Introduction
Previous work on natural materials has shown that natural fibers are a viable alternative to synthetic fibers due to well-known characteristics like high specific strength, availability, low cost, among others. Composites reinforced with natural fibers have driven interest of vehicle manufacturers also due to environmental concerns related to the end of the vehicle’s lifecycle, being already in use in the interior lining area of cars, buses, and trucks (Pickering et al. 2016, 98; Obed et al. 2016, 2553).
The curaua plant (Ananas comosus var. erectifolius) is a monocotyledon from the same family of pineapple, being native of the Amazon region. The leaves from this plant are rigid and have attracted interest from the Brazilian automotive industry in recent years (Pickering et al. 2016, 98). To improve the mechanical performance of the composite, various treatments can be carried out on natural fibers to increase their compatibility with polymer matrices. Alkaline treatments promote better fiber adhesion to the matrix by mechanical anchoring (Elenga et al. 2013, 2934). Isiaka et al. 2014, 1 reported that polyester matrix composites reinforced with chemically treated sisal fibers displayed higher tensile strength and elastic modulus, with less dispersion in the results due to fiber homogenization after treatment. Merlini et al. 2012, 339 observed similar result for epoxy composites reinforced with jute fibers treated with a 5% (w/v) NaOH solution. Beltrami et al. 2014, 388 observed 3% increase in tensile strength, 24% in elastic modulus, 30% in flexural strength and 12% in impact strength in biodegradable composites reinforced with curaua fibers treated with a 5% NaOH solution. The authors also observed that alkaline treatments at higher NaOH concentration (10% w/v) fragilized the fibers, resulting in weaker mechanical behavior.
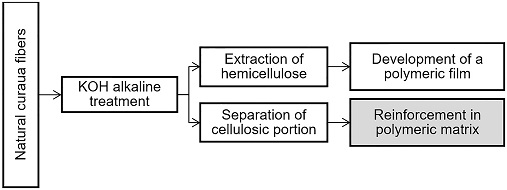
Alkaline treatments extract hemicellulose from the fibers, which can be reused in the manufacture of other materials (Beltrami et al. 2014, 388), and the cellulosic portion can be applied as reinforcement in a composite, as depicted in Figure 1. This way, all components of the fibers are utilized.
This refining process concept can be described as a biorefinery. That is, raw materials derived from biomass, such as lignocellulosic materials, are used to obtain added-value products such as fuels, energy, and chemicals, minimizing dependence on fossil sources. Most of the current biorefineries focus on the valorization of cellulose and hemicellulose and basic sugar platforms (Souto et al. 2015, 100). The biomass valorization concept developed in the present work is depicted in Figure 1: The extracted hemicellulose is transformed into a polymeric film, as found in the work of Roldi 2017, whereas the cellulosic portion of the treated curaua fibers, is studied in the present work as reinforcement in unsaturated polyester matrix composites, focusing on the effects of the KOH treatment on their mechanical and thermal properties.
Method
Materials
The curaua fibers used in this work were kindly provided by the Center for Support to Community Action Projects (CEAPAC) from Santarém, PA, Brazil as long fibers bundles (length: 80 cm) with impurities (leftovers of parenchymal cells) from the retting process. To clean the fibers and to disaggregate the leaves towards finer bundles, a soft carding process was carried out, avoiding damage to the fibers. The curaua fibers presented around 10% moisture, which is removed prior to the mixing with the resin. In addition, the polymer used as a matrix was an Arazyn 14.0 polyester resin with 1.8 wt% of hardener.
Fiber treatment and characterization
Samples (10 g) of fresh curaua fibers were magnetically stirred for 1 h in 200 mL of water at room temperature to swell the fibers (for better KOH absorption), as described in Bahcegul et al. 2011. Ten batches were made to obtain a large amount of treated fibers. The solution was filtered, and the fibers were magnetically stirred in 100 mL of 10% (w/v) KOH solution at room temperature for 3h, according to Roldi 2017. Afterwards, the insoluble alkaline fraction (cellulosic portion) was separated by filtration, washed three times with 200 mL of distilled water until a neutral pH was reached. The fibers were then air-dried for 5 days and oven-dried at 60 °C for 24 h.
