Introduction
The Athabasca oil sands deposits (colloquially called “tar sands”) in northeastern Alberta, Canada comprise a vast reserve of biodegraded petroleum, known as bitumen, that is surface-mined by several companies (reviewed by Foght et al., 2017). Bitumen extraction requires huge volumes of water, including recycled and makeup water from the Athabasca River to separate bitumen from the sand, clay and minerals of the ore. A light hydrocarbon diluent, the composition of which differs between mining companies, is used in one processing step to reduce bitumen viscosity and enhance separation of bitumen from fine solids. Following extraction, fluid fine tailings comprising suspended sand, solids, clays, unrecovered bitumen, and residual diluent are stored in large settling basins (tailings ponds) holding hundreds of millions of cubic meters of tailings. The solids separate from water relatively quickly initially, allowing the uppermost layer of process-affected water to be re-used in ore-processing activities. Thereafter, the solids (predominantly fine clay particles) suspended beneath the surface water layer form a semi-solid colloid that consolidates very slowly while expressing pore water; gravitational densification of fines to a “trafficable” physical state has been estimated to take 125-150 years (Eckert et al., 1996). Several chemical and physical methods have been developed to accelerate consolidation (e.g., BGC Engineering, 2010) but they can be cost-intensive and degrade the quality of recycle water. Thus, new technologies to enhance and accelerate separation of pore water from colloidal solids (“de-watering”) accompanied by faster aggregation and decreased volume of clay particles (“consolidation”) might augment existing tailings management practices.
Oil sands tailings ponds are biologically active and contain complex, predominantly anaerobic, communities of microorganisms (An et al., 2013; Ramos-Padrón et al., 2011; Holowenko et al., 2000; Penner and Foght, 2010). Each tailings pond appears to have a unique microbiota reflecting the ore source, bitumen extraction process and tailings operations (Wilson et al., 2016). The diverse bacterial communities in tailings ponds include nitrate-, iron-, sulfate-reducing, and hydrocarbon-degrading genera, and the Archaea are almost exclusively methanogens (reviewed by Foght et al., 2017). Analysis of metagenomic data has also been used to correlate community composition with microbial activity in tailings ponds (An et al., 2013; Wilson et al., 2016). It is presumed that certain hydrocarbons, predominantly from fugitive processing diluent, are the major microbial substrate in the tailings ponds, being biodegraded anaerobically to carbon dioxide and methane in situ. Syncrude’s Mildred Lake Settling Basin began to release bubbles of methane and carbon dioxide at the surface in the early 1990s and has been estimated to produce >40 million L methane/day (Holowenko et al., 2000; Siddique et al., 2008). Other tailings ponds receiving diluent-treated tailings also produce biogenic gases (Burkus et al., 2014; Kong et al., 2019).
Fedorak et al., (2003) observed in laboratory trials that the consolidation rate of methanogenic tailings was greater than that of non-methanogenic tailings, indicating that microbial activity enhances densification. Arkell et al., (2015) confirmed this observation by amending tailings with acetate, suggesting the potential for an on-site engineered process. Although using a pure substrate such as acetate is economically impractical for implementation at field scale, many agricultural industries produce large amounts of low- or negative-value by-products that are susceptible to anaerobic biodegradation. Siddique et al., (2014a, b) amended oil sands tailings with hydrolyzed canola meal and observed enhanced biodensification compared to an unamended control; a biogeochemical model involving various biodensification processes was developed to explain the phenomenon. Thus, engineered biodensification has potential environmental benefits by increasing the volume of pore water for reuse in the bitumen extraction process (thereby decreasing the fresh water demand) and reducing the legacy tailings inventory (thus reducing the land surface area required for storing tailings). An additional benefit could be production and capture of biogenic methane on-site as a clean-burning fuel, as commonly achieved in municipal biosolids waste treatment plants. However, exploiting this phenomenon on the vast scale of the oil sands tailings ponds would require a cost-effective carbon source and studies using tailings from different operators.
In the current study several agri-business by-products including canola meal, glycerol, blood meal, bone meal, whey powder and corn-based dried distillers’ grains were tested for their potential to stimulate tailings biodensification. These experiments are part of a larger study to identify the optimal conditions for biodensification of fine tailings and to address the physical and chemical factors that contribute to increased consolidation in microbially active tailings.
Methods
Sources of oil sands tailings and organic amendments
Tailings ponds operated by three different oil sands companies (Syncrude Canada Ltd., Suncor Energy Inc., and Shell Albian Sands) were sampled for mature fine tailings below the water:sediment interface (“mud line”) and process-affected water at the surface, using sterile containers filled to overflowing to eliminate aerobic headspace, per Foght et al., (2014). Prior to sub-sampling, the bulk tailings samples were gently stirred by hand under a stream of anaerobic N2 gas to avoid introducing oxygen. Gravimetric Dean-Stark analysis (as described by Foght et al., 2014) was performed to determine the water, solids and bitumen content of the tailings. Microbiological enumeration of four different metabolic classes of bacteria in the bulk tailings was performed as described below.
Six agribusiness feedstocks and one model substrate were tested as amendments in biodensification experiments. The agricultural by-products from commercial crops or agri-products (provided by Sanimax Canada) included: canola meal (post-oil extraction); hydrolyzed blood-meat meal and bone meal (slaughterhouse wastes, rendered safe to Canadian Food Inspection Agency standards); whey powder (dairy waste); corn-based dried distillers grains with solubles (corn-DDGS; brewing or bioethanol waste); and glycerol (representing biodiesel waste). Sodium acetate, a methanogenesis substrate, was used as a positive control amendment. The negative control treatment consisted of adding 10 mM 2-bromoethanesulfonate (BES) to specifically inhibit methanogens (Smith and Mah, 1978); BES was added in combination with acetate to distinguish it from a control comprising unamended (endogenous carbon) tailings. Unless otherwise indicated, pure chemicals were purchased from Fisher Scientific, Ontario, Canada.
