Introduction
The Gulf of Mexico (GoM) holds a variety of areas that represent critical habitats for many vertebrate animals (Durán-González et al. 2005, Rosenberg et al. 2009, Ellis et al. 2011). Its geographic location allows the movement of many bird species from the Florida Everglades and the Texan estuarine marshes in the north, to Laguna Madre in Tamaulipas, and as far as Celestún in the Yucatan Peninsula in the south (Buler and Moore 2011). Together these sites represent about 2,500 miles of habitat with different forest cover types and wetlands of fresh and saltwater (Yáñez-Arancibia and Day 2005, Mendoza-González et al. 2012). Such a diversity of wetland ecosystems supports a great richness of resident and migratory birds. For example, together, areas such as Laguna Madre in Tamaulipas and the lagoon system of Alvarado in Veracruz, among other sites in the GoM and the Mexican Caribbean, hold about 30%-40% of the recorded bird species in Mexico (Gallardo et al. 2009). Such species richness highlights the ecological importance of the coastal plains of both the GoM and the Yucatan Peninsula and their marine ecosystems. For instance, nearly 75% of the world’s population of Redhead Duck (Aythya americana) spends the non-breeding season feeding on the seagrasses (Hodule wrightii) of Laguna Madre (Garza-Torres 2001, Ballard et al. 2010). While in Laguna de Alvarado exists one of the lasts viable Mexican wild populations of the Muscovy Duck (Cairina moschata) (Conabio 2002). On the other hand, the vegetation characteristics of the Yucatan Peninsula allow the coexistence of several migratory and resident bird species (Peralta-Peláez et al. 2009), where migrants find the places and resources to rest and strength back again (Deppe and Rotenberry 2008, Greenberg et al. 1993).
Isla del Carmen is located at the southern side of the GoM in Campeche Mexico, within the natural protected area of Laguna de Términos. This area is about 700,000 hectares (ha) in size, and together with Pantános de Centla, supports one of the more extensive and best-preserved mangrove forests of Mexico (Soto-Galera et al. 2009, Pérez-Ceballos et al. 2013). Because of the diversity of aquatic, waterfowl, and marine bird species occurring there, and because of its characteristics for the conservation of the biodiversity, Laguna de Términos is also recognized as a RAMSAR site (Ramsar 2017). Although Isla del Carmen lies within the natural protected area, it has experienced a rapid and poorly planned urban growth during the last 40 years (Pérez-Ceballos et al. 2013), causing mangrove deforestation, filling and drainage of wetlands, and loss of breeding grounds for coastal and aquatic birds (Ramos Miranda and Villalobos-Zapata 2015). Nonetheless, Isla del Carmen still holds areas of lowland rainforests and coastal dunes, which contribute to the landscape’s heterogeneity in the GoM region (Villalobos-Zapata and Mendoza Vega, 2010).
Even though there are about 370 bird species recorded for the whole area of Laguna de Términos (Conabio, 2015, 2018), there is a gap in the study and monitoring of birds on the coast of Isla del Carmen for at least 15 years. Moreover, the most recent wildlife studies carried out in Isla del Carmen cover the topic of avian species richness only in a general way (see, for example, Amador-del Ángel et al., 2013, 2015). Such studies are checklists without a sampling design that allows the estimation of population trends, densities, or abundances, precluding comparison through time to make inferences about the changes in the bird community. Because birds are one of the animal groups facing significant pressures due to environmental and habitat changes (Julliard et al. 2004, Seress and Liker 2015), it is necessary to update the knowledge of this group of animals occurring along the coast of Isla del Carmen. Also, the presence of several species in Isla del Carmen mismatches the seasonality reported in the literature. Furthermore, some shorebird species considered winter visitors, might have local populations living there, with the potential to breed in the dunes or at the islets surrounding this island of the GoM.
This work aims to promote the conservation of migratory and resident bird species occurring at the coastline of the region of the GoM by contributing with new information about the bird community of Isla del Carmen. Because most of the previous studies in this island focused on the riverine area and mangrove systems (Amador-del Ángel et al. 2013), we surveyed land, shore, and marine birds at the beach areas and their nearby urban zones, which remain relatively unstudied.
