Introduction
Propagation by grafting is a frequently used method in agriculture because it shows several advantages, such as reducing the plant juvenile period to obtain fruit in less time; homogenizing and improving fruit quality; achieving strong plant structures; and conserving plants of genetic importance (Rojas et al. 2004).
In grafting, the plant donating and forming the root is called stock, scion, or rootstock; the part of the plant placed on the stock providing the variety for propagation is called graft, scion, or bud (Baraona and Sancho 2000). For successful grafting, both the rootstock and the graft should be compatible. Species of the same genus can be grafted perfectly between them, but those of different genera usually cannot although there are exceptions (Irigoyen and Cruz 2005).
Incompatibility of the graft causes economic loss and delay in launching new cultivations (Pereira et al. 2014). Thus, identifying the compatible rootstock and other benefits from the scion enhances grafting as an adequate propagation technique (Colla et al. 2010). For successful grafting between different plant species or varieties of plants, the ability to produce callus tissue starting from parenchyma cells and differentiating vascular tissue on the callus bridge is of great importance. On the other hand, the reduction of necrotic layers in the union of both grafting members improves the tissue in the graft (Cholid et al. 2014).
Jatropha curcas plants have shown potential for the production of biofuels and other products because the seed is rich in lipids (55-58%) and raw protein (31-34.5%) (Martínez-Herrera et al. 2006). Nevertheless, J. curcas is still in domestication period; its current varieties have low yield and production variability, and it shows a moderate tolerance to salinity of up to 50 mM of NaCl (Díaz-López et al. 2012). To improve its cultivation and adapt it to drought and salinity conditions requires genotypes with high yield, homogeneous maturity, resistance to pests and diseases, and tolerance to salinity (Quiroz, 2013).
On the other hand, Jatropha cinerea is a species distributed in wild populations of northwestern Mexico in saline soils; it can withstand long drought periods and tolerate up to 100 mM of NaCl (Hishida et al. 2013); it has medicinal and industrial uses (Yetman and Van, 2002; Quattrocchi, 2012). J. cinerea could be used as rootstock of J. curcas scion improving its tolerance to drought and salinity.
The grafting combination generally used for Jatropha planted in dry areas is a combination between the scion that has a high yield performance and the rootstock selected that is tolerant to the limited water availability (Cholid et al. 2014). Although J. curcas and J. cinerea belong to the same genus, they are morphologically distinct (leaves, flowers, fruits, and seeds), as well as the environmental conditions where they grow. Compatibility of the rootstock and the graft is essential for optimal growth, water capture, and nutrient transport (Martínez-Ballesta et al. 2010. In this work we have shown the grafting procedure and characteristics between J. curcas and J. cinerea plants, as well as descriptive anatomical study to allow explaining grafting compatibility of these two species.
Method
Plant material
The experiment was performed in the Laboratory of Plant Biotechnology at CIBNOR, La Paz, Baja California Sur, Mexico (N 24° 08’ 05.7” N, 110° 25’ 31.8” W; 6 m a.s.l.). Seeds of non-toxic J. curcas from cultivated plants were collected from an experimental lot in Estación Dimas, Sinaloa, México (N 23° 46’ 35.4” N, 106° 46’ 48.3” W; 42 m a.s.l.). J. cinerea seeds were collected from wild plants located in the town El Tambor, Navolato Sinaloa, México (N 24° 44’ 43.9” N, 108° 01’ 26.6” W; 5 m a.s.l.).
Seed germination
First, 200 seeds of each species were washed with detergent Axion® (Colgate-Palmolive, Mexico City, MX) for 10 min; then, they were treated with 10% commercial sodium hypochlorite Cloralex® (concentrated 4-6%) (Industrias Alen, Nuevo Leon, MX) for 15 min and rinsed five times with distilled water; after that, they were soaked in distilled water for a 24 h period at room temperature to soften the seed coat and allow a homogeneous germination (Willan 2000). The seeds were placed in sterile paper wetted in sterile water for their germination and incubated in a growth chamber at 25 ± 2° C in dark conditions. Once germination occurred, they were sowed in 250 mL polyethylene cups, perforated on the base and filled with vegetable transplant growing mix Sphagnum sp. moss Sogemix® (Canada). The germinated seeds were incubated in a growth chamber at 25 ± 2° C and periods of 12 h light and 12 h darkness. Once seedlings developed, they were placed in a polycarbonate greenhouse in light and temperature conditions at 28 ± 7° C.The germination percentage under these conditions was evaluated in both species.
