Introduction
During the first three-quarters of the last century, poaching drove the American crocodile, Crocodylus acutus (Reptilia: Crocodilidae), to the brink of extinction in Costa Rica (Sandoval et al., 2019, 2020). As a result, in 1992 the government enacted the law 7317 (Wildlife Conservation Law) aimed to protect the wildlife species, besides the crocodile is declared as vulnerable in the IUCN Red List, is on the II appendix of CITES and is declared endangered in Costa Rica´s Wildlife´s Conservation of Law (7317) (Sandoval et al., 2018). Studies of the last 2 decades indicate the application of the law 7317 was successful, and crocodile populations have increased (Bolaños, 2012a, b; Orozco, 2015; Sánchez-Ramírez, 2001; Sandoval et al., 2017; Valdelomar et al., 2012). In northwestern Costa Rica, populations of C. acutus are concentrated primarily in the coastal rivers of the Central Pacific (Abadía et al., 1998; Escobedo, 2005; Motte, 1994; Piedra, 2000; Porras, 2004; Sasa & Chaves, 1992; Torrealba et al., 1992) and in the North Pacific zone in the Tempisque Great Wetlands (Barrantes, 2008; Bolaños, 2012b; Mauger et al., 2012; Murray et al., 2015 Orozco, 2015; Peraza, 2015; Sánchez et al., 1996; Sánchez-Ramírez, 2001).
In Costa Rica, since the beginning of the nineties, a greater presence of crocodiles was identified in their distribution areas, as well as an increase in incidents against humans in the Northwestern and Central Pacific (Bolaños, 2012a; Porras et al., 2020; Sandoval et al., 2019). Many of these incidents have a direct relationship of humans invading the crocodile’s habitat in the study areas (Bolaños, 2012b, Porras et al., 2020). Currently, these interactions persist due to the extensive development of anthropic activities associated with roads, human population growth, agricultural activities in areas or margins of rivers, estuaries, and bodies of water. These activities have generated the alteration of the natural environment affecting the habitat of the species and enhancing the interaction with humans (Sandoval et al., 2019, 2020).
These zones have also registered a large increase in the human population, and the growth is associated with increases in agricultural conversion, urbanization, development of tourism and substantial improvements in road infrastructure (Barrantes, 2013; Bartels, 2012; Carrillo, 2013; Gisolf, 2018; Morales-Zúñiga, 2011; Sandoval et al., 2017, 2019, 2020). The changes in land use caused by these factors may lead to increases in landscape fragmentation and isolation of forest patches (Guzmán & Vega, 2015; Martínez-Salinas, 2008; Sandoval & Castillo, 2011; Sierra, 2016; Troche & Guarachi, 2001). However, since 1990, forest coverage has recovered, increasing to 52.4% of the country’s total area in 2015 (Camacho, 2015).
In this matter, landscape indexes allow to discriminate vegetation types, evaluate habitat heterogeneity conditions, besides habitat isolation, fragmentation, and complexity, which can give an idea of ecological conditions. Assessing the landscape, fragmentation and connectivity patterns may be a priority to ensure the maintenance of species and ecosystems. These tools, provide effective representations of the landscape structure, which could give significant information for improving conservation planning (Miranda et al., 2018; Rodríguez, 2018).
In the Costa Rica´s Central Pacific, crocodiles are associated with the presence of bodies of water, a high density of the drainage network and the presence of mangroves and estuaries, in altitudes less than 240 m asl (Sandoval et al., 2020). In addition to the drainage network (rivers, wetlands, and flood zones), the increase in forest coverage may increase the habitat available for crocodiles and thereby increase their abundance (Bolaños et al., 1997; Bolaños, 2012a; Sánchez-Ramírez, 2001), therefore, a landscape analysis for the American crocodile was conducted in northwestern Costa Rica to determine which landscape variables could benefit crocodile populations growth.