Methods
Study area
Our study area is a beach zone of approximately 120 ha, known as Playa Norte (PN), located at the north side of Ciudad del Carmen, within Isla del Carmen, Campeche (Figure 1). Although this is one of the most urbanized locations of Isla del Carmen, there are still some though fragmented coastal dune areas. The dune vegetation includes small patches of seagrape (Coccoloba uvifera) mixed with shoreline purslane (Sesuvium portulacastrum), bayhopes (Ipomea pes-caprae, Ipomea sp.), Mackenzie bean (Canavalia rosea), Gregg’s amaranth (Amaranthus greggii), sea lavender (Tournefortia gnaphalodes) and wild maracuja (Passiflora foetida) (Guadarrama et al. 2014). There is also a small patch (< 1 ha) of the button mangrove (Conocarpus erectus) on the west side of the study area. The height of the herbs and bushes ranges from approximately 3 cm to 135 cm, while the mangrove trees can reach about 3.5 m. The average vegetation cover in the study area is approximately 25% - 35% (López-Rosas et al. 2014). At the urbanized zone in the study area several introduced species such as the coconut tree (Cocos nucifera), the australian pine tree (Casuarina equisetifolia), the country almond tree (Terminalia cappata), as well as the native rosy trumpet tree (Tabebuia rosea) are present at the sidewalk and central ridges of the streets. The annual average rainfall is about 1,420 mm, and the annual mean temperature about 27°C (David and Kjerfve 1998).
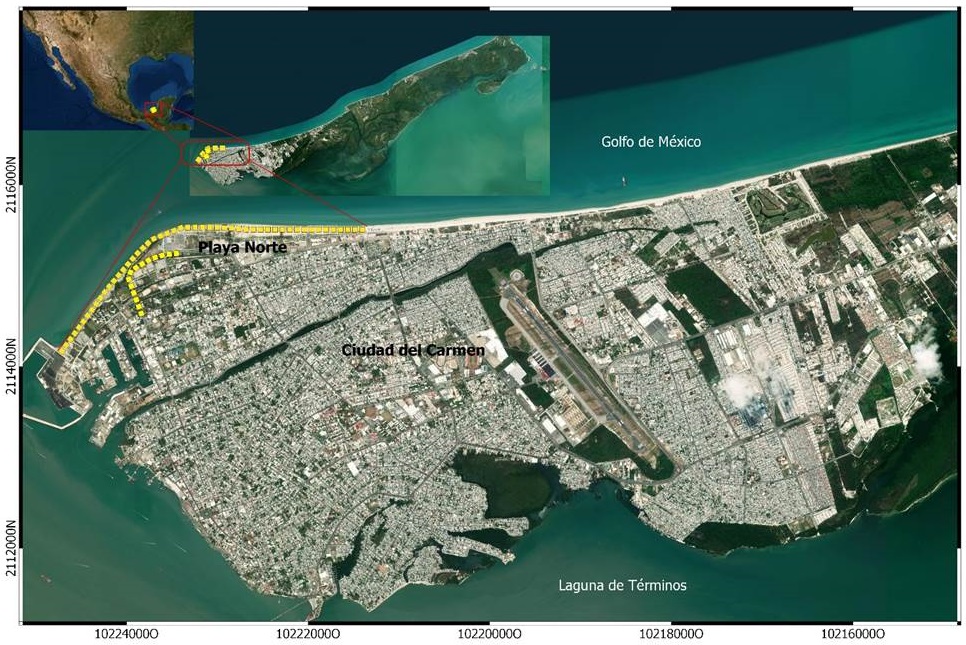
Figure 1 Study area and survey transects’ location in the shoreline and adjacent urban area of Playa Norte Ciudad del Carmen, Campeche. The yellow dotted lines show the location of the surveyed transects from January 2012 to December 2016.
Several disturbing activities for birds occur within the study area. The most evident are recreational-related, such as horse riding, ATV driving on the beach and dunes, and increased tourism density (López-Rosas et al. 2014). On the other hand, activities such as sand-dredging and building of port infrastructure, that might trigger important changes in the dynamics of the local marine currents, erosion and sedimentation patterns (Rosen and Vajda 1982), may affect the food availability and the habitat quality for many bird species.
