | 4 | |
| 35 °C | R. stolonifer AhRs-01 | _ | 0 | 0 | 0 |
| Colletotrichum sp. AhCx-02 | 1 | _ | 2 | 3 | |
| Colletotrichum sp. AhCx-03 | 1 | 2 | _ | 3 |
Note: * ID refers to the sum of scores at a certain temperature for each strain based on the interaction scores for each species. Types of interaction: a) Mutual intermingling [1]; b) mutual antagonism on contact [2]; c) mutual antagonism at a distance [3]; d) dominance on contact [4 for the dominant species, 0 for the inhibited species], and d) dominance at a distance [5 for the dominant species, 0 for the inhibited species].
The type of interactions was evaluated based on their growth rate at different conditions are shown in Table 3. At 13 °C the growth of R. stolonifer has been enhanced in contact with Colletotrichum AhCx-02 (P < 0.05), but not near Colletotrichum AhCx-03, while AhCx-02 has affected the strain AhCx-03 diminishing their growth rate (P < 0.05). Near the optimal conditions, the strain AhCx-02 enhances the growth rate of R. stolonifer with no significant differences while AhC-03 diminishes their growth rate (P < 0.05). On the other hand, the isolate AhCx-03 was affected by AhCx-02 also diminishing their growth rate (P < 0.05). Moreover, at 35 °C, the temperature appears to inhibit the growth of strain AhRs-01, and the strains AhCx-02 and AhCx-03 showed mutual antagonism. According to de Index of Dominance (ID) obtained, the strain AhCx-02 was more competitive at low temperature and optimal conditions than the others, while at 35°C, AhCx-02 and AhCx-03 coexist.
Microscopically, when a Colletotrichum sp. grew unpaired, the hyphae freely develop (Figure 4 A and B) as well as they are paired (Figure 4 C). The thin hyphae of R. stolonifer grows invading the space and intermingling with the Colletotrichum sp. (Figure 4 D). When the strain AhCx-03 was paired to AhCx-02 at 13 °C, only growth rate slows down (P < 0.05) with no apparent changes in the hyphae.
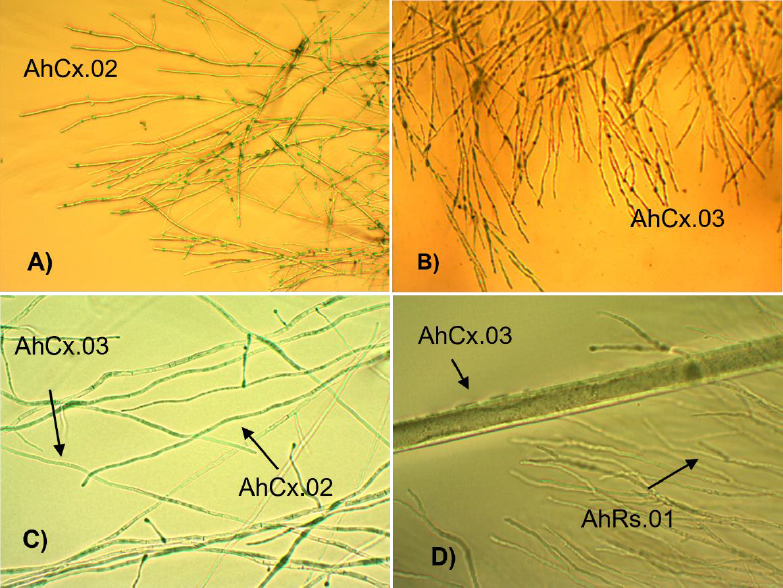
Figure 4 Micrographs (40 X) of A): «Colletotrichum» sp. AhCx-02; B): «Colletotrichum» sp. AhCx-03 individually cultured; C) «Colletotrichum» sp. AhCx-02 and AhCx-03 paired with each other, and D) «R. stolonifer» paired to Colletotrichum AhCx-03.
To validate the models, individual and mixed infections were done at room temperature on jackfruit. Individual infections were as expected (Figure 5). All infections start the day after being inoculated with a 100 % of incidence, being R. stolonifer the faster-growing fungus. On the fourth day after the inoculation, R. stolonifer showed the fruit surface covered by white fungal mycelia and black spores while the Colletotrichum species shows a white mycelium.

Figure 5 Infections caused by «R. stolonifer» (A and D); «Colletotrichum» sp. AhCx-02 (B and E) and «Colletotrichum» sp. AhCx.03 (C and F) on the second- and fourth-day following inoculation on jackfruit, respectively.
The radial growth rate of each isolate individually showed a different behavior comparing when they were mixed. Rhizopus stolonifer showed a halo of injury three times higher than the infection area. The other isolates grew slower than in the AJ medium (Table 4).