Canola meal, blood meal and bone meal were hydrolyzed as previously described (Siddique et al., 2014a) to render them water soluble and so avoid adding bulk to the tailings. Elemental analysis (C, H, N, and S) was performed on the organic amendments using a Carlo Erba EA 1108 elemental analyzer in the Analytical and Instrumentation Laboratory at the University of Alberta (Department of Chemistry) so that amendments could be added to tailings on a carbon-equivalent basis. The agricultural amendments (which contained 33.7 to 45.9% C; Supplementary Table S1) and pure glycerol were added individually at 400 mg C/L tailings; due to a calculation error, acetate was added at 72 mg C/L tailings.
Biodensification (de-watering and consolidation) columns
Amendments were dissolved individually in 75 mL of anoxic tailings pond water and gently mixed by hand into 1.5 L tailings under N2 gas to maintain anaerobic conditions. The amended tailings were transferred to sterile 2-L glass cylinders and capped with aluminum foil or several layers of latex secured with tape, to reduce water evaporation from the surface. An unamended (endogenous substrate) control that received tailings pond water but no organic substrate addition was prepared in parallel. Columns were incubated stationary in the dark at room temperature (~20°C), which is similar to the tailings temperature in situ (e.g., 12-21°C; Penner and Foght 2010). Initially the tailings were homogeneous with no pore water observed at the surface, but during incubation a water layer representing expressed pore water appeared above the mud line. The volumes of these two phases were measured at intervals during incubation for 56-188 d until de-watering and consolidation measurements reached a plateau.
At the end of incubation (168 d) of the Syncrude columns, the composition of randomly selected biogenic gas bubbles trapped in the tailings layer was measured by using a cannula to withdraw a portion of the bubble, followed by gas chromatography (GC) (Holowenko et al., 2000). During decommissioning of these Syncrude columns the overlying expressed pore water as well as sub-samples of the uppermost few cm and lowermost few cm of solid phase from each column were collected for standard chemical analyses by Syncrude Canada Ltd.
Measurement of cumulative methane production by tailings in microcosms
We could not directly measure methane production from the consolidation columns because they were not gas-tight and furthermore some gas remained trapped as gas voids within the solids. Therefore, we determined the methane-producing potential of amended tailings in sealed anaerobic microcosms. For these experiments, tailings samples were mixed with cognate anoxic tailings pond water at 3:1 by volume, then replicate 100-mL subsamples were added to sterilized 158-mL serum bottles having a headspace of 30% CO2/balance N2 (Holowenko et al., 2000). Organic amendments were added to the bottles based on the same carbon-equivalent basis as for the biodensification columns (400 mg C/L tailings) and acetate at 72 mg C/L. Microcosms were incubated stationary in the dark at room temperature (ca. 20°C). At intervals the bottle contents were mixed gently by inversion and 0.1 mL of headspace was removed using sterile needle and syringe for methane analysis using GC (Holowenko et al., 2000).
Microbial enumeration and identification (pyrosequencing of 16S rRNA genes)
Viable methanogens, sulfate-reducing prokaryotes, nitrate-reducing Bacteria and fermentative prokaryotes were enumerated in the solids fraction of initial bulk tailings samples and also after biodensification by using the 3-tube most probable number (MPN) method and previously described media (Hulecki et al., 2010; Penner and Foght, 2010). MPN tubes were incubated in the dark at room temperature (ca. 20°C) for 60 d before scoring; results were reported on a volumetric basis. Statistical differences between MPN values were determined using Cochran’s method (Cochran, 1950).
During decommissioning of consolidation columns, total genomic DNA was extracted from the solids phase in each column using a bead-beating method (Foght et al., 2004). Thereafter, pyrotag sequencing of 16S rRNA genes in total genomic DNA was performed as described by Siddique et al., (2014b). Briefly, triplicate aliquots (300 µl each) of every sample were extracted twice (total six extractions per sample), pooled, then PCR-amplified in triplicate using “universal” primers (An et al., 2013) and a touchdown method (Siddique et al., 2014b). Pyrosequencing of pooled amplicons was performed at Genome Quebec Innovation Centre, Montreal QC using a GX FLX Titanium Series XLR70 kit (Roche Diagnostic Corporation). Negative control extraction blanks and amplification reactions were included for each set of samples to ensure quality control. Sequence analysis was performed using the Phoenix 2 pipeline (Soh et al., 2013), which filters sequences through a series of stringent conditions, removes chimeras, and determines consensus sequences for each operational taxonomic unit using MOTHUR and the SILVA database. To compare communities in samples, non-metric multidimensional scaling analysis was implemented in Phoenix 2. Pyrotag sequences were submitted to the NCBI Short Read Archive under accession numbers SRR617546-SRR617557 (Albian); SRR617558-SRR617568 (Suncor) and SRR617569-SRR617579 (Syncrude).
Results
Chemical and microbial analyses of initial bulk tailings samples
The three oil sands tailings samples from Syncrude, Suncor and Albian contained similar proportions of solids upon receipt, ranging from 21.9-24.0 wt%, whereas the oil content (predominantly bitumen) varied by source, from 0.9-4.2 wt% (Table 1).