Materials and methods
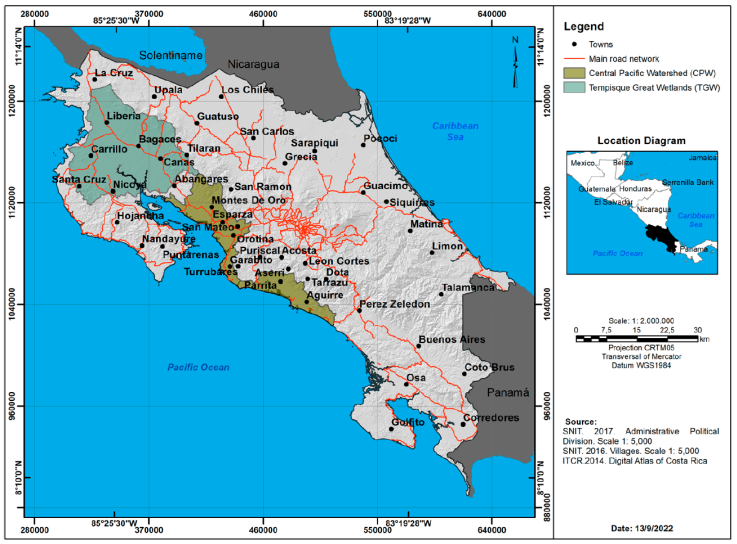
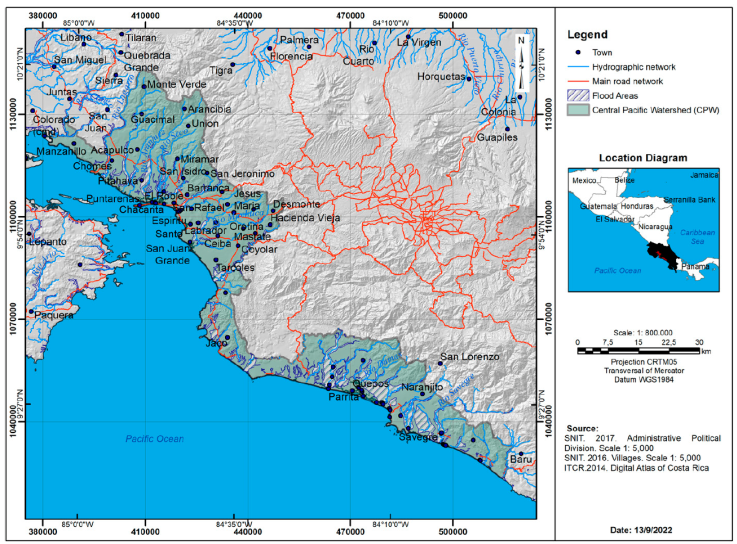
The study included the 2 areas with the most numerous crocodile populations in Costa Rica; the Tempisque Great Wetlands (TGW:10°12’00” N, 85°14’00” W) in the northwest sector of the Costa Rican North Pacific zone, in the inner region of the Nicoya Peninsula in Guanacaste Province, and the Central Pacific (CP: 94°47’00” N, 84°38’00” W) which is located south of the TGW and drains west into the Pacific Ocean (Fig. 1). Overall, the study area encompassed 9,815.32 km2. The area in the TGW was 5,381.89 km2, with 93% below 600 m asl, which is the limit of crocodile distribution in the Costa Rican Pacific area (Sandoval et al., 2019, 2020). The area in the CP was 4,433.43 km2, with 100% below 600 m asl. The CP has the second largest crocodile population in Costa Rica.