Characterization of cellulose, hemicellulose and lignin contents of the fibers was performed in triplicate following Morais et al. 2010: To determine the lignin content, 1 g fiber sample was placed into a mortar along with 17 mL of a 72% (w/w) sulfuric acid solution at 10-15 °C. The mixture was carefully macerated until no unsolubilized particles were visible, left to rest for 24 h, and transferred to a 500 mL boiling flask with 306 mL of distilled water to dilute the sulfuric acid solution to 4% (w/w). The boiling flask was connected to a simple condenser and heated for 4 h. After that, the solution was left to cool to room temperature and the mixture was washed with distilled water and filtered in a Büchner funnel until neutral pH was reached, oven-dried at 105 °C for 24 h and weighed. The lignin content was determined by:
Where FW is the weight of the clean and dry Büchner funnel, FLW is the weight of the funnel plus lignin after drying and SW is the weight of the initial dried sample.
Hemicellulose content is estimated by the difference between holocellulose and alpha-cellulose contents in the material (Morais et al. 2010). The fiber sample (3 g) was placed in a 500 mL erlenmeyer flask with 120 mL of distilled water, 2.5 g of sodium chlorite (NaClO2), 1 mL of glacial acetic acid, which was magnetically stirred at 70 °C. Additional 2.5 g of sodium chloride and 1 ml of glacial acetic acid were added after 1 h of heating. The solution was heated for fours more hours. The Erlenmeyer was left to cool to room temperature, opened under an exhaust vent and inserted into an ice bath for 30 min. The mixture was washed with distilled water and filtered in a Büchner funnel until neutral pH was reached, oven-dried at 105 °C for 24 h and weighed. The holocellulose content was determined by:
Where FW is the weight of the clean and dry Büchner funnel, FHW is the weight of the funnel plus holocellulose after drying and SW is the weight of the initial dried sample.
Alpha-cellulose is the non-degraded cellulose, which does not dissolve in 17.5% (w/v) aqueous sodium hydroxide solution (Morais et al. 2010). The previously-obtained dry holocellulose (1 g) was placed in a mortar with 15 mL of 17.5% (w/v) NaOH solution for 2 min. Then, the material was macerated for 8 min and the solution was transferred to a Büchner funnel, washed with distilled water, filtered until neutral pH was reached, and oven-dried at 105 °C for 24 h and weighed. The alpha-cellulose content was obtained by:
Where FW is the weight of the clean and dry Büchner funnel, FAW is the weight of the funnel plus alpha-cellulose after drying and SW is the weight of the initial dried sample.
FT-IR analysis was performed with a Thermo Scientific's Nicolet iS10 spectrophotometer with smart diffuse reflectance accessory. The samples were previously oven-dried at 60 °C for 24 h and placed in a desiccator to cool to room temperature. The analysis was performed in the 4000 and 400 cm-1 range at intervals of 4 cm-1 and 128 scans.
X-ray diffraction (XRD) was performed using a RIGAKU ULTIMAV diffractometer in reflection mode with 1.54 Å incident angle, CuKα radiation, 2θ incidence angle within 5°-50°, with a scan speed of 3°/min. The crystallinity index (% Xc) was obtained following Segal et al. 1959, 786, according to:
Where I002 (2θ = 22.5°) is the intensity of the crystalline peak and Iam (2θ = 18°) refers to the amorphous halo.
Scanning Electron Microscopy (SEM) images of the fiber samples were obtained after grinding and metalization in a JEOL microscope, model JSM-7001F, with an acceleration voltage of 15 kV and an emission current of 81 μA.
Composite manufacturing
The fibers were oven-dried at 60 °C for 24 h and allowed to cool to room temperature in a dessicator. The amounts required to obtain specimens for tensile (ASTM D638) and flexural (ASTM D790) test specimens were 1.2 g and 1.7 g , respectively, which correponds to a fiber weight content of 10%, which was selected based on previous trials. Arazyn 14.0 polyester resin was used, with 1.8% initiator in weight of resin according to specification (AraAshland 2007).
