Introduction
Mexican lizards constitute half of the reptile species in the country, whereas these represent almost 10% on a global scale (Flores-Villela & García-Vázquez, 2014; Reséndiz-López et al., 2021). In addition, of the 864 reptile species recorded in Mexico, a number that is still growing, 493 are endemic (Flores-Villela & García-Vázquez, 2014; Reséndiz-López et al., 2021). Around 20% of the total reptile species in Mexico can be found in insular systems, including 92 lizard species that represent 22% of the lizard species at the national level and 57% of insular reptiles, which is surprising considering that the islands represent 0.26% of the total territory (Calderón-Patrón, 2007; Flores-Villela & García-Vázquez, 2014).
Lizards inhabiting islands may confront particular ecological characteristics because the area of the island and the distance from the mainland limit the number of species inhabiting such isolated systems (Calderón-Patrón, 2007; Ibanez et al., 2018; Meiri, 2007). Thus, insular lizards frequently respond according to ecological theories. For example, density compensation results in dense lizard populations on the islands because of the decrease of rates of interspecific competition and predation (Connor et al., 2000; Itescu et al., 2017). This phenomenon, known as ecological release, may lead to the niche expansion of insular lizard species that may exploit or access resources that are occupied or not available on the mainland (Bolnick et al., 2010; Losos & Queiroz, 1997). These ecological aspects, which diverge between islands and the mainland, affect insular lizard populations, such as the increase of intraspecific competition (Itescu et al., 2017; Siliceo-Cantero et al., 2017), changes in reproductive traits like the increase in laying frequency (Novosolov & Meiri, 2013; Novosolov et al., 2013), increase of survival rates (Siliceo-Cantero & García, 2016), modification of their activity schedule and feeding habits (Siliceo-Cantero & García, 2015; Traveset, 1995), and morphological changes, which frequently include increased size (de Amorim et al., 2017; Siliceo-Cantero et al., 2016; Siliceo-Cantero et al., 2020). The most extreme effect of insularity, however, is the genetic divergence that results in the emergence of new species, contributing widely to biodiversity (Tallowin et al., 2017).
For such divergence, habitat use is one of the most important factors. Habitat use allows lizard species access to mates, prey, refuge, and thermoregulation sites that are crucial to their survival and fitness (Bruinjé et al., 2019; Muñoz & Losos, 2018). The habitat use, however, is affected by climate conditions and perch types provided by the environment. For example, for some lizard species, high environmental temperatures lead to a sedentary life style with the purpose of avoiding energy spent and the consequent dehydration (Lister & García, 1992). In fact, the high temperatures resulting from climate change have been emphasized as being responsible for the populations’ distribution reduction and species extinction (Sinervo et al., 2010). On the other hand, the perch types are correlated to locomotion in lizards, which is crucial for escaping from predators, as well as perch heights that improve the patrolling of their territory by searching for thermoregulation sites, prey, and mates (Collins et al., 2013; Gifford et al., 2012). These environmental variables (climate and perch types) may differ among the island and the mainland (Martín-Queller et al., 2017; Sagonas et al., 2013), therefore lizards must face these conditions through of behavioral and ecological changes (e.g., activity schedule and perch height). Thus, besides the direct contribution of insular systems for increasing biodiversity regarding species richness, these isolated areas support species with a divergent ecological role compared with their mainland counterparts, which is an important contribution to biodiversity.
In Mexico, relatively few studies have been performed on insular lizard communities despite the importance of islands in biodiversity and the occurrence of continental islands (Calderón-Patrón, 2007; Ureta et al., 2018). The main objective of this study is to present some traits of natural history from the lizard communities of 3 islands in western Mexico, in terms of the encounter frequency (as an approximation of population abundance) and age classes (adult, juvenile, and hatchling). Based on this, the insularity effect was Ctenosaura pectinata evaluated by comparing the encounter frequency, age classes, and ecological attributes such as basking behavior, activity related to climate, and the perch height of lizard species shared among the islands and the mainland.
Materials and methods
The study site comprises 3 islands (Cocinas, La Pajarera, and San Agustín) belonging to the Chamela Bay Islands Sanctuary (ChBIS) (Conanp, 2008), as well as one mainland site in the tropical dry forest of the Chamela- Cuixmala Biosphere Reserve (Ch-CBR). The islands off Chamela Bay are located together with the Ch-CBR, in the municipality of La Huerta, Jalisco, Mexico (Conanp, 2008) (Fig. 1). Cocinas Island, with 31.69 ha and a distance to the mainland of 1800 m, possesses 2 principal vegetation types: xeric shrubs and tropical dry forest (Conanp, 2008). La Pajarera Island, with 20.52 ha and 1,400 m to the mainland, is dominated by Guinea grasses and remnants of tropical dry forest (Hernández-Vázquez et al., 2017). San Agustín Island (regionally known as Don Panchito), with 3.3 ha and 450 m to the mainland, is covered by tropical dry forest (Conanp, 2008). The Ch-CBR presents an area extension of 13,142 ha, with tropical dry forest as the predominant vegetation type, which is characterized by a marked seasonality in precipitation that is concentrated within the year from June to September (Bullock, 1986; Conanp, 2008; Maass et al., 2018). In this region, the mean annual temperature is 24.9 °C, and the mean annual precipitation is 800 mm (Bullock, 1986; Conanp, 2008; Maass et al., 2018). In the Ch-CBR, 22 lizard species have been recorded (García & Ceballos, 1994), whereas on the islands only 4 species have been reported: Ctenosaura pectinata, Iguana rhinolopha (previously Iguana iguana; Breuil et al., 2020), Norops nebulosus (previously Anolis nebulosus; Nicholson et al., 2018), and Aspidoscelis lineattissima (Conanp, 2008).