Surveys
A five-year bird survey was conducted from the beginning of 2012 to the end of 2016 on the coastline of PN to assess species richness and abundance. We performed unidirectional surveys along two strip-transects of approximately 1 km and 4 km in length each (Figure 1).
The shorter transect included a section of the urban area adjacent to the beach. There, natural vegetation has been replaced by australian pine, coconut trees, and different ornamental tree species. Formerly flood plains adjacent to the main street of PN have been transformed into football fields or parking lots. The longer transect included coastal dune habitat where dominant vegetation is the sea grape and the button mangrove on the west side of the transect. On the east side of the study area, most vegetation has been removed, and the beach is used for touristic activities.
The surveys were systematically conducted by walk once a week every month during the entire five-year period. For each transect, a total of 48 replicates were carried out each year. Recording and counts of bird species were conducted from 07:00 to 10:00 hours (UTC-6/UTC-5 according to Mexico’s winter and summertime), by a single observer. Because bird groups with different detectability occur within the study area, we used two range distances to record bird species along the transects. For species such as egrets, ibises, sandpipers, willets, oystercatchers, and other shore and marine species, we recorded all seen or heard birds within the first 80 m on each side of the long transect. For land birds such as warblers, flycatchers, and other mid-sized and small species, we counted all birds seen or heard within the first 20 m on each side of the short or long transect (Nur et al. 1999). To ensure that the records did not exceed the range distances, we used a digital range finder to measure the distance from the observer to groups or individual birds.
We determined the seasonal status of all species according to the species’ account of Birds of North America (http://birdsna.org), and by literature (Howell and Webb 1995, Kaufman 2005). We used the following categories: winter visitor (WV), the species that spend the wintertime in the study area. Resident breeders (RB) are the species that inhabit permanently on the coast of Isla del Carmen. Summer residents (SR) include species that arrive in the mid-spring to breed in the area and leave in early autumn. Transient migrant (TM), the species that visit Isla del Carmen in winter only for a few days to rest and feed. Exotic species (EX) are the non-native species living in our study site. To verify whether the species on our list were previously recorded in our study area, we consulted the distribution and records of each species on the database of eBird (http://ebird.org/ebird/map). Determination of the conservation status was according to the Norma Oficial Mexicana NOM-059- SEMARNAT-2010, the Mexican list of native wildlife species under threat (Semarnat 2010), which includes categories such as threatened species, species under special protection, and species under extinction risk. The observed plumage characteristics were assessed using the identification field guides of Dunn and Alderfer (2011), Kaufman (2005), and Pyle (1997). To avoid confusion between incomplete basic plumage and reproductive plumage, we also investigated whether the birds in our list show one or two plumage types yearly (Pittaway 2000).
Data analysis
We estimated the Shannon-Wiener diversity index (H’) for each sampled year as:
All tests were significant at P < 0.05, if not indicated otherwise.
Results
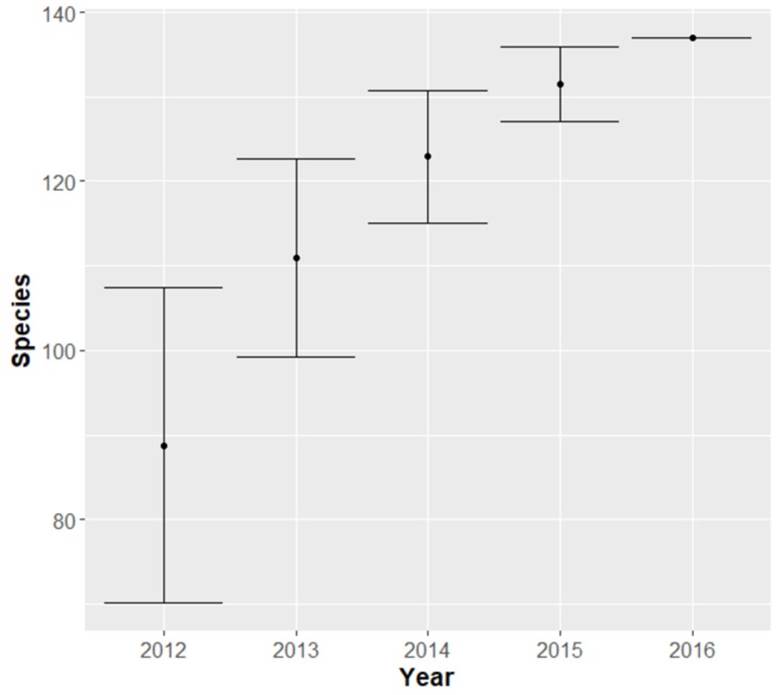
We recorded 137 species belonging to 33 families (Appendix 1). According to the species accumulation curve, longer monitoring could be necessary to find the true species richness in the study area (Appendix 2). However, for each sampling year, the observed species richness was higher or equal to the expected richness (Table 1). Thus, our results properly represent the species richness on the coastline of Isla del Carmen. Regarding the number of species, the richest families were Scolopacidae (n = 20), Parulidae (n = 16), and Laridae (n = 13).