The resin was mixed with the catalyst, the fibers were added and stirred, and the mixture was cast into the molds (Figure 2a). A layer of peel ply, a woven cloth that allows the removal of trapped air only, was placed on top, and breather fabric on the sides to absorb resin leakage (Figure 2b). The fiber/resin mixture was pressed into the molds to tightly pack it, controlling thickness and surface roughness (Figure 2c). The whole set was vacuum bagged (Figure 2d). After 24 h under vacuum, the specimens were taken out of the molds, and any resin flash was removed with a Dremel mini grinder. Finally, the samples underwent post-curing in an oven at 80 °C for 2 h.
Characterization of the composites
Thermogravimetry (TGA) and Differential Scanning Calorimetry (DSC) were used to characterize fibers, neat polyester, and composites. The samples (5 mg) were placed in alumina crucibles and the analyses were performed under nitrogen (N2) atmosphere (flow rate of 60 mL/min) from room temperature to 600 °C at a rate of 10 °C/min on TA Instruments SDT Q600 equipment.
Tensile tests were performed according to ASTM D683 in an Instron 8801 machine at a strain rate of 0.2 mm/min. Three-point flexural tests were performed according to ASTM D 790-86 in an EMIC DL 2000 machine at 2.7 mm/min. At least three measurements of width and height of the specimens’ cross sections were taken with a micrometer, and the average values were used. The tests were carried out at room temperature and, for each test, five specimens were evaluated. SEM images were taken of the fractured surfaces of the composites with the same microscope.
Results and Discussion
Characterization of the Curaua Fibers
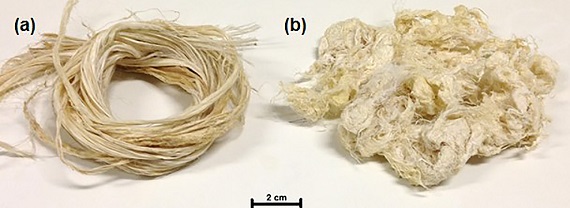
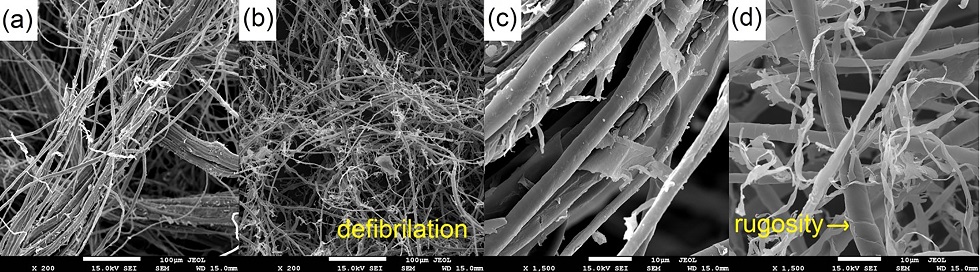
The chemical and morphological characterizations were performed for in natura and chemically treated curaua fibers. Figure 3 reveals that treatment of the fibers disaggregated them, with a trend towards agglomeration (Figure 3b).
Chemical composition of the fibers is presented in Table 1. The results for in natura curaua fibers, which were obtained by Oliveira 2016 and Souza 2016, are consistent with values reported in the literature, except for crystalline cellulose, whose value was about 5% lower than in (Rossa 2012; Corrêa 2010).
Table 1 Composition of in natura and chemically treated curaua fibers, data in dry basis [%w/w].
| Fibers | Amorphous cellulose | Hemicellulose | Alpha-cellulose | Lignin | Others |
|---|---|---|---|---|---|
| In natura* | ** | 15.94±1.39 | 65.68 ± 1.16 | 7.29±0.80 | 11.52±0.11 |
| Treated | 16.79 ± 0.24 | ** | 74.30±0.21 | 4.28±0.27 | 4.62±0.25 |
*The in natura fiber composition was reported by Oliveira 2016 and Souza 2016. **Not observed.