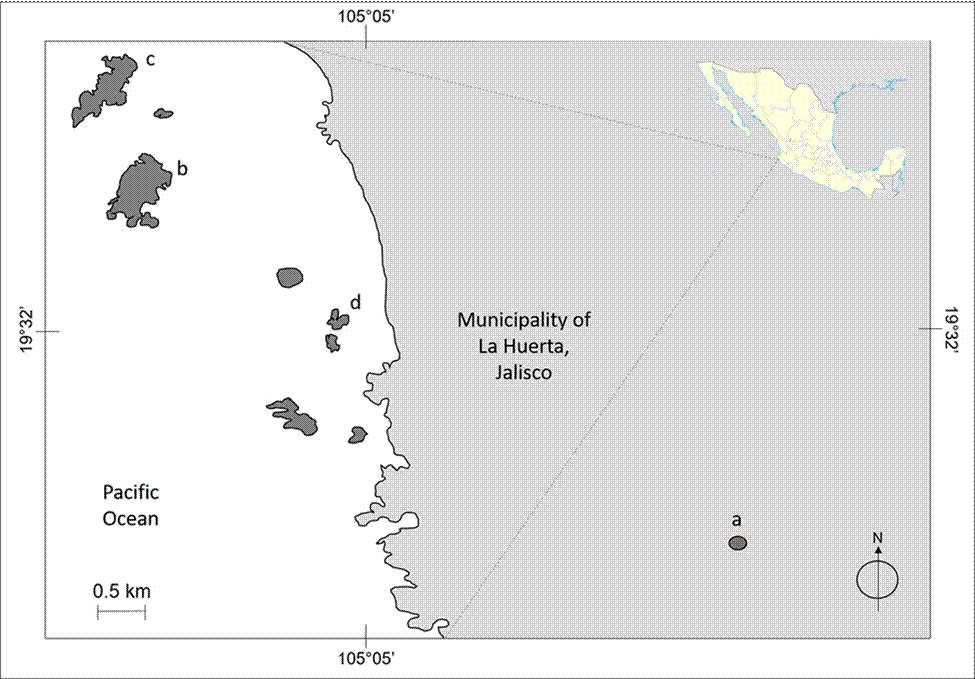
Figure 1 Study site: Pacific Coast of Jalisco, Mexico. The mainland (a) and the islands of Cocinas (b), La Pajarera, (c) and San Agustín (d).
The sampling of lizard species was performed during November 2018 and from March to May 2019. In every period, the sampling alternated among sites: the first day San Agustín Island was surveyed, the second day La Pajarera Island, the third day Cocinas Island, the fourth day the mainland, and so on. Two people with reptile sampling experience used the visual encounter survey method (Doan, 2003) to search intensively for lizards in established 200 m transects (4 per site) during 5 different schedules: 8 to 10h, 10 to 12h, 12 to 14h, 14 to 16h, and 16 to 18h. The survey schedules alternated among visits to the same island, using the identical survey schedule across all sites to compare the samplings. Thus, the sampling effort including the 4 sites was 251 person-hours (average 62.7 person-hours per site), which is comparable with other studies (e.g., Medina-Aguilar et al., 2011). For each lizard observed, we recorded the following information: species, time of observation, age classes (adult, juvenile, and hatchling), basking behavior (sun or shade), and perch height. The latter was categorized as low perch (bare ground, leaf litter, rock, thrown logs, and every substrate found at ground level), intermediate perch (grass, herb, shrub, and substrates found at an intermediate height), and high perch (cactus, tree, and substrates found at a high height). To identify species, we first took into account that lizards’ abundance in the TDF of Chamela, Jalisco, has recently (after 2015) decreased, recording only the following species: Aspidoscelis lineattissima, A. communis, Sceloporus utiformis, Phyllodactylus rupinus (previously Phyllodactylus lanei; Ramírez-Reyes et al., 2017), Ctenosaura pectinata, Urosaurus bicarinatus, Norops nebulosus, Aspidoscelis deppii, and Sceloporus melanorhinus (Marroquin-Paramo et al., 2021). Species that could be confused are A. lineattissima, A. communis, and A. deppii. We used the criteria of Garcia and Ceballos (1994), which indicate differences in tail coloration: blue in A. linettissima, red in A. communis, and bluish gray in A. deppii (Duellman & Wellman, 1960). These coloration patterns are brighter and more marked in juveniles and hatchlings (Duellman & Wellman, 1960; García & Ceballos, 1994). Another similar species is Holcosus miadis (previously Ameiva undulate; Meza-Lázaro & Nieto-Montes de Oca, 2015), which differs in body and tail coloration (García & Ceballos, 1994). Similarly, U. bicarinatus adults and juveniles of Sceloporus melanorhinus could be confused; however, in our field experience the head of the latter is larger in relation to the body. To identify the age classes, we principally used 3 aspects of the individuals: the lizard species’ size; their coloration, which is frequently brighter in hatchlings and juveniles; and the coloration and thickness of hatchling and juvenile tails, which allows higher mobility to escape predators (Bauwens & Castilla, 1998; Novosolov et al., 2016).