Table 1 Observed (SRobs) and expected (SRexp) species richness, and the evenness index (J) estimated for each sampling year (2012-2016) in the shoreline of Isla del Carmen Campeche.
| Years | 2012 | 2013 | 2014 | 2015 | 2016 |
|---|---|---|---|---|---|
| SRobs | 56.00 | 95.00 | 98.00 | 81.00 | 111.00 |
| SRexp | 51.89 | 85.29 | 86.79 | 81.00 | 108.79 |
| J | 0.47 | 0.58 | 0.58 | 0.67 | 0.61 |
The families with the greater abundances were Laridae, Scolopacidae and Pelecanidae (n = 21,823; n = 5,123; and n = 1,614 counts of individuals respectively). The analysis of the annual abundance showed a significant decrease at the end of the sampling period (Kruskal-Wallis test, χ2 = 39.23, df = 4, P = 0.001, Figure 2a). The top ten most abundant species (500-9600 individuals) were the Royal Tern (Thalasseus maximus), the Least Tern (Sternula antillarum), the Willet (Tringa semipalmata), the Semipalmated Plover (Charadrius semipalmatus), the Sanderling (Calidris alba), the Brown Pelican (Pelecanus occidentalis), the Laughing Gull (Leucophaeus atricilla), the Sandwich Tern (T. sandvicensis), the Black Skimmer (Rhynchops niger), and the Short-billed Dowitcher (Limnodromus griseus). The last four species showed a decreasing pattern in abundance over the sampling years (Figure 3). Nonetheless, the high abundance of species such as the Laughing Gull, the Willet, and the Brown Pelican, skew the evenness (Table 1). We did find evidence of significant proportional changes in H’ across the years. Only from 2013 to 2014, the change of H’ was negligible (Table 2). Although the inter-annual variation of H’ increased over time, we did detect a significant drop between 2015 and 2016 (Figure 2b).
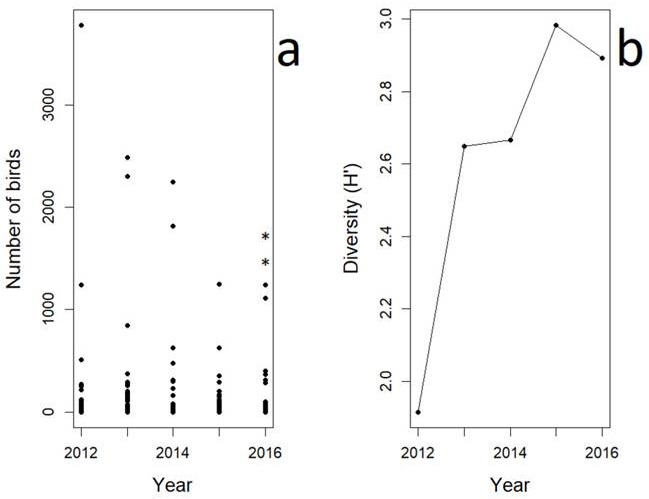
Figure 2 The abundance (a) and diversity (b) of the avian community of the shoreline of Isla del Carmen over a five-year sampling period. In (a), the black dots are counts of the different species in the study area, while the stars over the dots show significant differences in the annual abundance (P < 0.05). In (b), the black dots are the estimated values of H’.