Table 4 Radial growth rate of «R. stolonifer»; «Colletotrichum» sp. AhCx-01 and «Colletotrichum» sp. AhCx.03 on jackfruit at 25 °C, when grew individually and mixed, and their bias and accuracy factors comparing data in vitro and in vivo.
| Mold | *µ (mm/d) | R2 | Bf | Af |
|---|---|---|---|---|
| R. stolonifer | 19.18 ± 0.3 | > 0.95 | 2.2 | 2.2 |
| Colletotrichum sp. | 0.67 ± 0.2 | > 0.88 | 2.5 | 2.5 |
| Colletotrichum sp. | 0.70 ± 0.2 | > 0.85 | 2.6 | 2.6 |
| Fungal mix | 36.26 ± 3.5 | > 0.98 | # 0.72 | # 1.4 |
Note: *Values are means ± standard deviations. # The Bf and the Af was calculated comparing µ of the mixed infection on jackfruit with µ of R. stolonifer in AJ medium at 25 °C.
The bias and the accuracy factors (Bf and the Af ) were calculated for each isolate at room temperature to compare the predicted model in AJ medium. The values were > 2.2 indicating no correlations.
Otherwise, during the mixed infection with all spores, the symptoms were observed at the second day after the inoculation and the severity was greater showing a halo of injury twice the size of the initial infection. On the third day, the halo of injury was more than three times higher than the infection, and, on subsequent days, intermingles with the infection. The presence of R. stolonifer in the infection was evident (Figure 6) from the beginning. At the fifth day, the infection showed a cottony appearance around the edges, indicating the coexistence of Colletotrichum sp.

Figure 6 Infection on jackfruit with of «R. stolonifer» and «Colletotrichum» sp. AhCx-02 and AhCx-03 strains at the fourth day after inoculation.
The Bf and the Af for the mixed infection were calculated comparing with the faster-growing fungus Rhizopus AhR-01 in vitro. The values were Bf : 0.72 and Af : 1.4.
3.2 Discussion
To obtain a good quality jackfruit, it must be ripened on the tree. After harvesting, the fruit ripens in 3 - 7 days (Elevitch, Craig & Manner, 2012). Due to their short shelf life, rot diseases are an important problem resulting in huge losses due to deterioration (Amusa, Kehinde & Ashaye, 2002). The fungal isolates used in this research belong to the main one that causing rots on jackfruit in all producers zones (Nelson, 2005; Bhunjun et al., 2019). The environmental factors affect the growth of fungal disease and may vary on each other. Moreover, these factors influence the outcomes of spatial competition between fungal species. Rhizopus is a fast-growing fungus as it was observed in this work. Rhizopus stolonifer has been reported with the highest mycelial growth rate (μ opt = 94.32 mm/d), five times faster than the results obtained in this study. To our knowledge, there are only a few reports about the growth rate for Rhizopus species, and no jackfruit varieties have been reported to have resistance to the Rhizopus rot (Nelson, 2005).
On the other hand, our results showed that the two isolates of Colletotrichum used in this work had a similar behavior if they grow individually, even if their macroscopic appearance was different, reason for these strains to be chosen. The cultures are often highly divergent within a species. According to Weir et al. (2012), the differences are probably a reflection of different storage histories, especially with repeated sub-culturing, resulting in staling of the cultures, changes in the appearance and color of the mycelium, and variable in growth rate (Weir, Johnston & Damm, 2012). In this case, their growth rate was similar to those obtained for C. gloeosporioides isolated from papaya fruit at 25 °C (Sandoval-Contreras et al., 2020). Optimal temperatures for growth of C. gloeosporioides from avocado have been reported in a wide range: 25 to 35 °C (Judet-Correia et al., 2010) while for C. gloeosporioides from papaya at 27 - 31 °C (Sandoval-Contreras et al., 2020). No reports have been done for growth at 5 °C and no germination at 37 °C (Pitt & Hocking, 2009).
Regarding models of in vitro unpaired isolates, the minimum temperature estimated for all strains was near to the temperature for storage. The temperatures recommended for storage depend on the type of fruit and the variety, normally between 7 and 13 °C (Arias & Toledo, 2000). Rhizopus stolonifer has been reported to grow from 5 °C up to 30 °C or 35 - 37 °C, being their optimal growth at 25 °C, but at 37 °C usually colonies do not growth. According to Bautista-Baños et al. (2008), Rhizopus rot progression is temperature related with a maximal fungal growth at 27 °C, temperature higher than the theoretical optimal temperature obtained in this study (Bautista-Baños et al., 2008).