It is well known that ectotherms are strongly affected by the climate. Furthermore, some species such as those of the genus Aspidoscelis are recognized as active foragers, which means a constant movement between sunny and shaded places looking for food or patrolling territories (Díaz de la Vega-Perez et al., 2013). Therefore, to investigate whether the lizards’ activity is related to temperature or humidity, we measured the microenvironmental temperature and humidity for each site (the 3 islands and the mainland) with 2 HOBO MX2301A wireless temperature/humidity data loggers (Onset®, Bourne, U.S) that were programmed to take readings every 15 min during the samplings (from 8h to 18h). Data loggers were placed next to the 200-m transects (near the middle of transect) during the surveys: 1 data logger in the sun and the other in the shade, which we used as an approximation of the microenvironmental temperature and humidity in which lizards were active. The data logger position (sun or shade) will be a variable referred to as the basking place.
To offer a general panorama of the lizards’ community on the islands and the mainland, we reported descriptive results about the lizard species recorded, their encounter frequency (count of individuals observed and reported every hour from 8h to 17h), and their age classes (adult, juvenile and hatchling). Because only Aspidoscelis communis and Aspidoscelis lineattissima were recorded on all study islands and on the mainland, these were used to estimate the effect of insularity on ecological aspects. Thus, we compared the encounter frequency of both lizard species among the islands and the mainland by applying a deviance analysis using a χ2 test with generalized linear models (GLMs) and a Poisson error distribution. The dependent variable was the encounter frequency and sites the independent (Cocinas, La Pajarera, San Agustín, and the mainland). In the case of overdispersion in the error term, the model was rescaled to make it more conservative (Crawley, 2007). In addition, we compared the occurrence of every age class for both Aspidoscelis species among sites using a contingency table with a G-test for independence. This analysis was accompanied by a correspondence analysis to estimate whether a significant relationship existed between the sites and the age classes, using the FactoMineR package in R (Lê et al., 2008).
We compared the temperature and humidity among sites (islands and the mainland) and between basking place (shaded or sunny) by performing deviance analyses, using a χ2 test with a GLM and Poisson error distribution. Sites and basking place were the independent variables, whereas temperature and humidity were the dependents. The models were also rescaled when overdispersion was detected. Both variables were previously transformed using the log10 for temperature and the arcsine of the square root for humidity; however, the transformed variables were not normally distributed.
The basking behavior in both species was compared among sites by means of a binomial analysis of deviance using χ2 test with GLMs, where the response variable was composed of 0 for shade and 1 for sun; lizards in mottled or half sun were not used in the analysis. Additionally, we performed a post hoc Tukey test for paired comparisons. We estimated the effect of the microclimate on the lizard species’ activity by means of polynomial regressions using GLMs, where the independent variables were the microenvironmental temperature and humidity of both basking places and the average of these for the schedules detailed above, whereas the number of individuals observed every hour was the dependent variable. For every site and species, 6 polynomial regressions (previously exploratory performed linear regressions did not fit our data) were performed (3 for temperature and 3 for humidity including shaded, sunny, and the average). This means a total of 48 polynomial regressions.
Finally, we compared the perch height of Aspidoscelis lizards among sites by means of a contingency table with a G-test for independence. We used a significance level of α = 0.05. All analyses were performed in RStudio v3.6.3 (R Core Team, 2020).
Results
Lizard occurrence and their age classes
Overall, we recorded a total of 533 individuals of 11 species and 5 families: 155 on Cocinas (3 species, 2 families), 89 on La Pajarera (5 species, 3 families), 151 on San Agustín (5 species, 4 families), and 138 on the mainland (6 species, 2 families) (Table 1). Of the 11 species recorded on the mainland, Norops nebulosus, Aspidoscelis communis, A. lineattissima, Ctenosaura pectinata, Hemidactylus frenatus, Iguana rhinolopha, Phyllodactylus rupinus, and Urosaurus bicarinatus were found on the islands (Table 1). Therefore, Holcosus miadis, Sceloporus melanorhinus, and Sceloporus utiformis were observed only on the mainland (Table 1). Only 2 species (Aspodoscelis communis and A. lineattissima) were recorded on the mainland and the 3 islands (Table 1). Taking into account all species, the highest encounter frequency of lizards was recorded on both the mainland and San Agustín Island; however, the species A. communis was the most frequently observed on the mainland with the species U. bicarinatus as the most frequently observed on San Agustín Island (Table 1). La Pajarera Island showed the lowest encounter frequency regarding all lizard species (Table 1).
