Introduction
The Family Neanuridae has more than 1,500 species worldwide (Bellinger et al., 2020) with 6 subfamilies (Deharveng, 2004): 1) Caputanurininae (10 spp.) distributed only in the Sino-Japanese region, 2) Morulininae (21 spp.) in the Holarctic region, 3) Uchidanurinae (6 spp.) in Asia; and 3 cosmopolitan subfamilies: 4) Neanurinae (759 spp.), 5) Frieseinae (184 spp.), and 6) Pseudachorutinae (close to 500 spp.). The latter has many genera with very wide distributions, but some of these are restricted to the Northern or Southern hemispheres. They have a very variable development of furcula, number of eyes, presence or absence of postantennal organ, and simple or much modified mouthparts. Mandibles are small with a few or many apical teeth and no molar surface; maxillae often styliform, but sometimes with well-developed lamellae with teeth, serrate, or feathered. The mouth cone can be prominent in the shape of a beak or truncate. The genera Cassagnaurida, Delamarellina, and Notachorudina are known only from the Southern Hemisphere with a few species and most of them are poorly described.
Some subfamilies are easy to recognize: Neanurinae lack furcula and have strong reduction of eyes, mouth parts are transformed for sucking; Frieseinae have a variable number of eyes (8 + 8 to 0) and a different development of furcula or its complete absence and very characteristic maxillae; Pseudachorutinae have a variable development of furcula, eyes, and mouthparts, and are often easy to recognize by the protruding mouth (labrum and labium) in a beak. However, the division of this subfamily into tribes is still a subject for debate because many genera have uncertain taxonomic status and unclear affinities.
Massoud (1967), based on maxillae complexity, divided Pseudachorutinae into 2 tribes: Anuridini and Pseudachorutini. This division was rejected by D’Haese (2003), who proposed an alternative subdivision based on the presence or absence of some labial organs, but this information is lacking in many generic descriptions. The recent study of Pseudachorutinae is an important contribution to the taxonomy and distribution of the subfamily in the Neotropical region but no proposal for the division of tribes was made (Queiroz & Zeppelini, 2017). Greenslade (2015) transferred several genera formerly placed in Uchidanurinae, Acanthanura Börner (1906), Holacanthella Börner (1906), Megalanura Ellis & Bellinger 1973, and Womersleymeria Stach 1949, to Pseudachorutinae. Some endemic tropical and Patagonian genera were poorly defined and some descriptions were done with a few specimens and did not include important characters like chaetotaxy of antennae, labium, or body, the intraspecific variation was not considered either and no information on their development has been provided. This paper targeted for taxonomic revision the endemic Collembola from Patagonia, mainly those with very few representatives and that are more related to an ancient group occupying Australia and Oceania with a Pan-Antarctic distribution.
Materials and methods
Most of the specimens were collected by Mr. D. Burkhardt of the Museum of Natural History of Genève and later sent to me by Dr. Peter Swandinger for the study. Most of them were collected using pitfall traps preserved in 75% alcohol, and later cleared and mounted on permanent slides in Hoyer’s solution. The first results were given by Palacios-Vargas et al. (2013). The morphology of specimens was studied under a phase-contrast microscope and drawings were made using a camera lucida.
For dorsal chaetotaxy of Ant. IV, I followed Deharveng (1981) and Queiroz and Zeppelini (2017). A modified system of Smolis (2008) was used for the ventral chaetotaxy of Ant. IV and III. Intertergal areas have been named after the preceding segment from thoracic segment I to abdominal III.
Descriptions
CassagnauridaSalmon, 1964.
Diagnosis (modified fromMassoud, 1967). Large body with well developed paratergal and intertergal areas. With complex mouthparts. Buccal cone forming a small beak. Dorsally fused Ant. III and IV. Ant. IV with trilobed apical bulb, ventral file with many modified setae, dorsally 1 microsensillum, and 1 spherical organ (or), 6 sensilla poorly defined (S1-S4, S7, S8), 12 ordinary setae (a1-3, b1-3, c1-3, d1-3) plus seta i. Ant. III organ with 2 microsensilla displaced to level of setae i, Sgd displaced but not close to microsensilla; Sgv in normal position close to m’. Eyes 8 + 8, PAO small, with few simple vesicles; some plurichaetosis on head. Mandible with 10 teeth, basal one stronger with several small denticles ventrally; maxilla head with 1 small serrate lamella with a big basal tooth, 2 other lamellae fused, one with acuminate apex and the other with 2 small apical teeth. Labium moderately elongated, with setae A-G and short papillated seta L; setae B and D smaller than A and C. Body plurochaetotic, each intertergal area between Th. II-Abd. IV bearing 1 row of setae. Unguis with 2 pairs of strong lateral teeth. Ventral tube with 4 + 4 setae; tenaculum with 3 + 3 teeth. Well developed and complete furcula, manubrium plurochaetotic (23 + 23 setae), dens with 6 setae, mucro with 2 tapering lamellae with slightly hooked apex. Anal sacs present.