Top cleft
Thirty-day seedlings (N = 50) of both species were selected similar in stem diameter and height; J. cinerea seedlings were cut to use them as rootstock, and a V-shaped cut was made under the cotyledons discarding the aerial part to avoid growth of axillary shoots found in the knot of the cotyledon leaves. On the other hand, J. curcas (graft) plants were cut in an arrow shape above the cotyledons avoiding the bud union in such a way that it joined the rootstock, discarding the root (Dhillon et al. 2009), Cholid et al. 2014). The grafting parts were joint perfectly and covered with Parafilm® (American National Can, CT, U.S.A.) tape to hold them together and prevent the entrance of air. The seedlings recently grafted were placed in a growth chamber at a temperature of 27 ± 2° C and relative humidity from 70 to 80%. Grafted 3 month seedlings were transferred to 5 L of plastic pots with Sogemix® (CANADA) and perlite (Grupo Perlita, Torreón, Coahuila, MX) substrate (75% and 25%, V/V, respectively).
Plant growth measurement
The observations started 3 months after grafting. The parameters observed included: the percentage of grafted plant success, plant growth (plant height and stem diameter above the graft union) (Cholid et al. 2014). The statistical test ANOVA and Tukey’s test of mean comparison were performed on MINITAB 15 software to compare plant height and stem diameter of the Jatropha species studied.
Sample preparation for histological analyses
For the histological analysis, 1 cm of stem fragments were taken from J. curcas, J. cinerea (60 d after emergence), and grafted plants (30 d after grafting). The samples were preserved in formaldehyde-acetic acid-alcohol solution (FAA). Samples were processed to be cut with the paraffin inclusion technique. Fragments were dehydrated in series of ethyl alcohol in watery dilution at 70, 80, 90, 96°, and finally in absolute alcohol (ethanol) in 1 h periods for each concentration (D’ Ambrogio 1986). To clarify the material, pure xylol was used for 12 h. Paraffin infiltration was performed in a Thermo Scientific® (Thermo Fisher Scientific, Waltham, MA, U.S.A.) stove at 60 °C for 12 h. The paraffin inclusion system used with the LEICA® EG 1150 (Leica Biosystems, Germany) model in stainless steel molds; samples were embedded in paraffin and cooled down at 5° C; then, they were set in the freezer to obtain blocks.
The histological cuts were performed with a microtome LEICA® RM 2155 (Leica Biosystems, Germany) obtaining 4 µm cuts in thickness, transversally and longitudinally. The sections were mounted in slides and treated with alcohol. Dye was combined using fast green-safranin, especially for dyeing plant structures. The mounting medium used was distilled water and glycerine at 50% (D’ Ambrogio 1986). Observation was performed with an optical microscope Olympus® BX50 (Olympus Co. Japan) with lenses of 4X, 10X, 20X magnification with digital camera CoolSNAP-Pro (Roper Scientific, Inc. U.S.A.).
Results and discussion
Seed germination
Germination of J. curcas seeds (40%) occurred at day three while that of J. cinerea was only 10%. The rest of the germination for both species happened heterogeneously, obtaining 60% for J. curcas with a total of 120 plants and 25% for J. cinerea with a total of 50 plants in a period of 12 d. Hishida et al. (2013) observed that seed germination of J. curcas started at day three, reaching its maximum germination rate at day six, compared with J. cinerea where germination started at day 4 with a maximum rate at day 10. Two factors could be affecting this difference. The first one because the seed cover of J. curcas is thinner than that of J. cinerea since a great number of seeds of forest species do not germinate due to the hardness of the seed cover preventing the entrance of water (physical latency) and germinating unless seed scarification is performed (Poulsen and Stubsgaard 2000). The second factor could be seed quality; because J. curcas seeds are from cultivated plants, they reach physiological maturity faster. Budi et al. (2012), who studied J. curcas seed viability in different maturity stages in an experimental field, found that the best seed germination stage is physiological maturity (yellow fruit). J. cinerea seeds are collected from wild plants without any agronomic practice, obtaining heterogeneous fruits. Seedling emergence was from 7 to 10 d after sowing. Jatropha seed viability is reduced to storage periods greater than five months (Budi et al. 2012).