This may be justified considering the natural variations expected due to the influence of soil, post-harvest processing and fiber location in the plant body (Tomczak 2010). Crystalline cellulose represents 66% of the of the in natura fiber, which increases to about 74% after the chemical treatment, which is a positive aspect considering that crystalline cellulose is related to the mechanical performance of fibers. The alkaline treatment reduced the lignin content by approximately 30%, and removed impurities, resins, waxes, and fatty acids from the surface of the fibers (Marques et al. 2015, 41710). The term “others” in Table 1 refers to unidentifiable components.
Table 1 also indicates a rise in the content of amorphous cellulose (which includes hemicellulose) in the treated samples. This is an evidence of degradation of the crystalline cellulose, probably due to the severity of the treatment.
It is worth mentioning, however, that the method used for the lignocellulosic characterization of the fibers presents limitations (Morais et al. 2010). Lignocellulosic characterization by mass variation does not provide the exact cellulose and hemicellulose content, and only estimates amorphous and crystalline contents. Thus, the results may have been influenced by residues from the treatments.
KOH is a base which interacts more efficiently with hemicellulose rather than lignin. Hemicellulose is solubilized at low concentrations while lignin undergoes basic hydrolysis (Albinate et al 2013, 114). Some of the expected effects of this treatment are the disruption of the hydrogen bond in the hydroxyl (OH) groups of the fibers’ structure and the depolymerization of amorphous cellulose (Beltrami et al. 2014, 388; Marques et al. 2015, 41710).
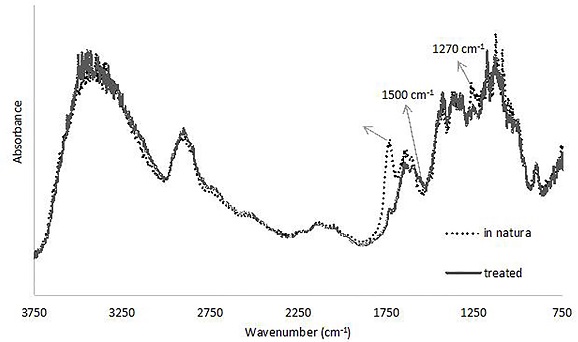
The removal of lignin may be observed in the infrared spectra of the fibers (Figure 4) due to the disappearance of the peaks at 1500 cm-1 and 1270 cm-1 and the decrease in intensity of the 1430 cm-1 band. These bands correspond to the vibration of the benzene ring and the stretches of the C-H and C-O bonds of the lignin’s acetyl group (Albinate et al 2013, 114). And significant removal of hemicellulose is also observed, mainly due to the great reduction in the peak at 1730 cm-1, which refers to its C=O and C-O bonds (Albinate et al 2013, 114).
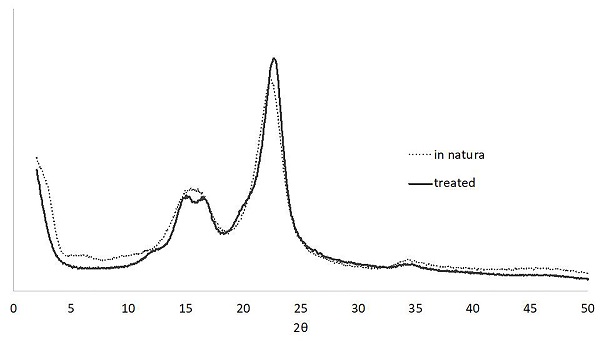
The X-ray diffraction profiles of the fibers (Figure 5) are mostly similar, with some distinction in the intensity of the peaks. Both fibers have well-defined peaks at 2θ of 15.5°, 22.5° and 34.3°.
The calculated crystallinity indexes were 72.88% and 75.28% for the in natura and the treated fibers, respectively. Therefore, even though the KOH treatment successfully removed amorphous components from the fibers, only a small increase in crystallinity occurred perhaps because the aggressive treatment that also degraded part of the crystalline cellulose. Some authors also reported that alkaline treatments weakens the hydrogen bonds in the molecular structure, increasing the amount of amorphous cellulose relative to crystalline cellulose (Santos et al. 2018; Vishtal and Retulainen 2014, 7951; Rosli et al. 2013, 1893). When inserted in an aqueous solution, the cellulosic structure undergoes swelling, which also contributes to crystallinity changes (Albinate et al. 2013, 114).