To analyze crocodile habitat, we followed the methodology proposed by Sandoval et al. (2019, 2020), consisting in an identification of zones of crocodile distribution, besides we integrated physical-geographic variables through Geographic Information Systems (GIS). In this way, a theoretical compilation was carried out to identify the physical-geographical variables that make up the crocodile’s habitat as well as the socio-environmental variables that influence the alteration and loss of habitat of the species, the variables selected were rivers, towns, wetlands, roads and highways, flood areas, and forest coverage. The databases used are compiled in the 2014 Costa Rican atlas (Ortiz et al., 2014). The programs QGIS 2.8.1 (QGIS Development Team) and ArcGis 10.5 (ESRI) were used for the landscape analysis. QGIS was used to create polygons of the TGW, including the Tempisque and Bebedero river watersheds, and the CP, including territory between the communities of Puntarenas and Quepos. In the polygons, several layers were generated from digital geographic databases of rivers (scale 1:200,000, 1:50,000), towns (all localities, villages, cities), wetlands, roads and highways, flood areas, and forest coverage (1997, 2000, 2005). We used additional layers of forest coverage for 2010 year from the digital database of the Fondo Nacional de Financiamiento Forestal (FONAFIFO) and for 2013 obtained from the digital database of the Sistema Nacional de Áreas de Conservación (SINAC).
We created new databases to estimate the area occupied by wetlands and flood zones, river density, and the number of towns, as well as road and highway networks in the Tempisque Great Wetlands (Fig. 2) and in the Central Pacific (Fig. 3). The area at or below 600 m asl was calculated, which is the maximum altitude inhabited by the American crocodile in Costa Rica (Sandoval et al., 2019, 2020). ArcGis was used to calculate the percentage of forest coverage under the 600 m asl in 1997, 2000, 2005, 2010, and 2013 in both sites (using the abovementioned databases). To quantify changes in the study area, forest cover change (km2) in the study area from 2000 to 2013 was calculated. Besides ArcGis (Patch anayst), we calculated landscape indexes to quantify forest fragmentation: shape complexity, Shape Index (SI): 1 when patches are completely circular or square and increases their value when moving away from geometric shapes. Fractal Dimension Index (FD): this index varies between 1 for Euclidean shapes and 2 fractal shapes. It is a quantitative measure of landscape complexity (Turner, 1989), also scale invariant and statistically robust (Ripple et al., 1991), which is why it is considered the most ad hoc descriptor to quantify the fragmentation of the different types of landscape (Rau & Gantz, 2001). Patch diversity, Shannon Diversity Index (SDI): it is 0 when the landscape contains only one ecosystem (there is no diversity), the SDI increases as the number of ecosystems of different types increases and or if the proportional distribution of the area of interest between the types of ecosystems is made more equitable. Patch distribution and abundance, Shannon Equity Index (SEI): it is equal to 0 when the landscape contains only 1 ecosystem and approaches 1 when the distribution of the area between the different types of ecosystems becomes larger.
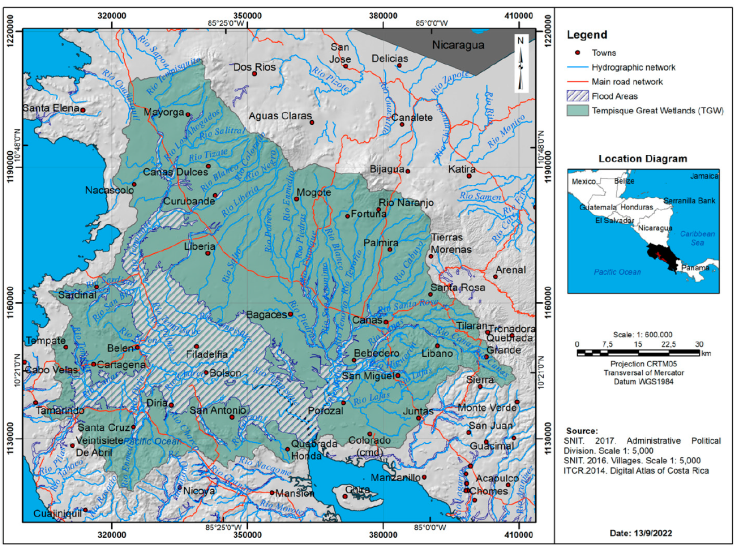
Figure 2 Hydrographic network, main roads network, flood areas in the Tempisque Great Wetlands (TGW).