Graft
We were observed a survival of 95% of the grafted plants. As the stem grew, callus formation in the graft union and detachment of the parafilm® (American National Can, CT, U.S.A.). The grafting success is determined by the scion and rootstock ability to develop a composite plant in the graft union (Figure 1). The transversal and longitudinal cuts performed to the stems of the grafted plants showed J. curcas tissue integration with J. cinerea rootstock when they developed jointly in only one plant. These macroscopic cuts (Figure 2), where we could confirm that the stems in the grafted area were completely joint at day 15 after grafting, showed successful compatibility with the stem tissues of both species. As mentioned previously, this union was formed by callus proliferation on the grafting area. Furthermore, dark parts or parts without tissue with holes were observed where a necrosis area appeared, typical of regeneration characteristics of mechanical plant tissue damage, including incompatible tissues (Poessel et al. 1996). Cholid et al. (2014) evaluated graft and rootstock compatibility of J. curcas using two grafting methods, whip grafting in diagonal cut and top cleft grafting in V-shaped cut with 1, 2, and 3 month rootstock, obtaining a grafted plant survival of 78.6%, they report that the best method for grafting was the V-shaped cut and the rootstock from 2-3 months with a survival percentage of 89.5 and 93.8%, respectively. The whip grafting method and the 1-month rootstock showed the lowest engraftment percentage of 66.5% (Cholid et al. 2014).

Fig. 1 (A) Grafted Jatropha plants in polyethylene cups, (B) Detail of stem grafted Jatropha, (C) Composite of Jatropha plants in 5 L pots with substrate.

Fig. 2 (A) Stem fragments from Jatropha species and (B) Transversal cuts of Jatropha cinerea, grafted Jatropha, and Jatropha curcas, respectively; (C) longitudinal cut of grafted Jatropha plants.
Grafted plant growth
The statistical analysis result at 3 months after grafting showed there were no significant differences between grafted and non-grafted plants on plant height and stem diameter (Table 1). The high compatibility of grafted plants showed similar growth in grafted and non-grafted plants. These results differ from those obtained by Cholid et al. (2014) who reported an average plant height of 74.42 cm and 2.74 cm of stem diameter, which might have resulted because the readings were made in plants 4 months of age of the same species.
Table 1. Effects of rootstock and graft on plants of Jatropha curcas and Jatropha cinerea.
| Species | Plant height (cm) | Stem diameter (mm) |
|---|---|---|
| Jatropha curcas | 50.16 a | 14.41 a |
| Jatropha cinerea | 50.50 a | 14.71 a |
| Grafted plants | 49.91 a | 14.69 a |
Values with same letters were not significantly different at 5%.
Histological cuts
In the transversal cuts of J. curcas and J. cinerea(Figure 3), well-differentiated tissue stem were observed from the exterior toward the interior: the epidermis was composed of cork bark in the most external part; the phellogen is the cork cambium. The phellodermis had several layers of cells in strata characterized by the presence of chloroplasts in its interior. The bark was composed of collenchyma and parenchyma cells in non-defined division patterns, which can be divided at random almost at any level and that could be observed up to where extra phloem fibers started. The phloem was located toward the interior with different cell types among the fibers, parenchyma, phloem parenchyma, eschlereid, phloem member elements, and their companion cells characterized by being in groups and with angular cells that have their origin in the vascular cambium. The vascular cambium showed continuously forming a cylinder around the stem, indicating the plant had reached a secondary development stage. The next layer was the xylem with few very large and solitary capillary vessels and radial cells with abundant starch grains dyed in purple, which could be functioning as osmolites to maintain the cellular volume and avoid hydric stress. Seki et al. (2007) mentioned osmotic adjustment is an important physiological mechanism by which plants synthesize and accumulate compounds acting as osmolites in the cells in response to hydric deficit.