Table 1 Composition of mature fine tailings and most probable number (MPN) of four anaerobic metabolic types in bulk samples received from different oil sands companies.
| Dean-Stark Gravimetric Analysis of Tailings (wt%)a |
MPN/mL x 10-3 (95% confidence limits) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Tailings source | Extraction Solvent b | Oil | Water | Solids | Total | Methano-gens | Sulfate-reducers | Nitrate-reducers | Fermenters |
| Syncrude | Naphtha | 1.6 | 72.5 | 23.6 | 97.7 | 2,300 (36-12,000) |
930 (150-3,800) |
280 (90-850) |
93 (15-380) |
| Suncor | Naphtha | 4.2 | 71.3 | 21.9 | 97.4 | 43 (7-210) |
24,000 (3,600-130,000) |
49 (17-130) |
430 (70-2,100) |
| Albian | C5 and
C6 paraffins |
0.9 | 73.6 | 24.0 | 98.4 | 8 (1-23) |
43 (7-210) |
220 (57-700) |
93 (15-380) |
a, performed per Foght et al., (2014); b, for additional detail see Burkus et al., (2014)
The initial bulk tailings samples harboured substantial numbers of methanogens, with Syncrude tailings having 2x106 MPN/mL and Suncor tailings having 4x104 MPN/mL; Albian tailings (a younger tailings pond; Foght et al., 2017) had the fewest methanogens at 8x103 MPN/mL (Table 1). Sulfate-reducing prokaryotes were prevalent in all samples, with Syncrude and Suncor tailings having 9x105 and 2x107 MPN/mL, respectively, and Albian tailings containing 4x104 MPN/mL. Nitrate-reducing prokaryotes and fermenters were also detected in all samples at 104-105 MPN/mL (Table 1). Thus, all tailings samples harboured a viable endogenous anaerobic microbiota, comparable in magnitude to previous surveys of tailings ponds (summarized by Foght et al., 2017). Based on the anaerobic types tested, Syncrude tailings were dominated by methanogens, Suncor tailings by sulfate-reducing prokaryotes, and Albian tailings were somewhat enriched in nitrate-reducers with a smaller microbiota overall.
Methane production from tailings incubated in microcosms
Because previous studies found a correlation between methanogenesis and biodensification (Fedorak et al., 2003; Siddique et al., 2014a, b), we measured the potential methanogenic activity of tailings samples amended with different agricultural by-products compared to three control treatments: unamended tailings as a control for endogenous substrates; acetate amendment as a positive control for methane production; and acetate plus BES to inhibit methanogens. For technical reasons methane production was measured in sealed anaerobic microcosms constructed in parallel with the consolidation columns rather than directly in the columns. However, production of methane in the columns is inferred from the occurrence of gas voids (particularly in the Syncrude tailings columns; see below).
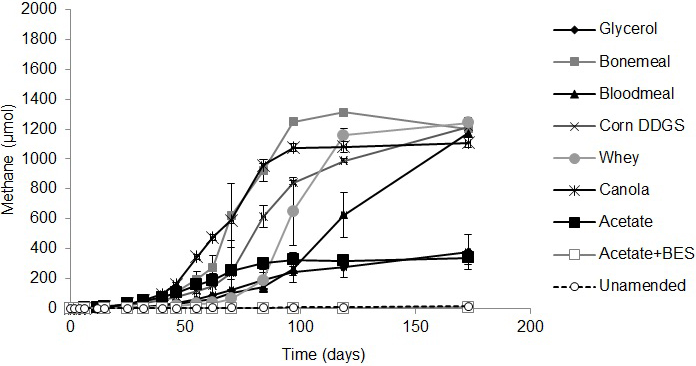
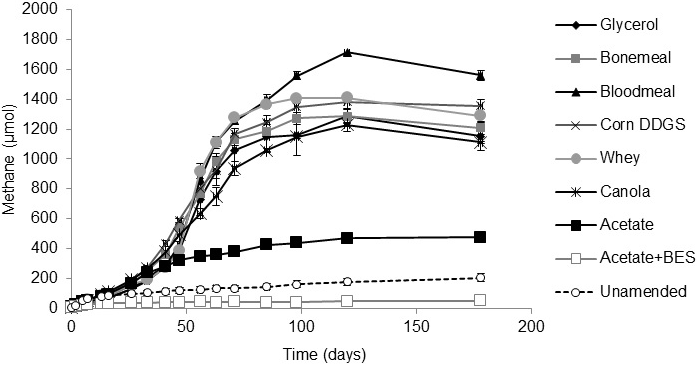
All three tailings samples produced methane in amended microcosms (Supplemental Figs. S1, S2, S3). Unamended (endogenous control) microcosms produced a small amount of background methane above that produced by BES-amended (negative control) cultures. More specifically, the Suncor and Syncrude tailings samples supplemented with glycerol, bone meal, blood meal, corn DDGS, whey or canola produced >1 mM methane during ~170 d incubation with a lag period of ~25 d before significant methane began to be produced (Supplemental Figs. S2, S3). Less methane was generated in the acetate-amended positive control microcosms because these cultures inadvertently received approximately one-sixth the amount of substrate (72 mg C/L) as the other amendments (400 mg C/L); arithmetically, the mass of methane produced in the acetate controls is proportional to that observed in the test microcosms. In contrast to Syncrude and Suncor tailings, the Albian tailings microcosms were slower to begin producing methane, having lag phases of 40-60 d, and exhibited substrate preference in the order canola>bone meal>corn DGGS>whey>blood meal, with glycerol being a poor methanogenic substrate (Supplemental Fig. S1). The observed methane yield compared with the theoretical maximum methane was calculated for the Syncrude microcosms using Buswell’s equation as implemented by Roberts (2002). After correcting for endogenous methane production in the unamended control microcosms, the agricultural amendments produced 56-129% of the maximum theoretical methane yield (Supplemental Table S2). Thus, the agricultural substrates supported similar methanogenesis by the Syncrude and Suncor microbiota but the Albian microbiota were more selective and slower to produce methane.