The data of 2 variables (SI, SDI) were not normally distributed, according to Shapiro-Wilks tests, and there was heteroscedasticity, according to Levine’s tests (SPSS). Therefore, nonparametric tests were used to analyze the data. The InfoStat 2018 (Universidad Nacional de Cordoba, Argentina) software was used to perform Kruskal-Wallis (K-W) tests on the fragmentation variables by region to determine whether there were differences between the variables by place and by year. In addition, the differences between the percentages of forest coverage among the different years, total area (km2), area < 600 m asl, area occupied by rivers and roads (km), the number of towns, and the area of wetlands and flood areas (Ha) were analyzed (K-W) tests.
Because crocodile density data in the country is scarce and because the dates of forest coverage analysis did not exactly correspond by year with the crocodile surveys, but did correspond by area and years, they were grouped and averaged in the intervals in which there were databases of change in forest coverage in the zone (1997, 2000, 2005, 2010, and 2013). To analyze the association between habitat variables and crocodile density, crocodile survey data published and unpublished over the years was compilated, and used, much of these surveys were made mostly by the authors or following the same methods (Table 1).
Table 1 Crocodile density in the Tempisque Great Wetlands and the Central Pacific in Costa Rica.
| Year | River | Density (ind/km) | Reference | |
|---|---|---|---|---|
| TGW | 1993 | Bebedero | 4.5 | Sánchez et al., 1993 |
| TGW | 1993 | Tempisque | 4.65 | Sánchez et al., 1993 |
| TGW | 2000 | Tempisque | 18.3 | Sánchez, 2001 |
| TGW | 2008 | Tempisque | 5.34 | Barrantes, 2008 |
| TGW | 2010 | Tempisque | 8.1 | Bolaños, 2012b |
| TGW | 2012 | Tempisque | 8.79 | Bolaños, 2012b |
| TGW | 2015 | Tempisque | 11.7 | Orozco, 2015 |
| TGW | 2015 | Tempisque | 12.9 | Peraza, 2015 |
| CP | 1992 | Tárcoles | 19.1 | Sasa and Chaves, 1992 |
| CP | 1992 | Tárcoles | 11.1 | Torrealba, 1992 |
| CP | 1994 | Tárcoles | 15.2 | Motte, 1994 |
| CP | 1998 | Tárcoles | 12 | Abadía, 1998 |
| CP | 2000 | Tulín | 8.14 | Piedra, 2000 |
| CP | 2000 | Jesús María | 2.39 | Piedra, 2000 |
| CP | 2000 | Tárcoles | 30.08 | Piedra, 2000 |
| CP | 2004 | Tulín | 5.58 | Porras, 2004 |
| CP | 2004 | Jesús María | 1.51 | Porras, 2004 |
| CP | 2004 | Tárcoles | 9.22 | Porras, 2004 |
| CP | 2005 | Parrita | 6.6 | Escobedo-Galván, 2005 |
| CP | 2005 | Parrita | 0.95 | Escobedo-Galván, 2005 |
| CP | 2009 | Tárcoles | 10.29 | Bolaños, 2015 |
The crocodile density data available were grouped per period, and it was matched with the nearest data of forest coverage register and the analysis was performed on the following: TGW: forest coverage 1997 year (average of 1993 crocodile density in Bebedero and Tempisque rivers), forest coverage 2000 year (2000 crocodile density in the Tempisque River), forest coverage 2005 year (2008 crocodile population in the Tempisque River), forest coverage 2010 year (average of 2010 and 2012 crocodile populations in the Tempisque River), and forest coverage 2013 year (average of 2 surveys in 2015 of the crocodile population in the Tempisque River); CP: forest coverage 1997 year (average of 1992, 1994, and 1998 crocodile populations in the Tárcoles River), forest coverage 2000 year (2000 surveys of crocodile populations in Tulín, Jesús María, and Tárcoles rivers), forest coverage 2005 year (2004 surveys of crocodile populations in Tulín, Jesús María, and Tárcoles rivers), forest coverage 2010 year (2009 survey of the Tárcoles River), and forest coverage 2013 year (2014 survey of the crocodile population in the Tárcoles River) (Table 1). Spearman correlation analysis (r s) were performed between crocodile densities and changes in the percentage of forest coverage and the fragmentation indices by year.
