Introduction
Myiasis, or infestations by dipterous larvae that feed on vertebrate tissues (Otranto, 2001), is a usual phenomenon in birds (Sabrosky et al., 1989; Dudaniec & Kleindorfer, 2006). It is most frequently caused by hematophagous larvae that are obligate feeders on nestling birds and include members of the families Calliphoridae (Protocalliphora), Muscidae (Philornis, Passeromyia, Mydaea), and Neottiophilidae (Neottiophilum, Actinoptera), of which Philornis is present in the Neotropics (Little, 2008).
The genus Philornis encompasses near 50 species that may be host-generalist or specialists (Löwenberg-Neto, 2008). With 3 types of larval habits (coprophagous, semi hematophagous, and subcutaneous; Dudaniec & Kleindorfer, 2006), infestation by Philornis larvae can affect nestlings differently, even leading to death (McNew & Clayton, 2018; Hayes et al., 2019). Detrimental consequences detected in infested nestlings include reduced hemoglobin and hematocrit levels (Dudaniec et al., 2006; Manzoli et al., 2018), lower growth rates and body mass (Fessl et al., 2006; Norris et al., 2010; Segura & Reboreda, 2011; Segura & Palacio, 2021), reduced feather and tarsus length (Koop et al., 2011), and decreased future reproduction (McNew et al., 2020). Whereas nestling birds seem the primary hosts of Philornis, parasitism of adult birds seems to be opportunistic and is more likely to occur in adults that spend long periods in the nest (Teixeira, 1999; Dudaniec & Kleindorfer, 2006).
The impact of parasitism by Philornis on nestlings is of particular concern in most already threatened bird species (Bulgarella et al., 2019). In the Galapagos islands, the invasive Philornis downsi is known to severely affect all 14 Darwin's finch species (Kleindorfer & Dudaniec, 2016). In continental Ecuador, Philornis falsificus and P. obscurus were found infesting nestlings in Guayas province, while P. grandis was recorded in nests from Pastaza and Napo provinces, in the Amazon basin (records compiled in Löwenberg-Neto & De Carvalho, 2013). In 2015, Bulgarella et al. found P. downsi larvae, along with pupal cases from other unidentified Philornis species in nests from Santa Elena and Guayas provinces. Finally, a Philornis larvae was found parasitizing a nestling of the Choco Screech-Owl (Megascops centralis) in Santa Elena province (Reyes & Astudillo-Sánchez, 2017). Thus, while over 145 studies have been carried on P. downsi (Quiroga et al., 2020), only 4 studies have addressed the presence of Philornis species in continental Ecuador. This situation reveals the notorious lack of information on parasitism by Philornis in continental Ecuador, where many bird species are of conservation concern (BirdLife International, 2021).
Our study is set at Bosque Protector Jerusalem (BPJ), the only track of protected Andean dry forest in the inter-Andean valleys of northern Ecuador. Between 2012 and 2014, we monitored the avian community at BPJ and surroundings, to generate a baseline for avian host-parasite dynamics in this poorly studied ecosystem. Here, we captured, inspected, and released adult birds with the next objectives: 1) detect parasitism by Philornis spp. in adult birds, 2) provide data on the prevalence of Philornis myiasis in adult birds, and 3) increase knowledge on the distribution and host range of the Philornis spp.
Materials and methods
Fieldwork was conducted at Bosque Protector Jerusalem (~10 km north of Quito; 00°00'05" N, 78°21'18" W; 2,300 m asl), a 1,110 ha protected dry forest, in the dry Andean valley of Guayllabamba, northern Ecuador. This area is located on a seasonal ecosystem, where the dry season extends from May to August (average annual rainfall of 125 mm), and the wet season extends from September to April (average annual rainfall of 360 mm) (Carvajal-Campos, 2009). Average annual temperature under shade is 19 °C (data for 2012-2013 provided by Instituto Nacional de Meteorología e Hidrología, INAMHI).