Table 1 Encounter frequency (mean of individuals per hour ± standard error) of lizard species on 3 islands off and the mainland of the coast of Jalisco, Mexico.
| Species | Cocinas | Mainland | La Pajarera | San Agustín |
|---|---|---|---|---|
| Aspidoscelis communis | 4.8 ± 2.5 | 8 ± 3.0 | 3.9 ± 2.0 | 0.1 ± 0.1 |
| Aspidoscelis lineattissima | 5 ± 2.0 | 3.2 ± 1.5 | 4.2 ± 1.7 | 0.8 ± 0.3 |
| Ctenosaura pectinata | 0.1 ± 0.1 | |||
| Hemidactylus frenatus | 0.3 ± 0.1 | |||
| Holcosus miadis | 0.3 ± 0.1 | |||
| Iguana rhinolopha | 0.4 ± 0.2 | |||
| Norops nebulosus | 0.8 ± 0.2 | |||
| Phyllodactylus rupinus | 0.2 ± 0.1 | 0.2 ± 0.1 | ||
| Sceloporus melanorhinus | 0.4 ± 0.2 | |||
| Sceloporus utiformis | 1.8 ± 0.7 | |||
| Urosaurus bicarinatus | 0.1 ± 0.1 | 11 ± 3.7 | ||
| Total | 11.5 ± 5.0 | 13.8 ± 4.7 | 8.8 ± 3.7 | 13.8 ± 4.1 |
Adults, juveniles, and hatchlings were observed in 9 of the 11 species recorded, whereas for the species A. undulata and C. pectinata only adults were observed (Fig. 2). The most predominant age class for the majority of species was adults, with the exception of N. nebulosus, S. melanorhinus, and S. utiformis. In the first 2 species, the predominant age classes were juveniles, whereas hatchlings were common in S. utiformis (Fig. 2).
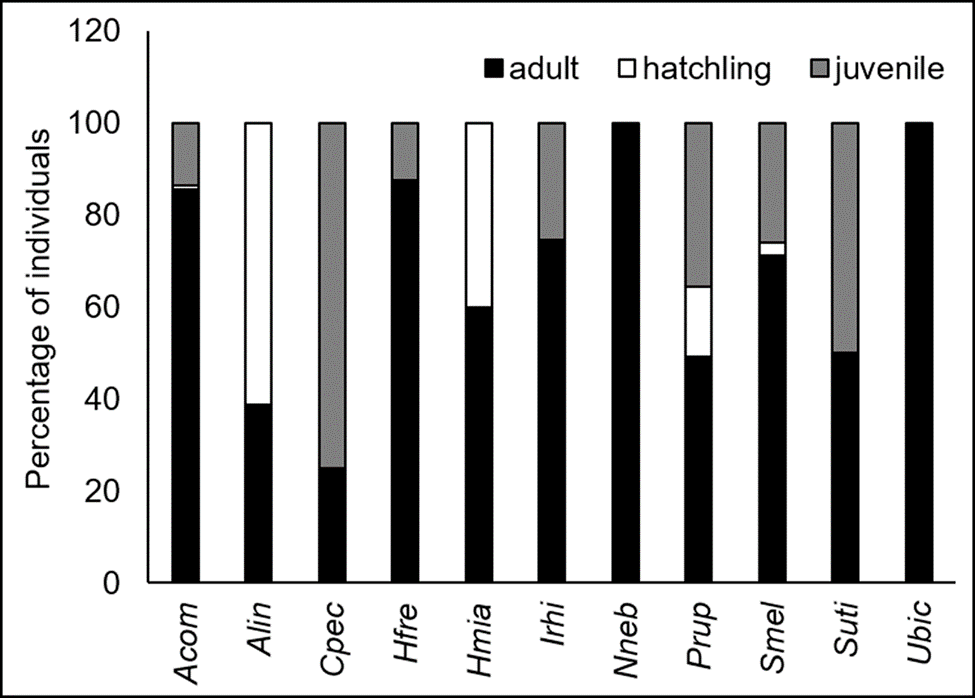
Figure 2 Percentage of individuals observed by age classes in the study area on the coast of Jalisco, Mexico. The species recorded were Aspidoscelis communis (Acom), Aspidoscelis lineattissima (Alin), Ctenosaura pectinata (Cpec), Hemidactylus frenatus (Hfre), Holcosus miadis (Hmia), Iguana rhinolopha (Irhi), Norops nebulosus (Nneb), Phyllodactylus rupinus (Prup), Sceloporus melanorhinus (Smel), Sceloporus utiformis (Suti), and Urosaurus bicarinatus (Ubic).
Insularity in lizard ecology
A total of 173 A. communis (80 on the mainland, 53 on Cocinas Island, 39 on La Pajarera Island, and 1 on San Agustín Island) and 140 A. lineattissima (32 on the mainland, 56 on Cocinas Island, 42 on La Pajarera Island, and 10 on San Agustín Island) were recorded. Only the encounter frequency of A. communis differed among sites (χ2 = 2.2, df = 3, p = 0.004), being higher on the mainland than on San Agustín Island (Tukey’s HSD tests: p = 0.004; Fig. 3) and marginally higher on Cocinas than on La Pajarera Island (Tukey’s HSD tests: p = 0.05; Fig. 3).