Results
The total kilometers of rivers and roads, wetland areas, and flood areas were higher in the TGW than in the CP, with only the number of towns higher in the CP, although they occupied a smaller area, but we did not find statistical difference between these variables (K-W; H = 1, df = 2, p > 0.9999) (Table 2).
Table 2 Geomorphologic environmental values in the Tempisque Great Wetlands (TGW) and the Central Pacific (CP), Costa Rica.
| Area | Total área (km2) | Area < 600 m asl (km2) | Rivers (km) | Roads (km) | Amount towns | Wetlands áreas (ha) | Flood áreas (ha) |
|---|---|---|---|---|---|---|---|
| CP | 4,433.43 | 4,433.43 | 4,757 | 3,510 | 424 | 90.1 | 205.53 |
| TGW | 5,381.89 | 4,987.74 | 6,522 | 5,550 | 383 | 186.75 | 628.92 |
The changes in forest coverage indicated forests increased (Table 3). In the TGW, there was significant increase in forest coverage (K-W; H = 4, df = 4, p < 0.01) from 15% in 1997 to 49% in 2013. In the CP, the forest coverage also increased significantly (K-W: H = 4, df = 4, p < 0.01) from 25% in 1997 to 50% in 2013.
Table 3 Forest coverage, fragmentation indices, Shannon diversity index, equity index, and average crocodile density in the Tempisque Great Wetlands (TGW) and Central Pacific (CP) in different years of the study.
| Year | 1997 | 2000 | 2005 | 2010 | 2013 |
|---|---|---|---|---|---|
| Forest cov (%), TGW | 15 | 19 | 48 | 41 | 49 |
| Forest cov (%), CP | 25 | 36 | 38 | 48 | 50 |
| FD-TGW | 1.47 | 1.51 | 1.53 | 1.54 | 1.63 |
| FD-CP | 1.41 | 1.48 | 1.41 | 1.45 | 1.49 |
| SI-TGW | 51.9 | 78.6 | 94.08 | 100.71 | 197.39 |
| SI-CP | 22.92 | 44.56 | 19.02 | 31.24 | 15.02 |
| SDI-TGW | 0.7 | 0.83 | 0.94 | 1.04 | 1.66 |
| SDI-CP | 0.71 | 0.78 | 1.18 | 0.8 | 1.74 |
| SEI-TGW | 0.36 | 0.43 | 0.43 | 0.53 | 0.76 |
| SEI-CP | 0.34 | 0.44 | 0.49 | 0.58 | 0.79 |
| Average crocodile density TGW (ind/km) | 4.57 | 12.3 | 5.34 | 6.96 | 12.3 |
| Average crocodile density CP (ind/km) | 14.3 | 13.24 | 4.77 | 9.5 | 11 |
SI: Shape index; FD the fractal dimension index; SDI: Shannon diversity index; SEI: the equity index.
In the TGW, 28.7% of the area experienced a change in land use between 2000 and 2013. These changes were primarily in forest coverage, bodies of water, mangroves, and flooded vegetation, with many of those areas changing to crops, pastures, and urban use. In 2013 in the TGW, only 30% of the area included land use types considered optimal for crocodiles, whereas almost 70% of the area was in crops, pastures, and urban use (Table 4). In the CP during the same year, approximately 31.4% of the area experienced changes in land use. The changes were primarily in bodies of water, forests, and mangroves, but in contrast to the TGW, there was little associated change in the areas of crops, pastures, and urban use. Although the change in land use in both areas was close to 30% (28.7% in TGW and 31.4% in CP), the greater area of the TGW resulted in a larger area experiencing changes (Table 5).