From December 2012 to June 2013, and February to August 2014, we placed 7 mist-nets (4 shelves; 12 m x 2.5 m; 36 mm mesh) for 72 h per month, for a total sampling effort of 1,008 h (or 72 h/net). We carefully inspected the body surface of captured birds for parasitism by Philornis spp. When found, subcutaneous larvae were removed from birds' bodies and stored in 70% ethanol. Bird taxonomy used in this study follows that of Remsen et al. (2021).
In October 2020, the collected larvae were molecularly identified by sequencing the second internal transcribed spacer region (ITS2) of the rRNA gene, a barcode for insects (García-Robledo et al., 2013). Genomic DNA was extracted from the whole larvae (1 per host) using a guanidine isothiocyanate protein precipitation, followed by isopropanol DNA precipitation protocol (Peñafiel et al., 2019). We amplified a portion of ITS2 using primers ITS2-LEcEn-F and ITS2-LEcEn-R (Monje et al., 2013). Amplification products were visualized in 2% agarose gel, and unincorporated primers and dNTPs were degraded using ExoSAP-IT PCR Product Cleanup Reagent (Applied Biosystems). Purified amplicons were sequenced with big-dye chemistry and PCR primers, using capillary electrophoresis in an ABI3730xl sequencer. Chromatograms were inspected in Geneious® 11.1.5 (Biomatters Ltd., Kearse et al., 2012). For some samples, we obtained good quality sequence readings for the forward DNA chain but not for the reverse; thus, we re-sequenced the forward chain to minimize the chance of sequencing errors.
To assess the identity of our larvae, we compared the sequences obtained to those available on GenBank (https://www.ncbi.nlm.nih.gov/genbank/) using BLAST (Basic Local Alignment Search Tool; https://blast.ncbi.nlm.nih.gov/Blast.cgi). To corroborate the identity of samples from a phylogenetic perspective, we performed a maximum likelihood (ML) analysis, as follows. We assembled a matrix with our novel sequences and those of Philornis (P. torquans, P. seguyi, Philornis sp. "Misiones", P. pici, P. porteri, and P. downsi) available on GenBank. All sequences from the same species and those obtained by us were aligned separately in Mafft (Katoh et al., 2019), using Auto alignment. Based on these preliminary alignments, we retained only the unique sequences (in terms of both nucleotide sequences and insertions/deletions). Given the presence of several ambiguous indels in ITS2 and the potential of these indels to generate very different tree topologies, we assembled 2 alignment matrices: one that contained the sequences more similar to our novel sequences (plus outgroup) and one that included sequences from all the species. Final alignments were also obtained with Mafft Auto feature. Using PartitionFinder 2 (Lanfear et al., 2016), we estimated the best-fit model of molecular evolution for both data matrices, which was the TIM + G model. For each dataset, we conducted a ML phylogenetic reconstruction as implemented in Garli 2.0 (Zwickl, 2006), using nst = 6 and gamma distribution (4 gamma categories); all other settings were left at default values. We ran 20 search replicates to obtain the ML tree, followed by 1,000 bootstrap replicates (1 search replicate each) to assess the nodal support for each clade.
Results
We sampled a total of1,429birds from 41 species. Among these, 15 individuals (14 adults and 1 immature) belonging to 6 species were infested by subcutaneous Philornis larvae. Parasitized species were: Troglodytes aedon (Troglodytidae), Conirostrum cinereum (Thraupidae), Geospizopsis plebejus (Thraupidae), Zonotrichia capensis (Passerellidae), Pheucticus chrysogaster (Cardinalidae), and Spinus magellanicus (Fringillidae) (Table 1; Fig. 1). The prevalence of myiasis by Philornis per bird species ranged from 0.9% (G. plebejus) to 6.9% (T. aedon), and intensity of parasitism ranged from 1 to 12 larvae (Table 1). Birds' abdomen was the area where more larvae were found (Table 1). The complete list of species and individuals examined in this study is provided in Appendix 1. Other individuals for which we found traces (i.e., rounded wounds in the skin) of likely myiasis are summarized in Appendix 2.