Type species: Cassagnaurida dentata (Cassagnau et Rapoport, 1962)
Remarks
In his key, Salmon (1964) delimited the new genus Cassagnaurida by PAO with 8 peripheral vesicles and fringed lamellae of maxilla shorter than maxilla head, whereas Pseudachorudina Stach, 1949 also has PAO with 15-20 peripheral vesicles and lamellae of maxilla longer than maxilla head. Massoud (1967) placed this genus very close to Ceratrimeria for having 8 + 8 eyes and the presence of postantennal organ. Two characters allow the genera to be distinguished: 1) maxilla of Ceratrimeria without fringed lamella and untoothed, while in Cassagnaurida it is toothed, and 2) the antennal segment IV has a ventral sensorial organ in Cassagnaurida.
Cassagnaurida dentata (Cassagnau et Rapoport, 1962)
Ceratrimeria dentataCassagnau et Rapoport, 1962 (Figs. 1-13)
Diagnosis. Body length 4 mm, dorso-ventral compressed. Color dark blue; intertergal areas from Th. I to Abd. III and well developed paratergal areas; moderate plurichaetosis in dorsal head, thorax, abdomen, and manubrium, but not on antennae, ventral head, ventral abdomen, legs, or ventral tube. Dorsal chaetotaxy with microsetae, in 2 rows on each segment, except Abd. IV which has 3 irregular rows; one row of setae in each intertergal area. Besides microsetae and sensorial setae, there are 2 macrosetae on each tergite. Tegumentary granulation very fine. Microsetae 25-40 µm; macrosetae 150 µm; ss 185-200 µm.
Ant. III and IV dorsally fused (Figs. 1, 3), Ant. III organ displaced to Ant. IV, 2 microsensilla in one depression but not cuticular fold, far above Sgv at level of seta i. Sgv and Sgd about the same length. Ant. I with 7 setae, Ant. II with 13 setae, Ant. III with 21 setae (Fig. 3). Apical bulb trilobed, 1 spherical organ “or” and 1 microsensillum; Sensilla of Ant. IV difficult to distinguish, only 2 well differentiated dorsal and several long acuminate setae. Sensorial ventral file with up to 225 modified setae in 8-9 irregular longitudinal rows (Fig. 2).
Head chaetotaxy in Fig. 7; 3 + 3 setae p and c, 3 ocular setae. 8 + 8 subequal eyes; very small circular PAO with 4-8 tubercles in a circle less than 1/4 the diameter of the closest eye (magnification in Fig. 7). Labrum formulae 4/2, 2, 4, 2, 2. Mandible with 10 teeth of different size, proximal one with ventral small denticulations (Fig. 5); elongated maxillary capitulum, with 3 lamellae, one finely serrate with 1 big curved basal tooth, and 2 long lamellae partially fused, one with acuminate tip and the other with 2 curved apical teeth (Fig. 4). Moderately elongated labium with setae A-G and papillated seta L; 2 pairs of postlabial setae (Fig. 6).
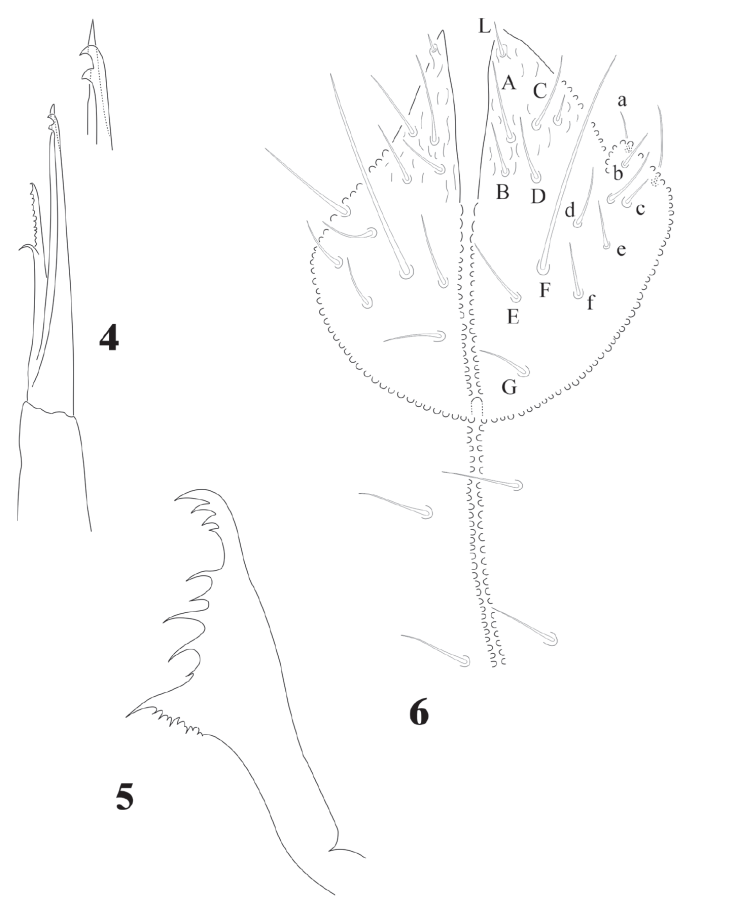
Figures 4-6 Cassagnaurida dentata. 4, Maxilla, with apex magnification; 5, mandible; 6, labium chaetotaxy and postlabial setae.