Table 4 Percentage of change in land use in the Tempisque Great Wetlands (TGW) between the years 2000 and 2013.
| Land use Tempisque Great Wetlands 2013 | Land use Tempisque Great Wetlands 2000 | Percentage of change |
|---|---|---|
| Low slope forest | High slope forest | 2.11 |
| Low slope forest | Bodie of water | 0.01 |
| Low slope forest | Crops | 1.03 |
| Low slope forest | Mangrove | 0.15 |
| Low slope forest | Low slope forest | 26.42 |
| Low slope forest | Anegade Vegetation | 0.04 |
| Bodie of water | Low slope forest | 0.02 |
| Bodie of water | Crops | 0.01 |
| Bodie of water | Mangrove | 0.01 |
| Bodie of water | Low Slope Pasture | 0.01 |
| Crops | High slope forest | 0.00 |
| Crops | Low slope forest | 5.70 |
| Crops | Bodie of water | 0.03 |
| Crops | Mangrove | 0.01 |
| Crops | Low slope forest | 26.35 |
| Crops | Anegade Vegetation | 0.01 |
| Mangrove | Low slope forest | 0.27 |
| Mangrove | Bodie of water | 0.03 |
| Mangrove | Crops | 0.01 |
| Mangrove | Low slope forest | 0.50 |
| Mangrove | Anegade vegetation | 0.17 |
| Low slope pasture | High slope forest | 2.35 |
| Low slope pasture | Low slope forest | 17.25 |
| Low slope pasture | Bodie of water | 0.00 |
| Low slope pasture | Crops | 3.28 |
| Low slope pasture | Mangrove | 0.33 |
| Urban | High slope forest | 0.03 |
| Urban | Low slope forest | 0.17 |
| Urban | Bodie of water | 0.02 |
| Urban | Crops | 0.74 |
| Urban | Low Slope Pasture | 0.62 |
| Anegade Vegetation | High slope forest | 0.00 |
| Anegade Vegetation | Low slope forest | 11.63 |
| Anegade Vegetation | Crops | 0.01 |
| Anegade Vegetation | Mangrove | 0.06 |
| Anegade Vegetation | Low Slope Pasture | 0.61 |
| Total | 100.00 |
Table 5 Percentage of change in forest coverage in the Central Pacific (CP) between the years 2000 and 2013.
| Land use central Pacific 2013 | Land use central Pacific 2000 | Percentage of change |
|---|---|---|
| High slope forest | Low slope forest | 1.37 |
| High slope forest | Clouds | 0.08 |
| High slope forest | High slope pasture | 3.70 |
| High slope forest | Low slope pasture | 2.32 |
| Low slope forest | High slope forest | 1.33 |
| Low slope forest | Bodie of water | 0.49 |
| Low slope forest | Crops | 0.10 |
| Low slope forest | Mangrove | 0.73 |
| Low slope forest | Clouds | 0.37 |
| Low slope forest | Low slope pasture | 57.11 |
| Bodie of water | High slope forest | 0.01 |
| Bodie of water | Low slope forest | 0.13 |
| Bodie of water | Crops | 0.07 |
| Bodie of water | Mangrove | 0.56 |
| Bodie of water | High slope pasture | 0.01 |
| Bodie of water | Low slope pasture | 0.56 |
| Crops | High slope forest | 0.14 |
| Crops | Low slope forest | 2.87 |
| Crops | Bodie of water | 0.13 |
| Crops | Mangrove | 0.17 |
| Crops | Clouds | 0.01 |
| Crops | Low slope pasture | 7.87 |
| Mangrove | High slope forest | 0.05 |
| Mangrove | Low slope forest | 0.71 |
| Mangrove | Bodie of water | 0.42 |
| Mangrove | Crops | 1.04 |
| Mangrove | Low slope pasture | 1.27 |
| High slope pasture | High slope forest | 0.01 |
| Low slope pasture | High slope forest | 0.41 |
| Low slope pasture | Low slope forest | 15.48 |
| Low slope pasture | Bodie of water | 0.23 |
| Low slope pasture | Crops | 0.02 |
| Low slope pasture | Mangrove | 0.18 |
| Low slope pasture | Clouds | 0.00 |
| Low slope pasture | Urban | 0.00 |
| Urban | Low slope forest | 0.02 |
| Urban | High slope pasture | 0.01 |
| Total | 100.00 |
The values of the fragmentation indices the FD and the SI as well as the SDI and the SEI increased in the years of the study therefore, the landscape complexity increased in both areas. The shapes of the fragments were not simple geometric forms, and the fragments were dominated by different types of land uses or forest coverage. In general, land use changed, and forests recovered in both zones, but there were no statistically significant differences between the indices in the TWG and the CP (K-W: H = 4, df = 4, p > 0.99) (Table 3).