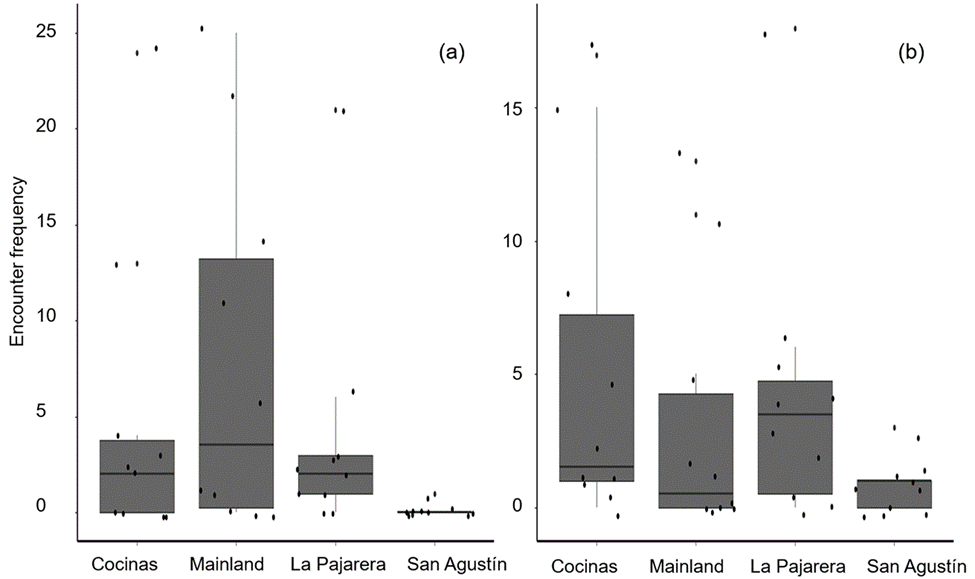
Figure 3 Encounter frequency in the lizards Aspidoscelis communis (a) and Aspidoscelis lineattissima (b) on 3 islands off and the mainland on the coast of Jalisco, Mexico. The horizontal line represents the median of the encounter frequency, the box indicates the first and third quartiles, and vertical lines indicate 1.5 times the interquartile range.
The occurrence of age classes significantly differed between sites for both A. communis (G-test = 31.3, df = 6, p < 0.001) and A. lineattissima (G-test = 37.7, df = 6, p < 0.001). In addition, the correspondence analysis indicates significant associations between sites and age classes for A. communis (χ2 = 29.85, p < 0.001) and A. lineattissima (χ2 = 32.67, p < 0.001). The pattern indicates a contrast between the islands (particularly Cocinas and La Pajarera) and the mainland, with more adults observed on the islands and more juveniles on the mainland (Fig. 4; Appendix 1).
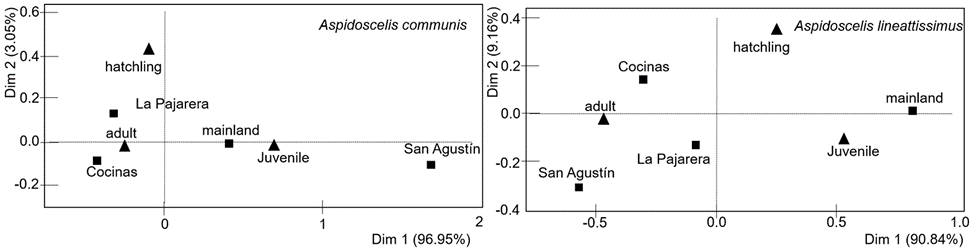
Figure 4 Correspondence analysis between sites (black squares) and age classes (black triangles) for the lizard species Aspidoscelis communis and Aspidoscelis lineattissima on 3 islands off and the mainland on the coast of Jalisco, Mexico.
Microenvironmental temperature differed among sites and between basking places (χ2 = 0.14, df = 3, p < 0.001). Particularly, the temperatures recorded in shaded places differed among sites (Tukey’s HSD tests: p < 0.001; Fig. 5). The temperature recorded in sunny places, however, did not differ among sites (Tukey’s HSD tests: p > 0.05; Fig. 5). Similarly, humidity differed among sites and between basking places (χ2 = 1.49, df = 3, p < 0.001). Paired comparisons indicated that humidity recorded under the shade differed among sites (Tukey’s HSD tests: p < 0.001), excluding the comparison between Cocinas and San Agustín (Tukey’s HSD tests: p > 0.05). The humidity recorded in sunny places did not differ among sites (Tukey’s HSD tests: p > 0.05; Fig. 5). Excluding the mainland, both temperature (Tukey’s HSD tests: p > 0.05) and humidity (Tukey’s HSD tests: p < 0.001) differed between shade and sun (Fig. 5).