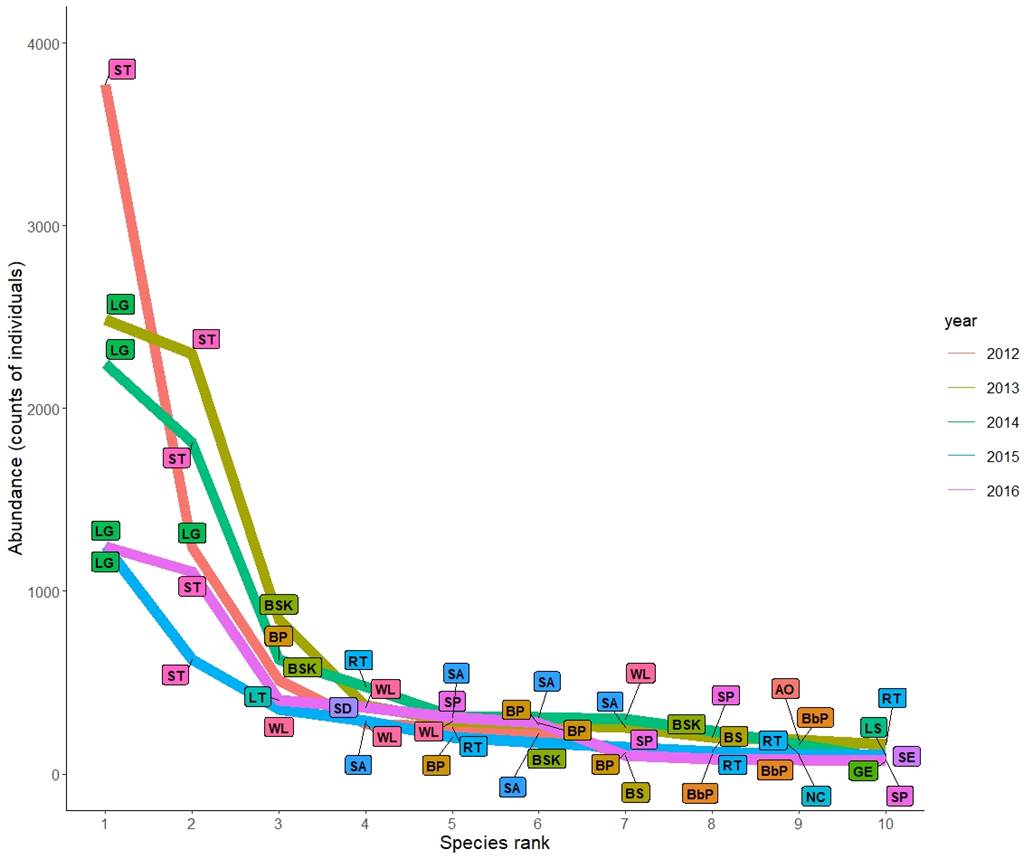
Figure 3 Rank plot of the ten most abundant bird species per year in the shoreline of Isla del Carmen. Each colored line represents a sampling year (2012-2016). The thinner black lines show the true position of each label. The bird species in the plot are: Sandwich tern (ST), Laughing gull (LG), Black skimmer (BSK), Brown pelican (BP), Least tern (LT), Willet (WL), Royal tern (RT), Short-billed dowitcher (SD), Sanderling (SA), Semipalmated plover (SP), Black-necked stilt (BS), American oystercatcher (AO), Black-bellied plover (BBP), Great egret (GE), Snowy egret (SE), Neotropical cormorant (nc), Least sandpiper (LS).