De-watering and consolidation of methanogenic tailings in column studies
The classic self-weighted (gravitational) densification behaviour of oil sands mature fine tailings is that expressed pore water accumulates above the mud line (i.e., cumulative values >0) and the underlying solids volume decreases (i.e., values <0) as fine clay particles pack more closely with expression of pore water. This pattern was observed in the Albian and Suncor tailings consolidation columns (Figs. 1A, B and 2A, B). As observed for methane production, the Albian tailings exhibited substrate preference, with acetate yielding the greatest volume of pore water (500 mL from an initial 1.5 L of amended tailings placed in the column) and the negative control (acetate + BES) producing the least pore water (~50 mL) during 100 d incubation (Fig. 1A). Correspondingly, acetate amendment resulted in the greatest consolidation of solids, decreasing by ~500 mL, whereas the BES control changed little (Fig. 1B). The unamended (endogenous control) Albian tailings showed slightly more biodensification than the negative control, but less than the other amended treatments.
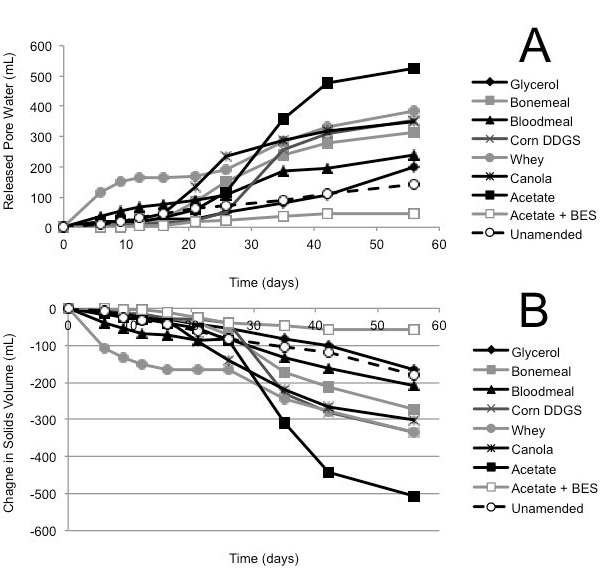
(A) Cumulative volume of expressed pore water measured above the mud line (i.e., de-watering); (B) Volume of solids layer below the mud line (i.e., consolidation). (C) MPN estimations of four metabolic types in tailings solids at the end of incubation in columns; error bars are 95% confidence intervals and asterisks (*) indicate MPN values that are statistically different from the unamended control value.
Fig. 1 (A, B). Biodensification of 1.5 L Albian tailings, amended with substrate or unamended, and incubated in columns for 56 d.
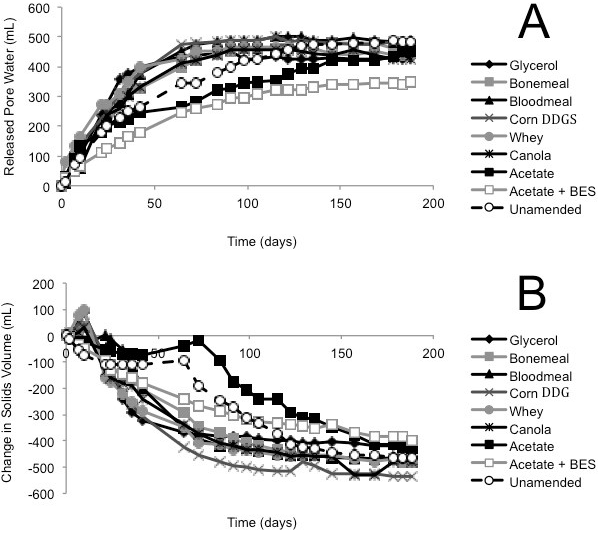
(A) Cumulative volume of expressed pore water measured above the mud line (i.e., de-watering); (B) Volume of solids layer below the mud line (i.e., consolidation). (C) MPN estimations of four metabolic types in tailings solids at the end of incubation in columns; error bars are 95% confidence intervals and asterisks (*) indicate MPN values that are statistically different from the unamended control value.
Fig. 2 (A, B). Biodensification of 1.5 L Suncor tailings, amended with substrate or unamended, and incubated in columns for 188 d.
In contrast, the Suncor tailings showed little substrate preference and even the BES negative control exhibited substantial biodensification (Fig. 2A, B). This outcome suggests that methanogenesis is a less important factor in Suncor tailings where sulfate-reducing prokaryotes predominate the endogenous microbiota by several orders of magnitude (Table 1). Instead, sulfate reduction and/or fermentation by facultative sulfate-reducing prokaryotes may be the dominant biodensification mechanism.
A different pattern of biodensification was observed in the Syncrude tailings columns. As expected, little pore water (<50 mL) accumulated in the BES negative control (Fig. 3A) but a larger proportion of pore water (~200 mL) was expressed from the unamended control column, suggesting that endogenous metabolism in Syncrude tailings is robust. Moreover, during the early stages of incubation, biogenic gases accumulated as gas voids visibly trapped in the solids layer in all amended Syncrude tailings except the BES negative control. This gas production resulted initially in increased rather than decreased solids volumes (Fig. 3B).The bloating effect diminished over time, presumably due to coalescence and ebullition of water-insoluble methane near the mud line and to CO2 dissolution into pore water (Siddique et al., 2014b), reducing the volume of gases trapped below the mud line. The composition of gases in these voids was tested during decommissioning of the Syncrude columns by sampling individual gas voids using a cannula and analyzing the gases by GC. The trapped bubbles comprised methane and CO2, with CH4:CO2 molar ratios ranging from 4 for corn DGGS to 13 for acetate (data not shown). Detection of methane in the gas voids confirmed that methanogenic conditions prevailed in the solid fraction of the columns even though they were not gas-tight.