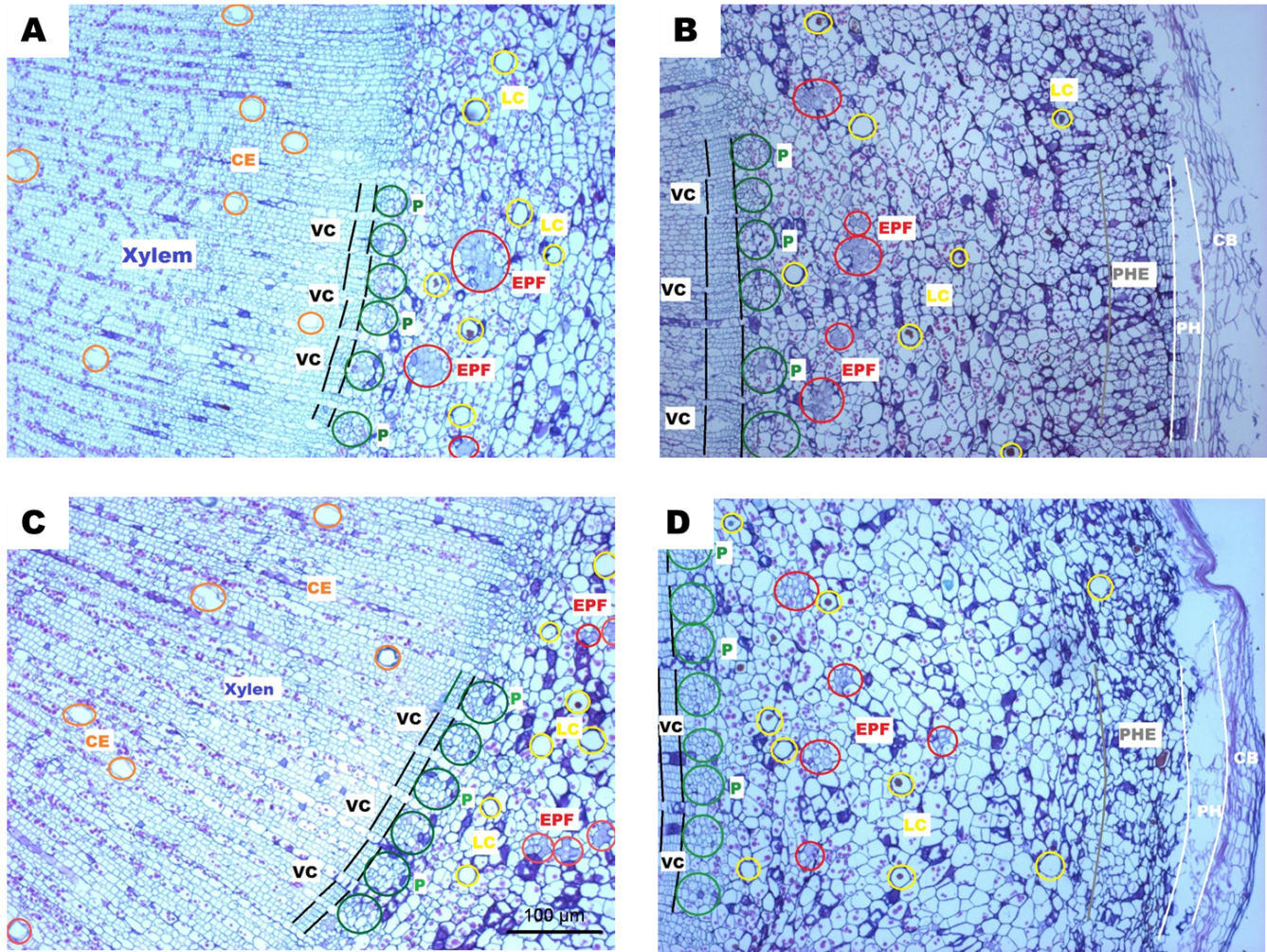
Fig. 3 (A), (B) Transversal cuts of Jatropha cinerea stem; (C) and D) transversal cut of Jatropha curcas stem; (CE = conducting elements, VC = vascular cambium, P = phloem, EPF = extra phloem fibers, LC = latex channels, PHE = phellodermis, PH = phellogen and CB = cork bark).