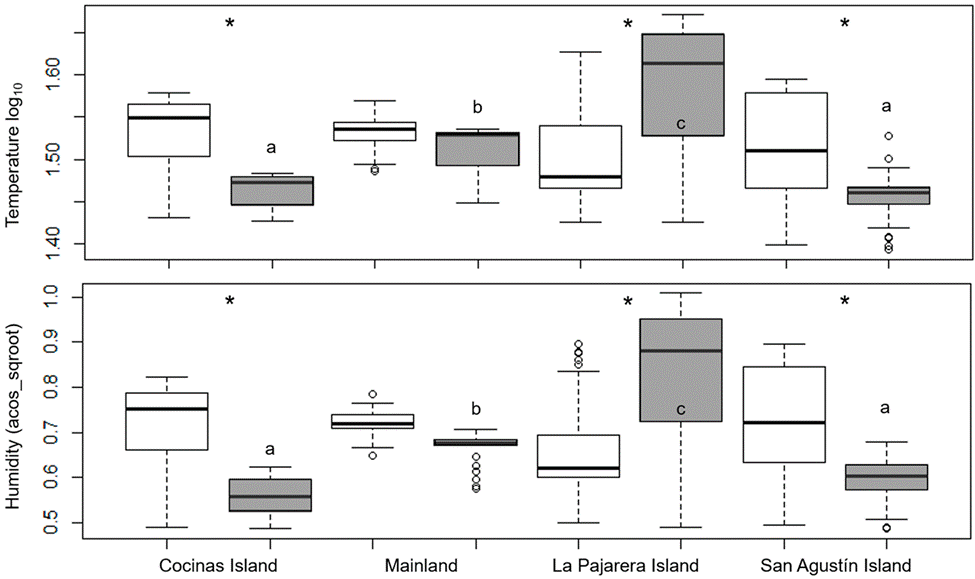
Figure 5 Microenvironmental temperature (upper panel) and humidity (lower panel) under the shade (gray boxes) and in sun (white boxes), for 3 islands off and the mainland of the coast of Jalisco, Mexico. Asterisks indicate differences between measurements taken in the shade and under the sun for each site. Letters indicate differences among sites, which only occur in the shade.
The basking behavior of A. communis and A. lineattissima did not differ among sites. Polynomic regressions showed, however, that the activity of these lizard species responds positively to intermediate temperatures and negatively to intermediate amounts of humidity throughout the day, particularly on Cocinas Island (Fig. 6).
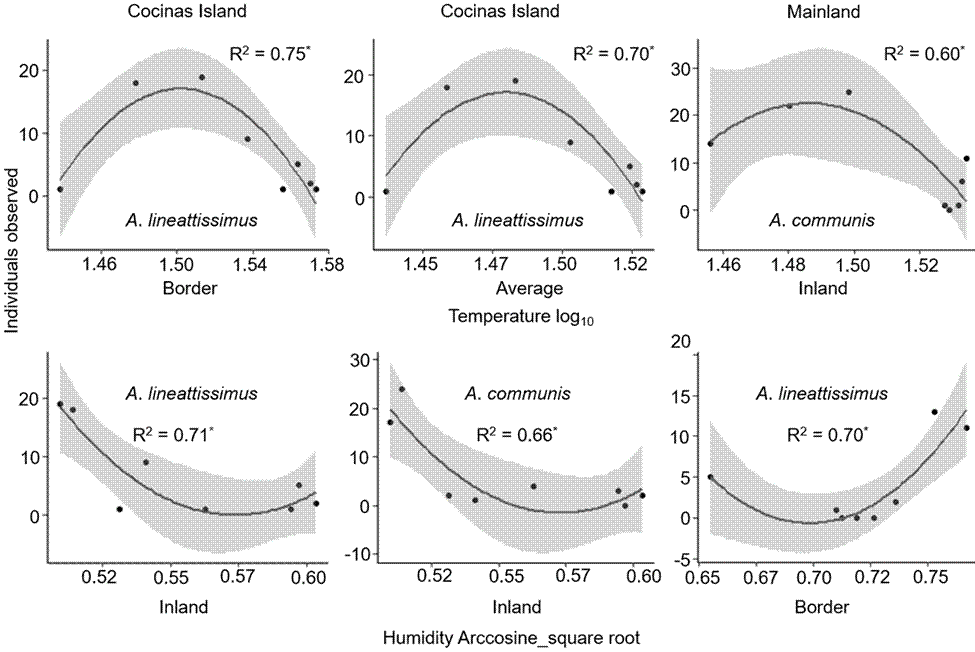
Figure 6 Relationship between the microenvironmental conditions (temperature and humidity) and the activity (number of individuals observed) of the lizards Aspidoscelis communis and Aspidoscelis lineattissima on Cocinas Island and the mainland (n = 8 for each relationship). The coefficient value of the polynomial regression and its significance is indicated (* indicates a p < 0.05). The gray band indicates 95% confidence interval.
The perch height of A. communis (G-test = 37.6, df = 6, p < 0.001) and A. lineattissima (G-test = 17.7, df = 6, p = 0.006) differed among sites. In addition, correspondence analysis indicates associations between sites and perch height for both A. communis (χ2 = 13.25, p = 0.039) and A. lineattissima (χ2 = 37.8, p < 0.001). The pattern indicates that these 2 terrestrial species used intermediate and high perches on the islands, particularly on Cocinas and La Pajarera, whereas on the mainland they were exclusively observed on low perches (Fig. 7; Appendix 2).