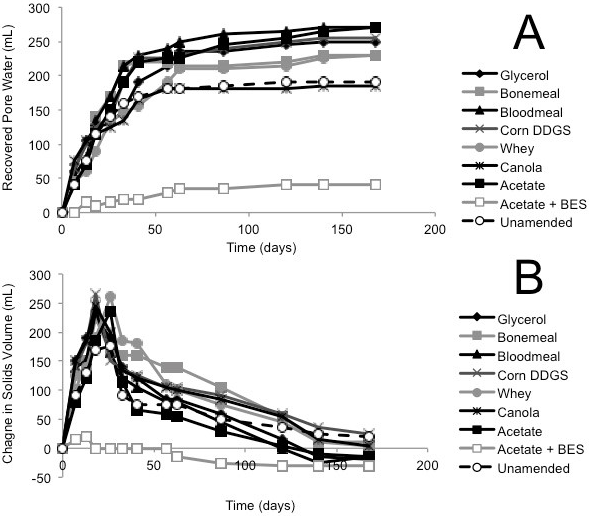
(A) Cumulative volume of expressed pore water measured above the mud line (i.e., de-watering); (B) Volume of solids layer below the mud line (i.e., consolidation). (C) MPN estimations of four metabolic types in tailings solids at the end of incubation in columns; error bars are 95% confidence intervals and asterisks (*) indicate MPN values that are statistically different from the unamended control value.
Fig. 3 (A, B). Biodensification of 1.5 L Syncrude tailings, amended with substrate or unamended, and incubated in columns for 168 d.
Effect of amendment on chemistry of pore water and solids in Syncrude biodensification columns
During decommissioning of the Syncrude biodensification columns after 168 d incubation, the overlying expressed pore water was collected for analysis of pH, conductivity, major soluble cations, anions, and metals (Supplemental Table S3). pH values were not substantially altered by amendment, but conductivity, magnesium, potassium, sodium, and bicarbonate concentrations were greater for all amended versus unamended tailings. There were also increased concentrations of ammonium in corn DDGS- and blood meal-amended tailings water and increased calcium in tailings amended with corn DDGS, whey, and canola meal; the addition of the methanogen inhibitor BES increased conductivity, sodium (the BES counter-ion), calcium and, of course, sulfur concentrations.
During decommissioning, two subsamples of the tailings solids were also collected: one near the mud line to determine whether an oxidation gradient existed in the columns or whether ebullition events affected the solids chemistry, and one near the bottom of the column to determine whether biogenic CO2 dissolution altered the chemistry of pore water in the tailings layer. The same suite of chemical analyses were performed as for the pore water sample by measuring bulk pH of the solids, and concentrations of major soluble anions, cations and metals in the pore water still entrained in the solids fractions, as well as gravimetric Dean-Stark analysis. Amended solids samples collected from both positions in the column showed minor differences compared with the corresponding unamended control tailings, including slightly lower pH, increased conductivity and increased cations and bicarbonate concentrations (Supplemental Tables S4, S5). Comparing Dean-Stark results from the BES-treated tailings to amended tailings shows the magnitude of methanogen-influenced biodensification.
Microbial enumeration and pyrotag sequencing of 16S rRNA genes
Microbial enumeration of tailings was performed during column decommissioning. MPN analysis revealed a general increase in fermentative bacteria and methanogens in tailings amended with complex agricultural by-products compared with the corresponding unamended control tailings and the BES negative control (Figs. 1C, 2C, 3C).
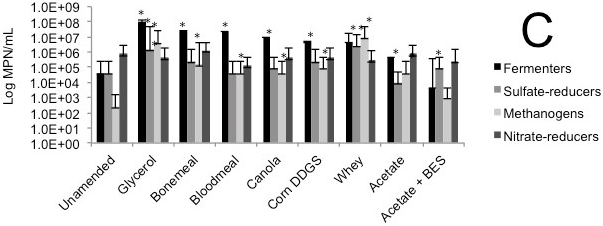
(A) Cumulative volume of expressed pore water measured above the mud line (i.e., de-watering); (B) Volume of solids layer below the mud line (i.e., consolidation). (C) MPN estimations of four metabolic types in tailings solids at the end of incubation in columns; error bars are 95% confidence intervals and asterisks (*) indicate MPN values that are statistically different from the unamended control value.
Fig. 1 (C). Biodensification of 1.5 L Albian tailings, amended with substrate or unamended, and incubated in columns for 56 d.
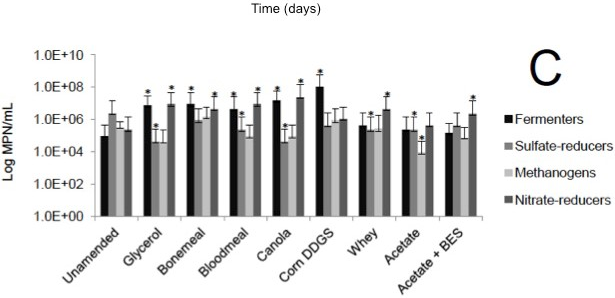
(A) Cumulative volume of expressed pore water measured above the mud line (i.e., de-watering); (B) Volume of solids layer below the mud line (i.e., consolidation). (C) MPN estimations of four metabolic types in tailings solids at the end of incubation in columns; error bars are 95% confidence intervals and asterisks (*) indicate MPN values that are statistically different from the unamended control value.
Fig. 2 (C). Biodensification of 1.5 L Suncor tailings, amended with substrate or unamended, and incubated in columns for 188 d.