In the current study, the impact of temperature on the growth rate of all isolated was evident. Rhizopus had a high growth rate compared to the other isolates. At the low temperature used in the experiments, all strains showed a certain growth rate, which increases as the temperature increases, then decreases as the temperature continues to increase. Similar results were obtained for C. gloeosporioides isolate from papaya fruit (Sandoval-Contreras et al., 2020). Comparative studies showed that Rhizopus had higher growth rates compared to other genera, taking a few days in became visible at the naked eye. Ochoa-Velasco et al. (2018) reports µ max = 0.31 (1/h) (~7.44 mm/d) for R. stolonifer at 28 °C. The mycelial growth rate for R. stolonifer on pears has been estimated at 3.93 mm/h (94.32 mm/d) (Sardella, Gatt & Valdramidis, 2018). On the other hand, similar results were found for C. gloeosporioides isolated from papaya. The maximal radial growth rate µ max was 3.6 mm/d (Teixeira et al., 2007) and between 2.3 and 4.3 mm/d (Sandoval-Contreras et al., 2020), both at 25 °C. High frequency of saprophytic fungi exists on fruit surface, but some of them are pathogens. Wind, rain, and insects dislodge and spread the fungal spores and expose the fruit to postharvest infections. Such conditions favor an increase in fruit mycobiota diversity increasing the risk or severity of rots. For this reason it was important to investigate the interaction of postharvest pathogenic molds (Lorenzini et al., 2013).
The main type of interaction between filamentous fungi is competition for space and nutrients. At first, resource exploitation and direct harmful replacement after physical contact exist, or antagonism at a distance if a volatile substance or diffusible chemical is produced. This ability to capture unoccupied space might be inhibited or enhanced by the presence of adjacent or distant mycelia. (Kolesidis et al., 2019). In the case of R. stolonifer, it belongs to the class of the Zygomycetes, with aseptate hyphae or very few septa that enhance rapid translocation and absorption of nutrients, thus allow rapidity of growth to the fungus (Pitt & Hocking, 2009; Sardella, Gatt & Valdramidis, 2018). In our study, R. stolonifer covers the plate in a few days, paired or unpaired, just as it did in vivo due to their fast growth. The presence of Colletotrichum affects its growth by a slight stimulation or by inhibiting it depending on the isolate, however, they coexist even in extreme conditions.
Regarding ID index, Colletotrichum isolate AhCx-02 shows more competitiveness face to the other Colletotrichum AhCx-03 isolate, but at high temperature, the interaction was not observed. The temperature is one of the main abiotic elements that influence the outcomes of spatial competition (Kolesidis et al., 2019) such as we observed in this study. Seasonal temperatures predispose the incidence of a fungal specie responsible for rot infection. Research about the interaction between C. acutatum, Botrytis cinerea, and Greeneria uvicola on berries, B. cinerea was reduced when co-inoculated with C. acutatum at 20 or 27 °C, but with G. uvicola was only reduced at 27 °C. They concluded that B. cinerea was the predominant pathogen at 20 °C, whereas at 27 °C predominates C. acutatum (Steel et al., 2011). It can be inferred that depending on the ambient temperature, a high virulent strain may appear and dominates over other species causing rots in fruits. Both isolates of Colletotrichum coexist with Rhizopus at 13 and 25 °C but no at 35 °C. Although Colletotrichum AhCx-03 inhibits the growth of Rhizopus, it continues its growth a little slower and they end up coexisting. To our knowledge, the mixed infection or interaction of Rhizopus and Colletotrichum on jackfruit have not been addressed. Stimulation of enzymatic activity in mixed infections of Pleurotus ostreatus and Ceriporiopsis subvermispora have been reported in the degradation of aspen wood (Chi, Hatakka & Maijala, 2007). In the same way, it may be a mixed fungal infection that enhances the enzymatic degradation of fruit. Spores of pathogens can cause a primary seasonally infection, but a subsequent infection caused by another pathogen produces a mixed infection increasing deterioration (Agrios, 2005). High temperatures did no favor Rhizopus growth because, in these conditions, Colletotrichum species were the dominant ones. At low temperatures or near optimal conditions, both Rhizopus and Colletotrichum coexist.
4. Conclusions
In conclusion, temperature influences competition between the studied species, being Colletotrichum sp. the most competitive fungus. On the other hand, R. stolonifer and Colletotrichum sp. appear to be more harmful to jackfruit when they coexist on jackfruit surface rather than when either is present alone. Understanding the interactions of epiphytic communities may contribute to the development of new postharvest control systems. Hierarchical or intransitive competition has yet to be studied in further detail; nevertheless, these findings may have relevance in the knowledge of postharvest treatment of jackfruit.











 text new page (beta)
text new page (beta)