Table 1 Birds showing Philornis myiasis at Bosque Protector Jerusalem. Specific ID is only provided for larvae identified molecularly.
| Species | Field number |
Age | Date | Observations | Prevalence | ID (haplotype) |
|---|---|---|---|---|---|---|
|
Troglodytes aedon |
HFC-745 |
Adult |
27 Apr 2013 |
1 in chest |
6.9% (2/29) |
P. torquans complex (A) |
|
|
HFC-859 |
Immature |
7 Jun 2013 |
1 in abdomen |
|
Philornis sp. |
|
Conirostrum cinereum |
HFC-121 |
Adult |
16 Dec 2012 |
1 in abdomen, bird showing incubation patch |
3.6% (1/28) |
Philornis sp. |
|
Geospizopsis plebejus |
HFC-386 |
Adult |
2 Feb 2013 |
1 in forehead; bird first captured on 2 Dec 2012 with no trace of infestation |
0.9% (1/107) |
Philornis sp. |
| |
HFC-10761 |
Adult |
19 Mar 2014 |
1 in chest, 5 in abdomen |
|
P. torquans complex (A) |
|
Zonotrichia capensis |
HFC-188 |
Adult |
27 Dec 2012 |
1 in abdomen, bird showing incubation patch |
2.6% (8/303) |
Philornis sp. |
| |
HFC-444 |
Adult |
10 Feb 2013 |
2 in abdomen; bird first captured on 12 Jan 2013 with no trace of infestation, showing incubation patch |
|
Philornis sp. |
| |
HFC-698 |
Adult |
14 Apr 13 |
12 in abdomen |
|
P. torquans complex (A) |
| |
HFC-736 |
Adult |
21 Apr 2013 |
4 in abdomen |
|
P. torquans complex (A) |
| |
HFC-904 |
Adult |
19 Feb 2014 |
4 in abdomen |
|
P. torquans complex (B) |
| |
HFC-1018 |
Adult |
11 Mar 2014 |
Near cloaca |
|
Philornis sp. |
| |
No number |
Adult |
4 May 2014 |
Larvae above cloaca; traces of former incubation patch |
|
Philornis sp. |
| |
HFC-1392 |
Adult |
6 Jun 2014 |
in abdomen |
|
P. torquans complex (A) |
|
Pheucticus chrysogaster |
HFC-248 |
Adult |
11 Jan 2013 |
1 in abdomen |
5.3% (1/19) |
Philornis sp. |
|
Spinus magellanicus |
HFC-226 | Adult | 10 Jan 2013 | 4 in abdomen | 1.2% (1/82) | Philornis sp. |
1 Captured 3.5 km outside Bosque Protector Jerusalem. Not included in the calculation of prevalence.
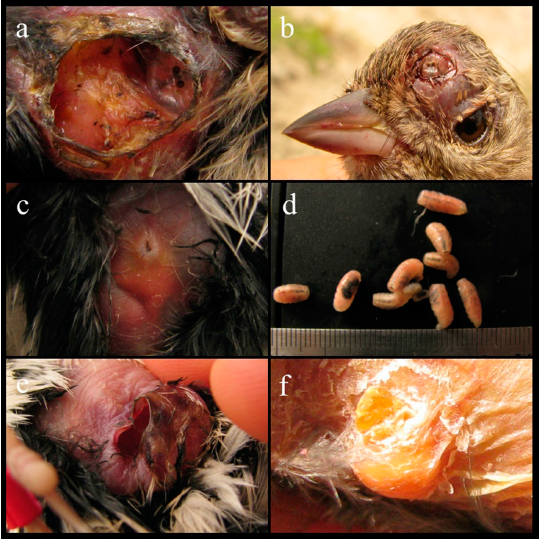
Figure 1 Philornis torquans larvae parasitizing: a) abdomen of Conirostrum cinereum HFC-121, larval orifice that still harbors a larva; b) Geospizopsis plebejus HFC-386; c) abdomen of Zonotrichia capensis HFC-188; d) larvae extracted from abdomen of Z. capensis HFC-698 and e) orifice after extraction, f) dead larvae exposed on the belly of Pheucticus chrysogaster HFC-248.