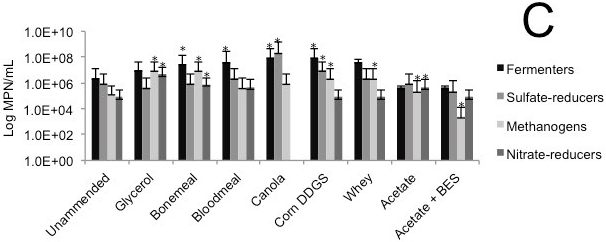
(A) Cumulative volume of expressed pore water measured above the mud line (i.e., de-watering); (B) Volume of solids layer below the mud line (i.e., consolidation). (C) MPN estimations of four metabolic types in tailings solids at the end of incubation in columns; error bars are 95% confidence intervals and asterisks (*) indicate MPN values that are statistically different from the unamended control value.
Fig. 3 (C). Biodensification of 1.5 L Syncrude tailings, amended with substrate or unamended, and incubated in columns for 168 d.
In addition to enumeration, samples of tailings solids were collected for pyrotag sequencing of 16S rRNA genes. Non-metric multidimensional scaling was applied to compare the microbial communities in Syncrude, Suncor and Albian tailings at a global level. This revealed that the three sources of tailings harboured distinct microbiota, and that all amendments shifted the microbial community compositions away from the unamended controls (Fig. 4). The BES negative control community shifted the greatest distance from the unamended community in both Albian and Syncrude tailings, whereas BES had less effect on the Suncor community that is dominated by sulfate-reducers (Table 1).
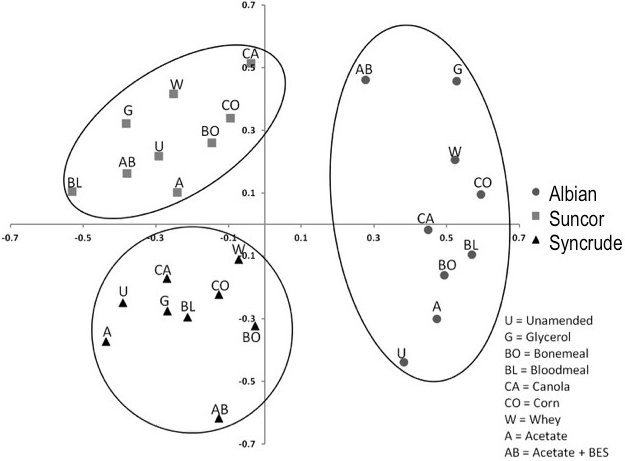
Fig. 4 Non-metric multidimensional scaling plot of 16S rRNA gene pyrosequences representing microbial communities in Syncrude, Suncor and Albian tailings solids amended with substrate or unamended, after incubation in columns.
Taxonomic analysis of the column tailings samples, identified to the family level, revealed that the unamended samples of all three tailings share a wide range of dominant taxa including those affiliated with known methanogens, fermenters, and syntrophs (Fig. 5). Syncrude and Suncor tailings communities had high proportions of reads affiliated with methanogens, particularly Methanosaetaceae. These proportions correlate with the MPN values for amended Syncrude tailings (Fig. 3C) but not for Suncor (Fig. 2C). The unamended Albian tailings had fewer methanogen-affiliated reads than Suncor and Syncrude tailings but higher proportions of Comamonadaceae (including Acidovorax) than either Syncrude or Suncor tailings. Unamended Syncrude tailings were enriched in reads affiliated with Desulfuromonadaceae versus the other two unamended tailings types. After incubation with amendments, Syncrude and Suncor tailings showed either decreased or unchanged proportions of methanogens but Albian tailings amended with bone meal, blood meal, canola, corn DGGS, or whey were enriched in methanogen sequences, particularly Methanoregula and Methanosaetaceae, All of the amendments resulted in a decrease in the proportion of Comamonadaceae reads in Albian tailings: however, many of the amendments stimulated an increase in Comamonadaceae in Syncrude tailings, with the largest increase observed in bone meal-amended samples (Fig. 5). All amendments also resulted in a decrease in Desulfuromonadaceae reads in Syncrude tailings and decreased Syntrophaceae reads in Suncor tailings.
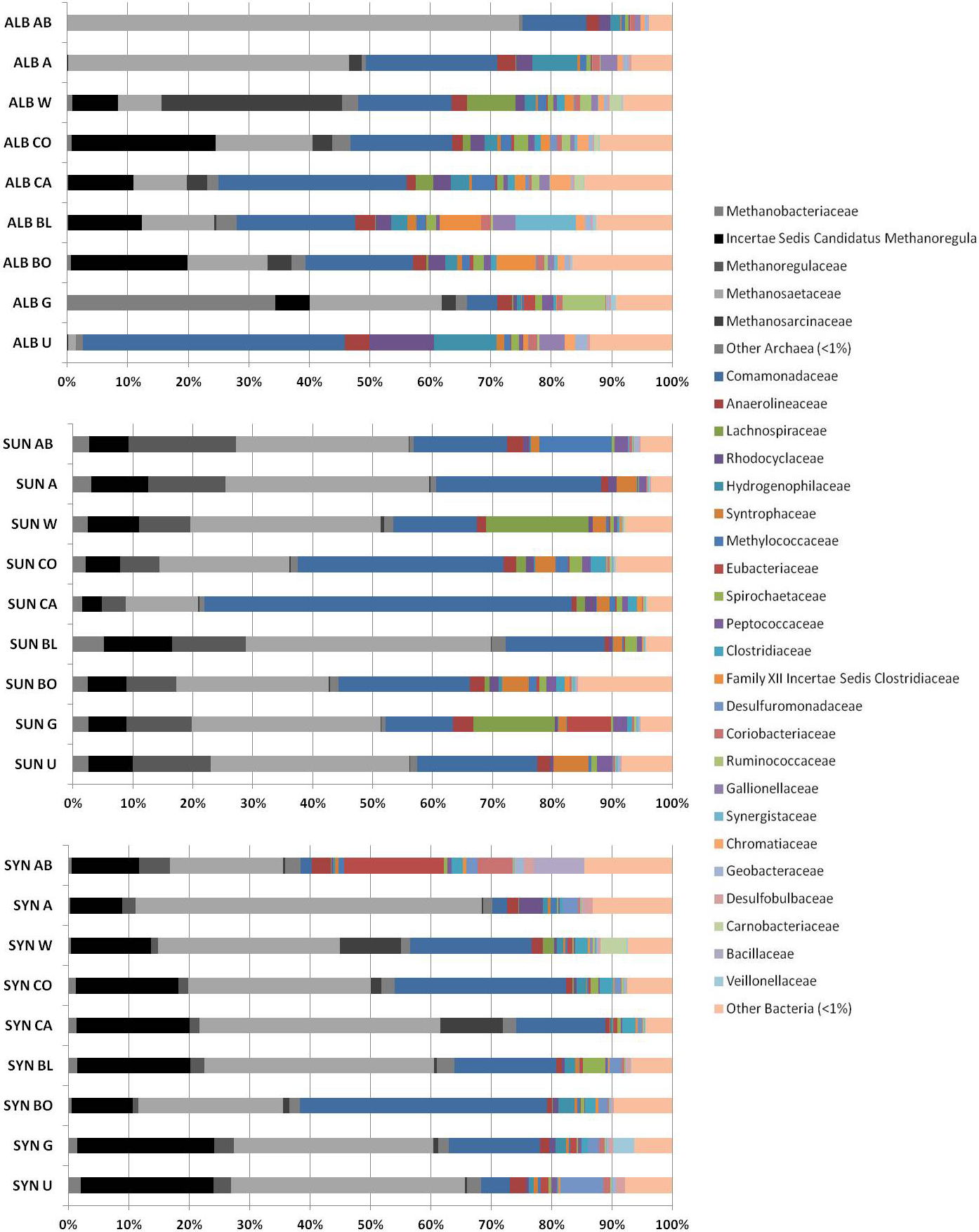
Fig. 5 Archaeal and bacterial 16S rRNA gene pyrosequences identified to family level in tailings samples unamended (U) or amended with glycerol (G); bone meal (BO); blood meal (BL); canola (CA); corn DDGS (CO); whey (W); acetate (A); or acetate plus BES (AB). ALB, Albian; SUN, Suncor; SYN, Syncrude. “Other Archaea” and “Other Bacteria” are the sum of all taxa present at <1% of total reads, as well as reads that could not be identified to family level.
Discussion