According to the crocodile populations that inhabit these areas, we found that in the CP, the crocodile population remained high, 10.17 ±7.68 ind/km on average in the density reports (Table 1). In the TGW, the average in crocodile population is 9.29 ± 4.81 ind/km, but the data showed that increased from 4.57 ind/km in 1993 to slightly more than 12 ind/km in 2013 (Table 3). We found no statistical difference between crocodile density in these areas ((K-W: H = 1, df = 2, p > 0.99).
In the TGW, the average crocodile density and forest coverage per year were positively correlated, with r s = 0.74, p > 0.37. In addition, the average crocodile density was also positively correlated with the FD and SI indices, with r s = 0.65, p > 0.89 for both. In the CP, the crocodile density and the forest coverage per year were also positively correlated, with r s = 0.48, p > 0.37. The average crocodile density was also correlated with the FD and SI indices, with rs = 0.93, p > 0.894 and 0.74, p > 829, respectively.
Discussion
Our data results are consistent with Ramírez (2008) in terms of the strong variations in the composition and structure of the landscape in the study area, due to an almost 50% reduction in the size of the cattle herd between 1990 and 2006. With the substantial contraction in cattle activity, the change in forest coverage from 1997 to 2013 showed that forests regenerated in the areas formerly dedicated to pastures, beginning with the recovery of small fragments. These results of forest fragmentation obtained are like those reported by Gardner et al. (2018) and Laurance et al. (2006).
The changes in the landscape structure could be due to improvements and increases in communication routes, and Guanacaste and Puntarenas Provinces (location of the TGW and CP) becoming a touristic destination. Besides, in the TGW, the development of aquaculture, attracted larger investment, higher visitation, and infrastructure development (Ramírez, 2008). As a result of such changes in land use, fragmentation of the landscape became inevitable, and the increases in fragmentation indices in this paper support that conclusion. As well, Sandoval et al. (2019, 2020) found a similar situation in the CP.
The changes in forest coverage in this study differ from those in several other studies in Costa Rica (Bonilla & Rosero-Bixby, 2004; Carrillo & Vaughan, 1994; Harrison, 1991; Sandoval & Castillo, 2011), and showed a change in the trend of vegetation cover change from that observed in 1960 to1980, in which there was an important loss in forest coverage. In this study, in general, the recovery in forest coverage began in 1997, but the recovery was heterogeneous, as the fragmentation indexes suggested. Although the increases in forest fragmentation, with associated numerous small fragments, were linked with changes in land use, the data may indicate that forest fragmentation could be a part of the reasons for the increases in crocodile populations in the study area.
The increases in crocodile populations could have happened because they are generalists, which tend to be abundant in human-altered areas and regenerating fragments of forest, such as those found in the TGW and some parts of the CP (Karchesy et al., 2016; Ryall & Farig, 2006; Schwenk et al., 2012). Food supplies, as well as mating and breeding areas, among other factors, have increased at other crocodilian distribution centers with human activities (Pooley, 2016; Pooley et al., 1989), as well as those in Costa Rica and Panama (Sandoval et al., 2017, 2019, 2020; Venegas-Anaya et al., 2015).