We extracted, amplified, and sequenced DNA from larvae found in 14 individuals. However, the DNA in the larvae of 8 individuals was degraded because their preserving ethanol evaporated during at least part of the storage period. Thus, we were able to obtain ITS2 sequences for larvae found on 6 individuals: 1 T. aedon, 1 G. plebejus, and 4 Z. capensis (GenBank accession numbers MW853826- MW853831). From the sequences of these 6 larvae, we obtained 2 unique haplotypes, A and B (different by 1 indel; Table 1) identified by BLAST as belonging to P. torquans. Also, phylogenetically, they were placed within the P. torquans clade (Fig. 2A, B), which was sister to the only available haplotype of P. seguyi in the reduced-dataset tree (Fig. 2A). In that same tree, Philornis sp. "Misiones" showed as sister to P. seguyi + P. torquans. We also found a few mostly unambiguous indels (Fig. 3) that may prove phylogenetically informative in the future, when more extensive haplotype sampling is available. The sequences obtained in this study allowed us to identify the sampled larvae as part of the P. torquans complex that includes both P. torquans and P. seguyi (Quiroga et al., 2016; see Discussion).
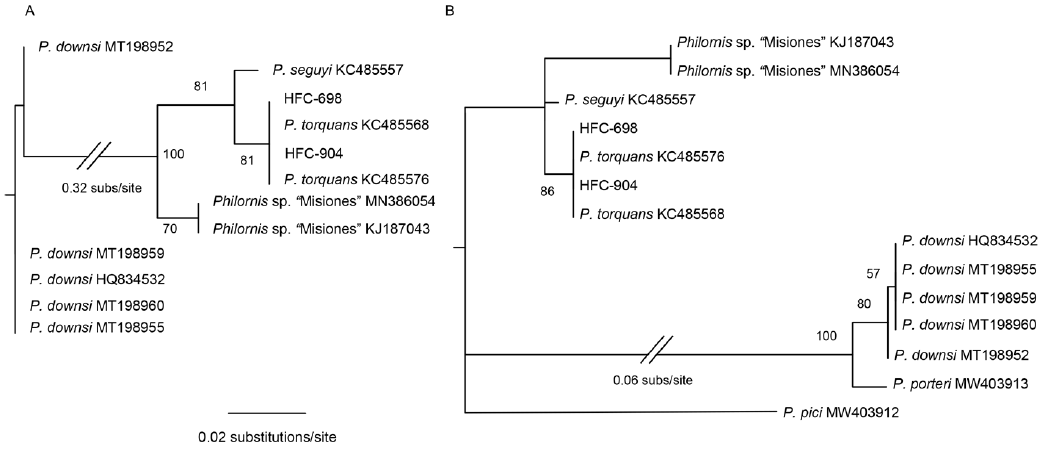
Figure 2 Phylogenetic reconstructions by maximum likelihood showing the position of our novel sequences in the context of the unique haplotypes of other Philornis species available on GenBank. A, Shows most closely related sequences, with P. downsi as outgroup; B, shows all Philornis included in this study.

Figure 3 Indels found in the alignment of P. seguyi, P. torquans, Philornis sp. "Misiones" and our novel samples; upper numbers indicate sequence position with respect to P. torquans ITS2 sequence KC485568. A, 2 different indels in Philornis sp. "Misiones"; B, 2 synapomorphic (?) indels in Philornis sp. "Misiones" and others in P. torquans and P. seguyi; C, 2 synapomorphic (?) indels in Philornis sp. "Misiones"; D, 2 synapomorphic (?) indels in Philornis sp. "Misiones" and others in P. torquans and P. seguyi; sequences of HFC-698 and 904 ended at position 408.