Previous studies of biodensification of oil sands tailings ponds have been based on experiments using a limited number of tailings ponds and organic substrates (e.g.,Fedorak et al., 2003; Siddique et al., 2014a, b; Arkell et al., 2015). The current study expanded the range of substrates and tested different tailings types that initially had similar physical characteristics (Table 1, Dean-Stark analysis) but were from three companies using unique combinations of ore extraction and post-extraction tailings management methods. Syncrude and Suncor use naphtha (of different compositions) as the extraction diluent, whereas Albian uses a light aliphatic diluent (Table 1). Suncor adds gypsum (calcium sulfate) to fluid tailings as they are deposited in the tailings pond, whereas Albian amends fresh tailings with citrate (reviewed by Foght et al., 2017). At time of sampling the Syncrude pond was ~40 years old whereas the Suncor and Albian tailings ponds were ~15 years old. Such processing and age differences contribute to the distinct endogenous microbiota in the ponds, both taxonomically (Fig. 4 and Wilson et al., 2016) and numerically (Table 1). In fact, the shorter lag phase before onset of methanogenesis and the maximum rate of methane production in Syncrude and Suncor microcosms (Supplementary Figs. S1, S2, S3) correlated with the greater MPN estimates of methanogens in these tailings (Table 1). Thus, we expected - and observed - different responses of the tailings samples to amendment with different organic substrates based on tailings sources and their microbiota.
The agribusiness amendments were selected based on their regional availability and low (or negative) value in their respective industries. Acetate was chosen as the positive control substrate because it was previously demonstrated to be effective in small-scale biodensification trials with Syncrude tailings (Arkell et al., 2015). BES was selected for the negative control treatment under the assumption that it would suppress biodensification as it did in previous experiments with Syncrude tailings (Arkell et al., 2015). In both the Albian and Syncrude tailings columns, little or no methane production (Supplementary Figs. S1 and S3) and slow biodensification (Figs. 1 and 3) were observed in the BES-inhibited controls, with no evidence of gas void formation in the BES-containing columns, suggesting that methanogenesis was the main source of biogenic gas in these substrate-amended tailings. Because BES specifically affects methanogens (Smith and Mah, 1978), whose inhibition can have upstream effects on the activity of synergistic anaerobes, any settling observed in the BES-inhibited tailings presumably represents primarily gravitational (self-weighted) consolidation and de-watering, without a significant contribution by the methanogenic microbial community. Interestingly however, in the current study, BES was far less effective at stalling biodensification of Suncor tailings (Fig. 2) that are dominated by sulfate-reducing prokaryotes and fermenters (Table 1). This indicates that methanogenesis per se is not essential for biodensification and suggests that anaerobic metabolism generating CO2 and/or organic acid wastes may contribute to or substitute for methanogenic activity during biodensification, given an appropriate microbial community composition.
The column method used to quantify biodensification at small scale could be criticized for the fact that the headspace and pore water are not incubated under strictly anaerobic conditions, since the columns are sealed (initially under a N2 headspace) using only a foil or thick latex cap to prevent evaporative losses from the water surface. However, although the water and upper few mm of solids may have been suboxic rather than anoxic, it was obvious that the underlying tailings remained sufficiently anaerobic (due to minimal gas penetration into the dense tailings) to permit production of gas bubbles comprising methane and CO2 to form throughout the solids layer.