Discussion
The 3 studied islands cover a total area of 55.72 ha, representing 0.42% of the area of the Chamela-Cuixmala Biosphere Reserve (García & Ceballos, 1994; Conanp, 2008). Thus, the islands contribute widely to regional herpetofauna biodiversity since they maintain 40% of the lizard genera and 36% of the lizard species recorded on the coast of Jalisco, including 4 species protected by the Mexican law (NOM-059-SEMARNAT-2010) (Semarnat, 2010). Lizard species observed on the mainland had an encounter frequency from 0.1 to 8 lizards per hectare, so species not observed in that site during the present study but recorded in previous regional studies could have a lower encounter frequency. Conversely, they could inhabit a different vegetation type than the tropical dry forest, where our surveys were made (García & Ceballos, 1994). In addition, after 2 hurricanes (Jova in 2011, and Patricia in 2015), the occurrence of some lizard species decreased, negatively affecting the encounter frequency (Suazo-Ortuño et al., 2018). On the islands, encounter frequency indicated that there are 3 dominant species. For example, on Cocinas and La Pajarera, the species most frequently observed were A. communis and A. lineattissima, whereas on San Agustín Island the most frequently observed was U. bicarinatus. A common pattern of the 3 lizard species is their affinity or tolerance for high temperatures (García, 2008; Navarro-García et al., 2008), which could explain their success (high encounter frequency) on the study islands that possess a more open canopy in comparison with the tropical dry forest of the mainland, which allows the increase of microenvironmental temperatures (Conanp, 2008; De Frenne et al., 2013). Furthermore, the fact that San Agustín Island is covered by tropical dry forest would explain the higher encounter frequency of arboreal lizards such as U. bicarinatus, whereas on the islands of Cocinas and La Pajarera this vegetation type is rarely represented. Thus, the encounter frequency of terrestrial species is higher (Conanp, 2008).
Our surveys were made during November and from March to May, which correspond to the end of the rainy season when reproduction occurs and during the dry season when food resources are low (Bullock, 1986; Lister & García, 1992). The surveys in these 2 periods allowed us to record adults, juveniles, and hatchlings in every site; however, for some species (with very few individuals recorded) only adults were observed, which does not necessarily indicate that juveniles or hatchlings were nor present on the islands. It is well known that on islands intraspecific competition plays an important role in population structure. In fact, cases of cannibalism have been reported; therefore, juveniles and hatchlings must possess behavioral strategies to avoid competition with adults (Donihue et al., 2016; Mateo & Pleguezuelos, 2015). On the other hand, it is also indicated that insularity may modify the reproductive traits of insular lizards (Novosolov & Meiri, 2013; Novosolov et al., 2013).
Comparison of the lizards’ ecology among islands and the mainland
Aspidoscelis communis and A. lineattissima were recorded from all sites. Contrary to our expectations, these species demonstrated similar encounter frequency among sites, with the exception of A. communis on San Agustín Island. Thus, encounter frequency recorded in the 2 species did not fit with the density compensation (Case, 1975), which could result from a low food supply that several insular systems show (Valido & Olesen, 2007; Van Damme, 1999). The low encounter frequency recorded on San Agustín Island might be caused by less than optimal temperatures for this lizard species. Aspidoscelis lizards have been linked to high environmental temperatures, whereas the island is covered by tropical dry forest, and, according to our results, microenvironmental temperature is significantly reduced under the shade produced by the vegetation on the islands (Güizado-Rodríguez et al., 2014; Navarro-García et al., 2008).
The contrasting age classes recorded between the islands and the mainland were unexpected because of surveys being conducted in the same time periods for all study sites. Two hypotheses could be proposed for this result. Insular lizard populations may change their activity patterns compared with their mainland counterparts (Floyd & Jenssen, 1983; Siliceo-Cantero & García, 2015). This could lead adult insular lizards to be active for a longer period of time compared with the population on the mainland where more juveniles and hatchlings were observed as active. This longer period of activity of adult lizards could increase the intraspecific competition that often results in cannibalism, leading hatchlings and juveniles to change their habitat use to avoid competition (Cooper et al., 2015; Delaney & Warner, 2017; Donihue et al., 2016). Our detection of this could decrease during the surveys. On San Agustín Island, there were more observed juveniles of A. communis than expected, but this might be because only 1 juvenile individual of this species was recorded. On the other hand, several studies indicate that reproductive traits may diverge in time of occurrence between insular and mainland populations, which is attributed to different selection pressures such as low predation rates (Novosolov & Meiri, 2013; Novosolov et al., 2013). Thus, our result might suggest that insularity modifies reproductive traits in generalist species such as A. communis and A. lineattissima when reproduction occurs; however, more detailed research must be done to confirm this finding.
In the present study, microclimate characteristics offered for ectothermic species such as A. communis and A. lineattissima differed among islands and the mainland, particularly in shaded places in the function of the vegetation cover that also differs among sites (Conanp, 2008; De Frenne et al., 2013); however, the basking behavior of both species was not affected by such microclimate differences. A plausible explanation is that lizards of this genus are recognized as heliothermic and active foragers, which means a frequent movement between shaded and sunny places while searching for prey (Díaz de la Vega- Perez et al., 2013; Granados-González et al., 2020). This habit clearly could homogenize the basking behavior of A. communis and A. lineattissima among the islands and the mainland and could also be an advantage that allows them to thrive on the study islands.