SEM images of the fibers are shown in Figure 6. In their natural state (Figure 6 a, c), the fibrils are enveloped by an external layer comprised of resins, waxes and fatty acids (Marques et al. 2015, 41710). After treatment, defibrillation (characteristic of hemicellulose removal) and separation of fibrils (Figure 6b) are seen. KOH mainly interacts with hemicellulose and lignin (components that promote adhesion between fibrils), but ultimately exposes cellulose fibrils to the chemical attack (Figure 6d). These factors increase roughness and contact surface of the fibrils, which potentially increase mechanical anchoring of the polymer matrix onto the fibers (Albinate et al. 2013, 114; Beltramiet et al. 2014, 388; Carvalho et al. 2010, 1143).
Composite Characterization
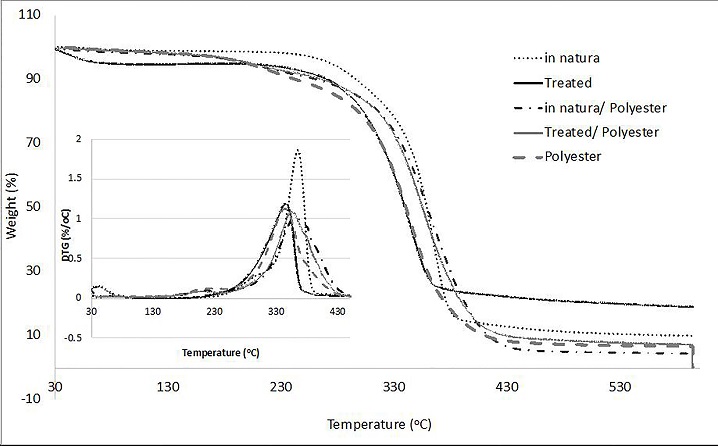
The thermal behavior of both fibers was similar (Figure 7), with differences in initial degradation temperature, number of degradation stages, amount of residue and thermal stabilities (Table 2). The chemically treated fiber showed a decrease in thermal stability at 30 °C , with the onset of degradation at 200 °C, against 230 °C for the in natura fiber (Table 2). The peak mass loss rate, Tpeak, occurs at 345 °C (1.18%/°C) for the treated fiber and at 365 °C (1.86%/°C) for the in natura fiber. Nevertheless, the treated fiber starts degrading earlier and reach total degradation at 360 °C against 380 °C for the in natura fiber. This is related to the removal of lignin (which pyrolyzes at higher temperatures) and of the surface layer of fatty acids and waxes.
Table 2 Main thermal degradation stages of fibers, resin and composites obtained from TGA.
| Degradation stages | ||||||||||
| 1st | 2nd | 3rd | 4th | Thermal stability | Residue | |||||
| Tonset | Tpeak | Tonset | Tpeak | Tonset | Tpeak | Tonset | Tpeak | |||
| t | (°C) | t | (°C) | t | (°C) | t | (°C) | (°C) | (%) | |
| (C°) | (°C) | (C°) | (C°) | |||||||
| in natura fiber | 4 | 6 | 268 | 90 | 45 | 65 | - | - | 230 | 12.04 |
| Treated fiber | 0 | 3 | - | - | 303 | 45 | - | - | 200 | 28.03 |
| Polyester | 47 | 60 | 182 | 218 | 312 | 343 | - | - | 120 | 6.36 |
| in natura fiber/ polyester | - | 25 | 179 | 212 | 315 | 360 | 332 | 380 | 116 | 4.95 |
| Treated fiber/ polyester | - | 26 | 175 | 209 | 316 | 355 | 321 | 376 | 110 | 5.25 |
Fiber components (hemicellulose, cellulose, and lignin) degraded at different temperatures. Lignin starts deteriorating immediately and maintains a nearly constant degradation rate (due to its various oxygen functional groups with different thermal stabilities), followed by hemicellulose at 230 °C and by cellulose at 300 °C (Brebu and Vasile 2010, 353). Analysis of the DTG curve reveals three degradation stages: an initial mass loss due to loss of moisture (demonstrating a higher moisture content in the treated fiber), the hemicellulose degradation (not observed for the treated fiber due to the lack of hemicellulose), and later the cellulose degradation, which began, for the treated fiber, at lower temperature than expected, evidencing cellulose degradation (decrease in length of the crystalline cellulose chains).