A small degree of de-watering and consolidation was observed in all unamended control tailings columns with all three tailings sources. This is interpreted to be due to metabolism of endogenous hydrocarbons (e.g., residual diluent; Siddique et al., 2006, 2007, 2011) supporting microbial activity. In contrast, each tailings type responded differently to amendment. Albian tailings exhibited substrate specificity for methanogenesis, with very little methane being produced in unamended and BES-treated microcosms (Supplemental Fig. S1). Notably, acetate was the most effective amendment for Albian tailings biodensification (Fig. 1) even though it was inadvertently amended at approximately one-sixth the concentration (by C content) of the other amendments. This preference may result from Albian tailings having been exposed in situ to citrate as a water conditioning agent (Li, 2010), which may have enriched for microbes capable of fermenting acetate. Correspondingly, MPN enumeration of Albian tailings from the columns showed significant enrichment of methanogens and fermenters for all amendments (Fig. 1C). Suncor tailings exhibited a slight preference for blood meal for methane production (Supplementary Fig. S2) but a slight preference for canola for biodensification (Fig. 2A, B). MPN enumeration of Suncor columns showed significant increases in fermenters and nitrate-reducers for most amendments (Fig. 2C) but little change in proportions of methanogens and an overall trend to decreased proportions of sulfate-reducers. Bearing in mind that both nitrate- and sulfate-reduction can be facultative properties, and that some taxa may be enumerated by more than one type of MPN medium, the shift towards more fermentation capability is likely the stronger observation. Syncrude tailings showed no strong substrate preferences for either methane production (Supplementary Fig. S3) or biodensification (Fig. 3). In fact, even the unamended control tailings exhibited biodensification similar to the amended columns. No consistent patterns of enrichment were noted in Syncrude MPN enumerations (Fig. 3C).
Non-metric multidimensional scaling analysis of 16S rRNA pyrosequences from the tailings after incubation in columns confirmed the unique compositions of the initial endogenous microbiota (i.e., unamended control communities) and showed that amendments caused the community compositions to shift (Fig. 4). Interestingly, this analysis also indicates that amendments did not simply select for the same taxa regardless of tailings source: the communities remained unique even after amendment with the same substrates or inhibitor. Examining the taxa at the family level (Fig. 5) revealed the complexity of the prokaryotic communities. In general, substrate amendment increased the proportion of archaeal to bacterial sequence reads but did not perturb the bacterial community in any consistent patterns. For example, Albian and Suncor tailings amended with canola were enriched in Comamonadaceae, but Syncrude tailings were not; Syncrude amended with bone meal was enriched in Acidovorax but Albian and Suncor were not. Because these communities are represented by a single time point at the end of incubation, no strong conclusions can be drawn from the molecular analysis. Additionally, the full metabolic potential of many of the anaerobic taxa detected is incompletely known, so correlating their presence with the observed behaviour of the tailings is tenuous. Future studies must include more comprehensive replication, temporal sampling and analysis of the microbial communities.
Chemical analyses of the changes in pore water and solid phase chemistry of biodensified oil sands tailings previously revealed that microbial activity also altered the clay chemistry of tailings (Siddique et al., 2014 a, b) where dissolution of biogenic CO2 lowered the pH of tailings, dissolving carbonate minerals and releasing calcium, magnesium and bicarbonate ions, which reduced the surface charge potential (repulsive forces) of the clay particles. Transformation of FeIII minerals to amorphous FeII minerals, and the formation of FeS, also reduced the surface charge potential of clay particles to aid the aggregation of clays in tailings and could be related to the formation of de-watering channels in microbially active tailings. The mechanism underlying biodensification of oil sands tailings appears to be a complex combination of geobiological processes activated by indigenous microorganisms. The detailed chemical analyses of the pore water retained in tailings from the upper and lower column reported here (Supplementary Tables S4 and S5) showed similar increases in cations and bicarbonate as well as greater conductivity and lower pH in amended tailings compared with unamended control tailings (which also showed endogenous biodensification activity). Future studies should examine the geochemistry of the solids phase to determine whether the factors for different tailings sources and amendments are the same as reported for Syncrude with canola amendment (Siddique et al., 2014b).
Regardless of recent developments of physico-chemical processes for de-watering tailings, oil sands surface mining companies continue to generate and deposit fresh tailings, adding to the inventory of microbially active tailings ponds. Amendment of oil sands fine tailings with agri-business by-products has been shown here to enhance the production of methane, and in turn the consolidation and de-watering (biodensification) of tailings. Accelerated de-watering of tailings would increase the water available for reuse in oil sands extraction processes thus decreasing the demand for make-up water from the Athabasca River, and reduce the volume of existing tailings. The success of organic amendment suggests that a flow-through biodensification system analogous to sewage treatment facilities could be possible for oil sands tailings treatment. The generation of methane as a clean-burning on-site fuel could be an additional benefit to exploiting biodensification of methanogenic tailings. However, to implement an oil sands tailings treatment technology that accelerates consolidation and water recovery from amended tailings, a better understanding of the physical, chemical and microbiological processes of biodensification is required. It is important to recognize that each tailings pond is unique in terms of endogenous microbiota and metabolic potential, requiring optimization of amendment type and concentration for each pond. In addition, the consequences of altered pore water and tailings solids chemistry for water re-use and ultimate solids reclamation must be considered.
Supplemental information
Four Tables (S1-S4), and three Figures (S1-S3) are provided as supplemental information.











 nueva página del texto (beta)
nueva página del texto (beta)