On the other hand, latex channels were observed from the peridermis up to the medulla; because the latex function is not clear, researchers suggest some of its components could have an important role in the mechanisms of healing wounds and/or providing mechanical defence against predators and pathogens (Hagel et al. 2008). It is known that the family Euphorbiaceae has resiniferous channels from which latex of some genera as Euphorbia, Hevea, Jatropha, and Manihot is used in traditional medicine because of its healing properties. Thus, the presence of articulated and non-articulated laticifers in Euphorbiaceae has been reported (Demarco et al. 2013).
While comparing the different tissues in both species, collenchyma cells of J. curcas lacked starch grains, and starch amount was lower in parenchyma cells compared to the cells of J. cinerea in both tissues where they were present in greater amount. Another difference was the width of the vascular cambium where J. cinerea doubled the number of cambium cells compared to those of J. curcas(Figure 3).
We can conclude that although J. curcas and J. cinerea plants showed different size in tissues when the cut for grafting was performed at 30 d after emergence, they anatomically showed the same tissues well differentiated, formed by the same cell types, and fixed on similar strata. Some cellular contents as starch grains were present in radial parenchyma cells and chloroplasts in layers not deep from the phelloderm.
The longitudinal cuts performed to J. curcas showed a phloem with cells containing starch grains larger than those observed in J. cinerea cells although as mentioned before, the amplitude of the vascular cambium is greater in this species. Groups of radial medullary cells in J. curcas xylem were observed horizontally in greater quantity compared to those of J. cinerea, which showed a smaller number of radial cells piled up diagonally (Figure 4). The grafted plants showed a high survival percentage because J. cinerea rootstock has a wider vascular cambium than that of J. curcas. When the vascular cambium area of J. cinerea made contact with the graft, there was a high probability of matching with the cambium area of J. curcas, allowing a fast forming callus for tissue regeneration in the wound.