The polyester resin has three thermal degradation stages (Figure 7), the first onset at 60 °C (due to loss of moisture), the second at 182 °C (peaks at 218 °C), and the third onset at 312 °C (peaks at 343 °C at 1.125% /°C) (Table 2). The second stage is associated with the breakage of cross-links in the resin (Lo and Hoa 2006) and the third is related to the depolymerization and degradation of the polyester resin itself. A fourth thermal degradation stage is observed in the composites, referring to the fiber degradation.
The two composite samples showed similar behavior, differing only after the third degradation stage. Both composites had similar thermal stability, 110 °C and 116 °C (Table 2). Since thermal stability of the resin is lower than the fibers, initial degradation of the composite is related to the resin. The peak mass loss rate of the composite reinforced with treated fibers occurs at 355 C (1.08%/°C), and at 360 °C (1.01%/°C) for the in natura fibers. Therefore, the treated fiber composite has higher DTG peak and degrades more rapidly, which may be justified considering the fibers to be enveloped by the resin, which acts as a protective layer.
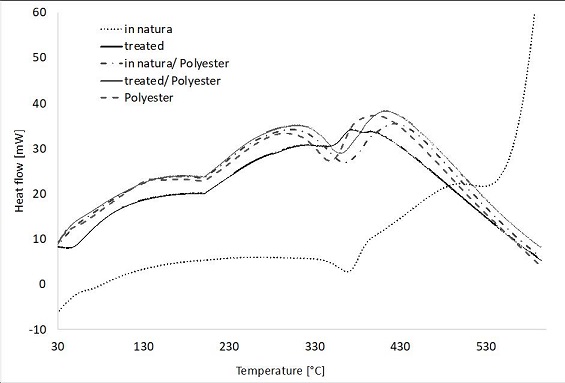
Regarding DSC analyses (Figure 8), the first endothermic event observed for the treated fiber, which is not present in in natura fiber, indicates again higher moisture content for the former. The second event of the treated fiber (peak at 350 °C) coincides with the 3rd degradation stage. This event peaks at 370 °C for the in natura fiber. After that, the in natura fiber showed a final endothermic peak at 540 °C, triggering a large exothermic event to the near-complete degradation of the material, whereas the treated fiber did not show any late exothermic event which may be related to the formation of potassium oxide residue (of very high degradation temperature). The amount of residue was 28.0% for the treated fiber and 12.0% for the in natura fiber (Table 2).
For the unreinforced resin, a large endothermic peak is observed at 346 °C, corresponding to the resin’s third degradation stage, and no degradation was found after 400 °C. Baseline deviation (the horizontal portion of the curve) is due to the laxity from molecular oscillations during the glass transition phase (Almeida et al. 2012, 20). The composites presented an endothermic event at 360-365 °C, and degradation ceased at 413-424 °C.
Table 3 presents the results of mechanical characterization of the composites. All tested samples presented a fragile behavior, typical of thermoset matrices. Although no significant differences were observed in maximum stress (Table 3), the composites reinforced with treated fibers presented a more homogeneous behavior. There were no significant differences in tensile modulus and maximum tensile strain, and tensile strength varied within 10.7%, still inside the deviation range. And no significant changes were observed in flexural behavior. Therefore, the chemical treatment of the fibers did not impact the mechanical behavior the composites.