Our results agree with several studies on lizards of the Aspidoscelis genus that documented the relationship between environmental and corporal temperature (Güizado-Rodriguez & Casas-Andreu, 2007; Güizado-Rodríguez et al., 2014). In our study, lizards of both species showed higher activity at intermediate levels of temperature and low activity at intermediate humidity amounts. The activity temperatures of these lizard species (29 to 33 °C) agree with other studies on several Aspidoscelis species (30 to 35 °C; Díaz de la Vega-Perez et al., 2013). Only on Cocinas Island and the mainland we found a relationship between microclimate and recorded lizard activity, suggesting high thermal acclimation and/or plasticity (Güizado-Rodriguez & Casas-Andreu, 2007). The temperatures range (e.g., 27.6 to 47.2 °C in A. lineattissima) would allow higher invasion success of new habitats, such as the study islands (Ceballos et al., 2015; Muñoz et al., 2016; Olsson et al., 2018; Riddle, 2018; Walkup et al., 2017).
In addition to physical characteristics such as temperature and humidity, ecological factors like predation or competition play an important role in the ecological behavior of lizard species. In the study area, a recent study indicates that interspecific competition and predation are less intense in insular lizards than mainland ones, whereas intraspecific competition plays an important role as an ecological driver in insular lizard ecology (Siliceo-Cantero et al., 2017). Our results indicate that few species compose lizard communities on the studied islands, which is a recognized factor that allows ecological release and niche expansion (Campbell-Staton et al., 2016; Velasco et al., 2019). The lack of several arboreal and semi-arboreal species on the islands that are present in the TDF of Chamela-Cuixmala Biosphere Reserve allows the use of these kinds of perches by other species, such as A. communis and A. lineattissima, which are abundant on the islands. The fact that these species used low, intermediate, and high perches on the islands, whereas on the mainland they exclusively used low perches, suggests niche expansion related to perch height.
The different perch axes (height, texture, location, etc.) are important resources on islands that often lack resources (Valido & Olesen, 2007; Van Damme, 1999). Perch heights on the islands might provide thermoregulatory and feeding advantages for Aspidoscelis lizards since the sunlight hits the canopy better than the ground and allows better surveillance for searching for prey (Lister & García, 1992; Lopez-Darias et al., 2012). These advantages clearly have important effects on lizards’ fitness, since dominant individuals would use high perches. Perch use and morphology have been recognized as key drivers in evolutionary divergence in insular lizard species (Losos, 2009). In fact, they could be responsible for differences found in morphology between insular and mainland populations of A. lineattissima (Hernández-Salinas et al., 2014).
Studies on island lizard ecology indicate that insularity may imply an increase in population abundance and changes in reproductive traits (Itescu et al., 2017; Novosolov & Meiri, 2013; Novosolov et al., 2016). Ecological aspects, such as thermoregulatory behavior, activity, and the perch height of insular lizard species, may also differ from their mainland counterparts (Irschick et al., 1997; Sagonas et al., 2013; Siliceo-Cantero & García, 2015). The response of generalist lizard species such as A. communis and A. lineattissima to insularity did not support the density compensation theory since no higher encounter frequency (a variable indirectly related to population abundance) was observed on the islands as compared with the mainland (Case, 1975; Novosolov et al., 2016). Results in perch height support the niche expansion theory and are helped perhaps by the generalist habits of both species (Bolnick et al., 2010; Losos & Queiroz, 1997). Thus, insularity not only morphologically and genetically enriches biodiversity but also ecologically, which is where we expect that our results contributed to the current knowledge.
It is concluded that 3 islands with a total area of 551,200 m2 are capable of maintaining one-third of the lizard species inhabiting the coast of Jalisco, Mexico, contributing to the conservation of at least 4 lizard species protected by Mexican law. The islands individually support a range of 2 to 3 lizard species and share few among them, which highlights the importance of protecting the insular complex as a whole.
The islands provide different microclimate conditions for the lizard species inhabiting them. The lizard species A. communis and A. lineattissima are successful colonizers of the studied islands since they presented high encounter frequencies and were present in every site. These species showed no changes in their basking behavior among islands and the mainland, but their activity may be affected by microclimate conditions. These features are related to the generalist habits and the preference for hotter sites in both lizard species, which allow favoring the establishment in new environments such as the insular systems.
Our results showed signs of change in the phenology of the reproductive season of these lizard species because of insularity and a clear expansion of the perch height on insular systems. Therefore, our study contributes to the ecological knowledge of the lizard community and to support ecological theories such as niche expansion caused by ecological release on islands. The expansion of the perch height used by terrestrial lizard species on the study islands is a particular example of it. In addition, this study contributes to strengthening the perception of the islands as biological reservoirs since lizards could be considered different ecological species compared with their mainland counterparts.











 nova página do texto(beta)
nova página do texto(beta)