Table 2 The estimated proportional change of bird diversity (M = D min )/D max ) between pairs of years (below diagonal) in the shoreline of Isla del Carmen Campeche, and the significance of this change (above diagonal) according to Hutcheson’s t test.
| Year | 2012 | 2013 | 2014 | 2015 | 2016 |
|---|---|---|---|---|---|
| 2012 | * | < 0.001 | < 0.001 | < 0.001 | < 0.001 |
| 2013 | 0.49 | * | 0.478 | < 0.001 | < 0.001 |
| 2014 | 0.47 | 0.95 | * | < 0.001 | < 0.001 |
| 2015 | 0.34 | 0.69 | 0.73 | * | 0.003 |
| 2016 | 0.37 | 0.76 | 0.80 | 0.91 | * |
Less than half (40%) of the recorded species are residents in the study area, being Ardeidae the family with more resident species. About 56% of the recorded species are considered WV or TM, mainly belonging to Scolopacidae and Parulidae (n = 18 and n =15, respectively, Appendix 1). About 3% of the species on the coastline of Isla del Carmen are non-native birds. Specifically, we recorded the Eurasian Collared-Dove (Streptopelia decaocto), the Rock Pigeon (Columba livia), the Burrowing Parrot (Cyanoliseus patagonus) and the Monk Parakeet (Myiopsitta monachus). We did observe all these species feeding and breeding (as indicated by the presence of juveniles and nests) within the urbanized areas adjacent to the beach during our study. Approximately 33% of the recorded species showed reproductive plumage (Supporting file Table S1). However, only eight species, including the Ruddy Turnstone (Arenaria interpres), the Sanderling, the Semipalmated Plover, the Black-bellied Plover (Pluvialis squatarola), and the Willet, considered WV in our study area, showed reproductive plumage during the local breeding season (April to September, Table 3) in at least three months of each of the five-year study period.
Table 3 Number of individuals of different species showing reproductive plumage during the local breeding season in the shoreline of Isla del Carmen Campeche through years (2012-2016). Only sightings of species showing signs or reproductive activity for ≥ three months per season, or ≥ 25 total individuals are shown.
| Apr | May | Jun | Jul | Aug | Sep | Total | |
|---|---|---|---|---|---|---|---|
| Charadriidae | |||||||
| Charadrius semipalmatus | 0 | 14 | 4 | 0 | 2 | 3 | 23 |
| Pluvialis squatarola | 2 | 26 | 1 | 0 | 15 | 9 | 53 |
| Laridae | |||||||
| Sternula antillarum | 68 | 65 | 123 | 126 | 86 | 66 | 534 |
| Scolopacidae | |||||||
| Actitis macularius | 8 | 17 | 1 | 4 | 8 | 3 | 41 |
| Arenaria interpres | 0 | 12 | 2 | 2 | 22 | 2 | 40 |
| Calidris alba | 0 | 6 | 0 | 2 | 17 | 0 | 25 |
| Tringa semipalmata | 0 | 0 | 0 | 5 | 31 | 40 | 76 |
| Total | 78 | 140 | 131 | 139 | 181 | 123 | 792 |
According to eBird database, there are no previous records in our study area for 23 species reported here (Appendix 1). Charadriidae, Scolopacidae, Laridae, and Tyrannidae were the families with most of the novel records at this location of the GoM. Eighteen of the recorded species are under some conservation status: threatened species = 4, extinction risk = 5, and special protection = 9. Finally, we obtained a collection of digital photographic material of each one of the species reported here. Such a collection was deposited at the ICMyL-UNAM Estación el Carmen.
Discussion
We recorded 137 bird species at the beaches of Isla del Carmen, Campeche. This diversity represents about 34% of the avian families, and almost 12% of the bird species recognized in Mexico (Navarro and Sánchez-Gónzalez 2003, Navarro-Sigüenza et al. 2014). Moreover, our findings represent 31% to 41% of the avian richness reported for the GoM region (Gallardo et al. 2009).
Although today the urbanization severely modifies about 20% (3,000 ha) of the surface of Isla del Carmen, the diversity found in our study site is comparable with that reported for other islands of the Caribbean (Appendix 3). The number of bird species at the beaches of Isla del Carmen is also similar to the richness at some areas of evergreen forest in the inland of Tabasco (González-Valdivia et al. 2012). Such diversity is possible because of the high proportion of migratory birds (57%) that use the island as a stopover site, winter refuge, or as breeding ground during the summer (Langin et al. 2009, Sheehy et al. 2011). Inter-annual variation in abundance over the years was due to lower counts of species that usually flock in big groups, such as pelicans, sandpipers, terns, and plovers, which were in high numbers at the beginning of our study. Such changes are likely the result of an increased tourism density, but additional studies are required to support this hypothesis. Through the survey time, the lower dominance of birds such as the Sandwich and Royal terns , the Brown Pelican and the Black Skimmer, allowed the values of H’ and J’ to increase. However, the proportion of species recorded only three times or less (25%) in a five years period, kept the evenness only moderately balanced.