Discussion
We report, for the first time in continental Ecuador, parasitism by Philornis in adult birds of 6 species of passerine birds (T. aedon, C. cinereum, G. plebejus, Z. capensis, P. chrysogaster, and S. magellanicus). There are records Philornis myiasis in nestlings of T. aedon (Young, 1993; Quiroga & Reboreda, 2012), Z. capensis, and S. magellanicus (Salvador & Bodrati, 2013) in other latitudes (Costa Rica, Argentina), but never in adult birds. Although Philornis infecting non-nestlings is a rare event, there are at least 15 additional bird species for which adult infestation, by at least 5 species of Philornis, have been documented (Huber et al., 2010; Quiroga et al., 2020). The infestation prevalences found herein are close to the ranges summarized in Quiroga et al. (2020), when considering species with 19 or more individuals analyzed (i.e., 0.2-8.75%), except for the high prevalence shown by Margarops fuscatus (31%; Arendt, 1985).
Although parasitism by Philornis in adults may be more common than previously thought (Quiroga et al., 2020), nestlings seem to be more susceptible than mature birds, as they stay for a longer time in the nest and are less mobile, and then more vulnerable to infestation (Herrera & Bermúdez, 2012). In our study, at least 4 individuals showed myiasis in the abdomen with current or traces of former incubation patch and in the forehead and near the cloaca, areas that are usually less protected by contour feathers. These surfaces may be more easily reached by Philornis larvae that emerge from eggs laid in nest material (Quiroga et al., 2020).
Philornis torquans was formerly reported in Argentina and Brazil (Nielsen, 1912; Löwenberg-Neto & De Carvalho, 2013), but now it is considered a complex of cryptic species from southern South America named the P. torquans complex (Quiroga et al., 2016). Here, we report the P. torquans complex in Ecuador for the first time, broadening its distribution range. This complex has been previously found affecting 34 species of birds, including T. aedon and Z. capensis (Cuervo et al., 2020). Thus, our findings expand the host range of the P. torquans complex by including a new host: G. plebejus. Furthermore, 3 other bird species -C. cinereum, P. chrysogaster, and S. magellanicus- also presented myiasis by Philornis in the same locality. The presence of the P. torquans complex along the Central and Northern Andes was predicted by the abiotic niche model of Cuervo et al. (2020). While this model was developed to predict the distribution of the P. torquans complex in southern South America, the results suggested (with some uncertainty) that this complex of cryptic species may be present in our study area. The finding of the P. torquans complex in the Andean dry forest of northern Ecuador represents the first independent confirmation of this model. This result is also consistent with those of Percara et al. (in press) who reported the P. torquans complex covering heterogeneous habitats and environmental conditions (average mean temperature 10-28 °C, mean rainfall 100-2,000 mm/year), thus encompassing the environmental conditions present at BPJ.
Few field studies have focused on the prevalence of Philornis in both adults and nestlings. To our knowledge, the most extensive research is that of Arendt (1985) on Margarops fuscatus, in Puerto Rico. In that study, adult prevalence ranged 0-92%, depending on the month of the year, whereas nestling prevalence ranged 81-100% for the same site and time period. Thus, the presence of myiasis in adults at this Andean dry forest may signal a broader Philornis infestation in the nestlings of those species (Texeira, 1999) and, likely, the nestlings of other species. Moreover, in this seasonally dry forest, infected adults may act as reservoirs for the parasites, allowing them to maintain viable parasite populations year-round. Although our sampling of the area was designed to cover the diversity of micro habitats in this dry forest, year-round sampling, including the inspection of nests and adults, is needed to support this hypothesis.
Bird populations living in this Andean dry valley are experiencing continuous landscape transformation (Aguirre et al., 2006), the presence of avian malaria parasites in high prevalence and intensity (Cadena et al., 2019), the likely expansion of the Shiny Cowbird, Molothrus bonariensis (Medrano-Vizcaíno et al., 2020; J.F. Freile pers. comm.), and probably, the non-documented effects of global climate change. Thus, they may be exposed to the synergistic effects of these factors and Philornis infestations, which are likely increasing the pressures on their reproductive effort. For these reasons, for a broader perspective on the ecology of this community and other communities along the dry Andean valleys, future studies should focus on studying how Philornis infestation, habitat degradation, malaria and cowbird parasitism, climate change, and their interactions may be affecting bird populations.











 nueva página del texto (beta)
nueva página del texto (beta)