Table 3 Tensile and flexural properties of the composite materials.
| Resin reinforcement | in natura fibers | Treated fibers |
|---|---|---|
| Tensile strength (MPa) | 21.91±0.79 | 19.57±1.58 |
| Tensile modulus (GPa) | 1.15±0.51 | 1.12±0.20 |
| Maximum tensile strain (%) | 2.27±0.59 | 2.23±0.45 |
| Flexural strength (MPa) | 40.82±3.39 | 40.57±1.33 |
| Flexural modulus (GPa) | 3.67±0.34 | 3.77±0.22 |
| Maximum flexural strain (%) | 1.16±0.18 | 1.08±0.75 |
Several factors may have contributed to the poor performance of the material, such as limitations due to the molding process adopted, random distribution of fibers, limited fiber length, modification of the fibers’ internal structure due to the treatment and low reinforcement content. Nevertheless, the tensile and flexural parameters reported in this paper are within an acceptable range comparing with those found in the literature (Rodrigues 2008) for unsaturated polyester reinforced with randomly oriented short natural fibers.
The predominant failure mechanism in the composites with in natura fibers was fiber pull-out (Figure 9a), evidencing low fiber/matrix adhesion. In the composite with treated fibers, fiber pull-out (red arrows) is still seen, but fractured fibers (yellow arrows) are more predominant (Figure 9b). Detached fibers arranged transversely to the load direction, which end up acting as defect propagators and contribute to early failure, are also seen, along with some voids (blue arrows) (Figure 9b).
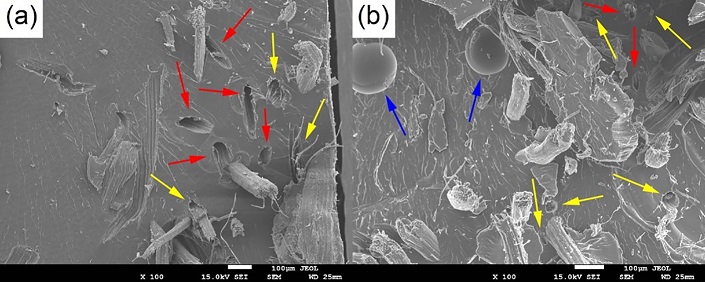
Figure 9 SEM images of the fracture surface of composite samples reinforced with in natura (a) and chemically treated curaua fibers (b). 100× magnification.
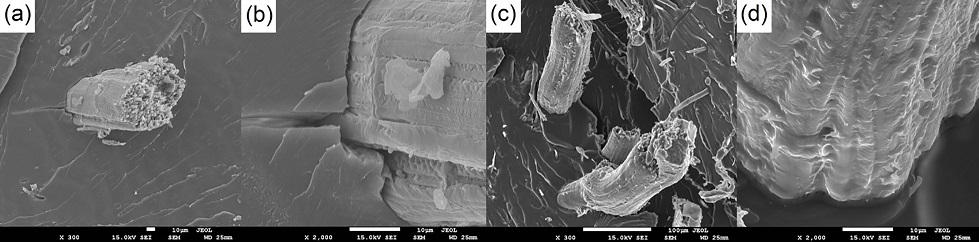
The fiber/matrix interface characteristics are better visualized in Figure 10. Better adhesion is observed for the treated fibers due to their greater roughness. Figure 10b, for instance, shows the propagation of a crack at the interface of in natura fiber.
Conclusions
The KOH chemical treatment on curaua was highly selective with the fibers’ hemicellulose (which was fully extracted), and 30% of the lignin content was also solubilized. A significant attack of the fibers’ structure was observed, which yielded degraded crystalline cellulose and decreased thermal stability (30 °C). Defibrillation, removal of the surface coating layer (resins, waxes and fatty acids) and exposure of the fibrils were also observed.
Despite the fact that a great part of the amorphous components of the fibers was extracted, there were no significant changes in crystallinity index, confirming fiber degradation. The increased roughness and fibril contact surface brought by the chemical treatment promoted better fiber/matrix adhesion which was however not sufficient to improve the mechanical properties of the composite, negatively impacted by the fiber degradation. The total removal of hemicellulose negatively affects the aplication of these fibers as reinforcement, however, the high cellulose content can be useful for other applications.











 nueva página del texto (beta)
nueva página del texto (beta)