We did observe significant differences in the proportional change of H’ between 2012 and the remaining years. Such a result was due to an increased number of passerine species over time. Although most of the beach and the nearby urban areas lost their natural vegetation cover (mainly mangrove) in the last decade, fragmented dune vegetation remains and shows signs of recovery. These few remnants at the outer edge of the urban zone are attractive to resident and migrant birds, and favor the detection of an increased number of small species such as vegetation gleaners (Tavares et al. 2015), thus, increasing the estimates of H’. However, we also observed a significant decrease in H’ from 2015 to 2016, as indicated by the drop of the M value. As variation in species richness and evenness relate to productivity, niche and food availability (Symonds and Johnson 2008), the observed differences could be linked to the change of land use of the coastal dunes, to the human activities carried out in the study area, or both (Weston et al. 2014). Unfortunately, there are no previous studies in, or close to our study area, to make inferences about whether our estimations lay within the normal parameters of variation in the bird community of Isla del Carmen.
Conversely, the increase of the recreational ground area (which includes the building of palapas, parking lots) and the constant presence of people, along with the artisanal fishing activities near to the beach, are potential attractors to synanthropic species. It is demonstrated that urban areas and intercity roads facilitate foraging activities of synanthropic birds of different guilds (D’Amico et al. 2013), especially those with generalist habits (grackles and gulls). Moreover, the abundance of some piscivorous species such as pelicans and gulls can be increased in areas close to human settlements (0-2 km), but might negatively affect less-disturbance tolerant species such as terns, skimmers, herons, and willets within distances under 2 km (Lafferty 2001, Tavares et al. 2015). Additionally, the abundance of water birds can decrease by temporal or definitive habitat loss due to sea-level rise or coastal erosion (Nores 2011). On Isla del Carmen, there is a continuous process of erosion of the shoreline. For instance, between 1975 and 2007 Isla del Carmen lost beach area in a range of 8-177 meters at different points on the coast of the island (Torres Rodríguez et al. 2011). Thus, the inter-annual bird abundance observed may be linked to such erosive processes.
Most of the recorded species under protection status by Mexican (Semarnat 2010) and international agencies (IUCN 2016) are shorebirds. Therefore, the coast of Isla del Carmen, as well as the adjacent habitats in Laguna de Términos are critical for the conservation of the avian richness moving along the GoM region. The threats to the species of national and international conservation concern range from habitat loss by dredging, invasive plant species, urban, industrial, and agricultural development, to the commercial and touristic development of the coastal areas, as well as the illegal species trade (IUCN 2016). At least several of these activities occur in our study site. On the other hand, the literature classifies species such as the Ruddy Turnstone, the Sanderling, the Semipalmated Plover, the Black-bellied Plover, and the Willet as WV or TM in our study area (Kaufman 2005, Dunn and Alderfer 2011). However, we did observe individuals showing reproductive plumage within the local breeding season (April to September), suggesting that these species could have reproductive activity locally, at nearby zones in the islets or coastal dunes of Laguna de Términos. Nevertheless, further monitoring is required.
Although Isla del Carmen supports about 12% of the species richness of Mexico and around a third of the species diversity present in the GoM region, assessments on the habitat quality and food availability for several bird species are required, especially for less well-known shorebird species in the study area. To better understand the factors related to the inter-annual variation of diversity and abundance of the coastal bird community in Isla del Carmen, further monitoring and analyses are encouraged. Our findings contribute to updating the knowledge on the avian community in the southern GoM, as well as on the distributional range of species of conservation concern.











 nueva página del texto (beta)
nueva página del texto (beta)