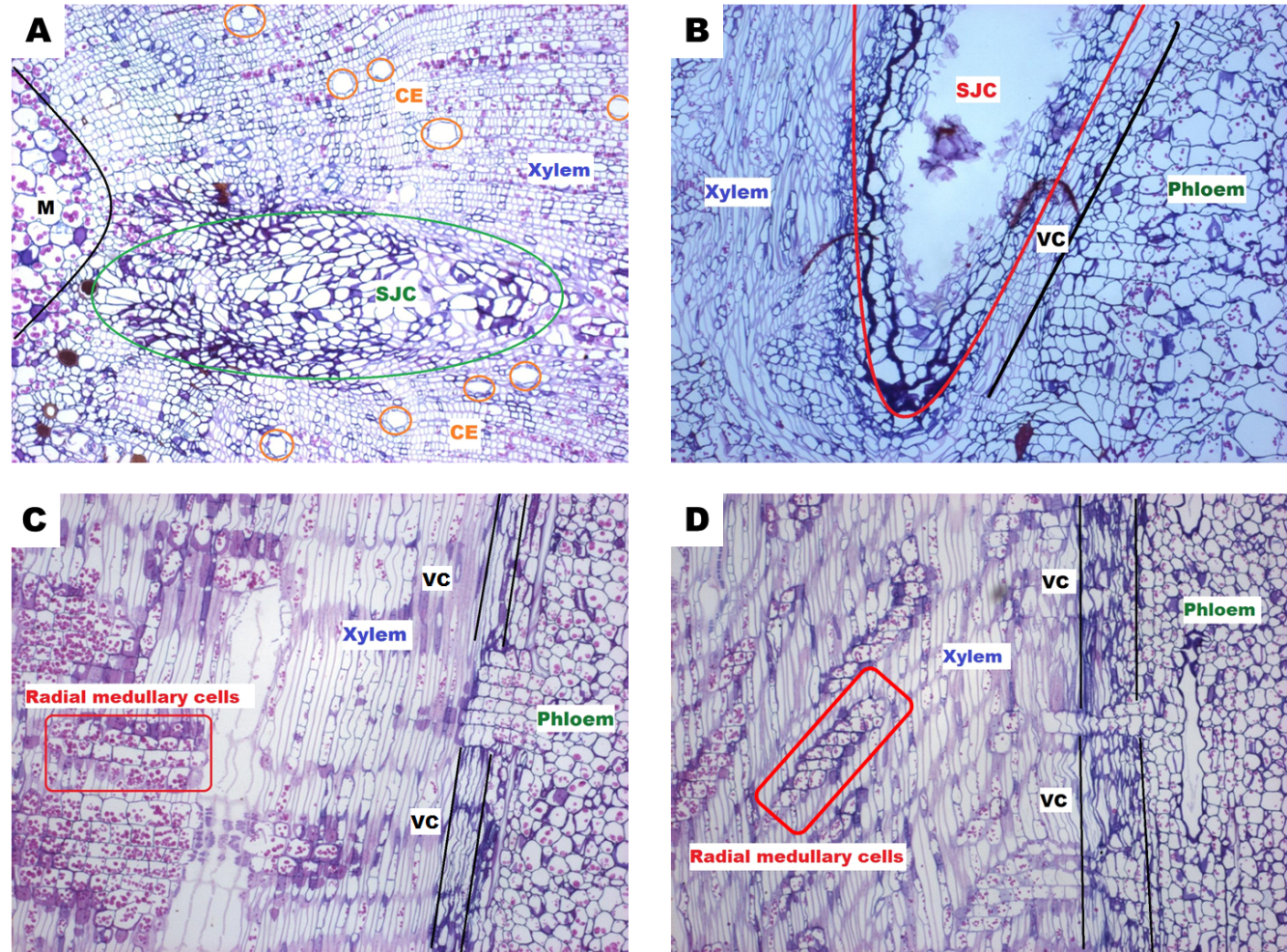
Fig. 4 (A) Transversal cut of grafted Jatropha stem; (B) longitudinal cut of grafted Jatropha stem; (C) longitudinal cut of Jatropha curcas and (D) longitudinal cut of Jatropha cinerea stem; (CE = conducting elements, VC = vascular cambium, M = medulla, SJC = scion J. curcas.
In general terms, grafting between J. curcas on J. cinerea was successful mainly because the following characteristics took place: (1) rootstock and graft belonged to the same genus; (2) plants of the same age and diameter were used; (3) anatomically both species showed tissues formed by similar cells distributed in the same order in the stem, which increased the probability of forming interconnections in less time. Another possibility was the amplitude of the vascular cambium and the invariable presence of parenchyma cells in almost all the tissues in both species that could split and generate layers, which jointly formed the callus. The callus can differentiate itself in other more specialized cell types or take an active role in transport by the presence of connections that make lateral transport possible without the need of conducting elements.
According to Cholid et al 2014, the causes of grafting incompatibility can be (1) physiological and biochemical factors; (2) modifications of cells and tissues in the graft union; and (3) cellular recognition between grafting parts. They also mentioned that grafting success depends on compatibility of the graft union in terms of rapid formation of the conductor vascular tissues between the two sections that allow recovery of the root and the aerial art of the grafted plant.
Conclusions
Grafting compatibility between genotypes of J. curcas and J. cinerea was achieved by histological assays, obtaining a survival 95% of grafted plants and compatibility of the vascular tissues to regenerate and develop composite plants. Considering that each of the species studied developed in different conditions of hydric and saline stress, in future studies we will show adaptation of grafted plants in these stress conditions to evaluate if this methodology will benefit the development of these species and favor seed production.











 nueva página del texto (beta)
nueva página del texto (beta)