Our data suggest that the changes in forest coverage in TGW and CP, fragmented the landscape and isolated areas of forest as Martínez-Salinas (2008), Schneider (2001), and Troche and Guarachi (2001), mentioned. The recovery of small fragments of forest, the changes detected in the habitat, could explain the increases in the fragmentation indexes observed in this study. With increases in forest fragments, depending on their size and shape, generalist species such as crocodiles can benefit and their population can grow easily (Beier, 1993; Fahrig, 2002; Pincheira et al., 2009; Rau & Gantz, 2001; Simonetti & Mella, 1997). In particular, a fragmented landscape with human activities as TGW a CP, may benefit crocodiles by increasing the availability of unexpected food coming from the human settlements, and some prey wandering around also looking for their own food (Amarasinghe et al., 2015; Pooley, 2016; Pooley et al., 2017).
However, it is important to consider in addition to the arguments about the changes in the habitat, that the increase in crocodile populations in the study areas might be due to the elimination of intensive hunting. Fukuda et al. (2011) found that with the cessation of hunting, the populations of other crocodilian species began to recover, therefore, in addition to new habitats created in fragmented and anthropic areas as TGW and CP, the absence of hunting could be a reason to explain the increases in crocodile populations in Costa Rica (Sandoval et al., 2019, 2020).
Although crocodiles in northwestern Costa Rica were severely affected by poaching, it seems that the recent habitat modifications have fortunately increased their populations. It has been proven that the species might even prosper better in altered zones, a conclusion also reached by many others (González-Maya et al., 2015; Morales, 2013; Orozco, 2015; Quirós-Arias, 2017; Sandoval et al., 2017, 2019, 2020; Valdelomar et al., 2012; Valverde, 2018). In fact, crocodiles in the CP and the TGW are abundant in zones with a high grade of urbanization and habitat alteration (as well was found in other areas in Central America) (Barrantes, 2008; Bolaños, 2011; Bolaños et al., 1997; King et al., 1990; Morales, 2013; Orozco, 2015; Sánchez et al., 1996; Sánchez-Ramírez, 2001; Sandoval et al., 2017, 2019, 2020; Valdelomar et al., 2012). Bolaños et al. (2019) found similar results in the Costa Rican Central Caribbean zone, where crocodiles were more abundant in disturbed areas than in those well preserved.
The area of the TGW and CP has high permeability because of the high density of watercourses (Monge-Nájera & Gómez (2007); and this type of matrix may facilitate the territorial movements of crocodiles). Therefore, even with alteration of their habitat, crocodiles should be able to move relatively free. In addition, Clark and Sues (1989) and Reiser and Peacock (1985), found that crocodiles can easily overcome obstacles and leave the water and walk, there should be relatively few habitat alterations that would limit their movements.
Thus, with abundant watercourses and wetlands in our study area, plus little effect of changes in forest coverage, and fragmentation in general, the American crocodile could move freely throughout the entire habitat, which might explain the increase in interactions with humans (Bolaños, 2012a, b; Morales, 2013; Porras et al., 2020; Sandoval et al., 2017, 2019, 2020; Valdelomar et al., 2012).
Based on these results, it is proposed that, in addition to the protection provided by law 7317 (Wildlife Conservation), the crocodile in the TGW and CP as an opportunistic species, can benefit from changes in the habitat. For example, there is an increase in easily captured prey, such as domestic animals, in the disturbed areas associated with forest fragmentation, towns, roads, and highways, which can reduce intra- and interspecific competition in different habitats (Amarasinghe et al., 2015; Carrillo, 2013; Morales, 2013; Pooley, 2016; Pooley et al., 2017; Sandoval et al., 2017, 2019, 2020; Valdelomar et al., 2012).
Our data can support the idea that land use changes in TGW and CP can be correlated with the increase of crocodile populations, these situations can influence the conflicts and interactions between humans and crocodiles, and those are likely to increase as crocodile populations increase under the current altered conditions (Bolaños, 2012a, b; Morales, 2013; Porras et al., 2020; Sandoval et al., 2017, 2019, 2020; Valdelomar et al., 2012). In conclusion, crocodile populations in Costa Rica could benefit from habitat fragmentation and human activities as changes in the landscape may increase the resources that can promote population growth.











 nueva página del texto (beta)
nueva página del texto (beta)