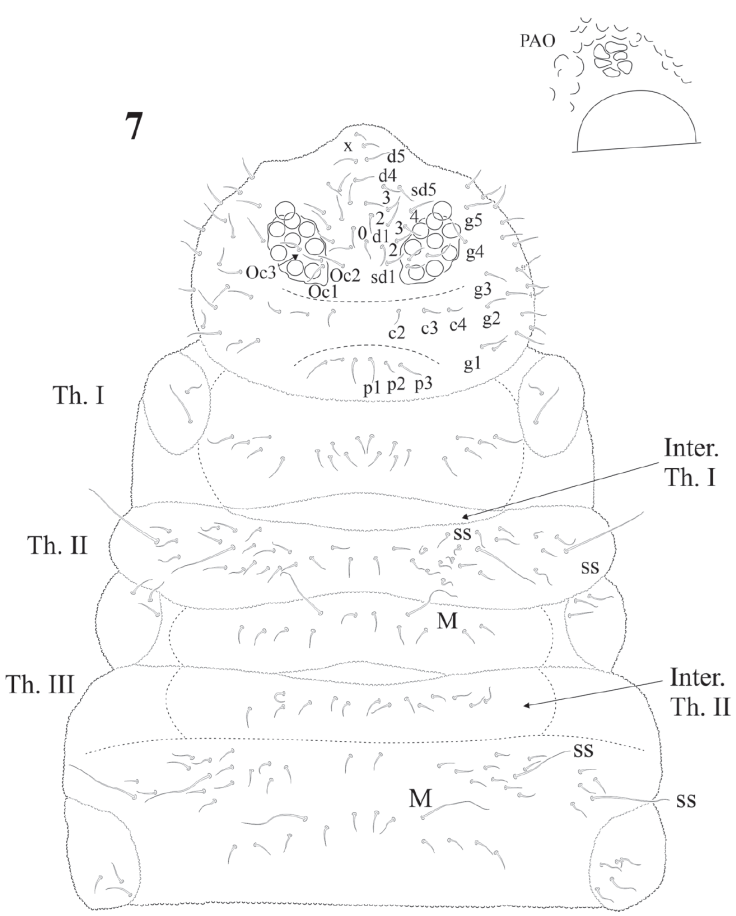
Figure 7 Cassagnaurida dentata. Head and thorax chaetotaxy with intertergal areas marked, magnification of PAO and nearest eye.
The sensillar formula by demitergite as: 0, 2, 2/1, 1, 1, 1, 1 and 1 pair of strong macrosetae from Th. II to Abd. IV, giving the appearance of having 3 ss on Th. II and III and 2 pairs on each abdominal segment (Figs. 7, 8). Thoracic chaetotaxy on figure 7. Th. I with 10 + 10 setae in 2 irregular rows and 2 lateral setae on each paratergal area, one elongated. Th. II with 22 + 22 setae in 3 irregular rows, 2 + 2 sensorial setae and 1 pair of macrosetae. Paratergal areas of Th. II with 3 subequal setae, intertergal area Th. II-III with 7 + 7 setae. Th. III with 20 + 20 setae in 2 irregular rows and 2 + 2 sensorial setae and 1 pair of macrosetae, intertergal area Th. III-Abd. I with 7 + 7 setae. Paratergal areas with 3-5 setae each.
Chaetotaxy from subcoxae to tibiotarsi I-III: 0?, 3, 6, 12, 19; 3, 8, 6, 12, 19; 3, 8, 6, 11, 18. (Figs. 9, 12). Femora with one ventral acuminate t.h. on each leg (Fig. 12). Tita. with 2 long acuminate t.h. ventrally on proximal verticil (Figs. 9,12). Seta M between both t.h. unguis with 1 median tooth and 2 pairs of strong lateral teeth on each side, better seen in ventral view (Fig. 9). Pretarsus with 2 setae.
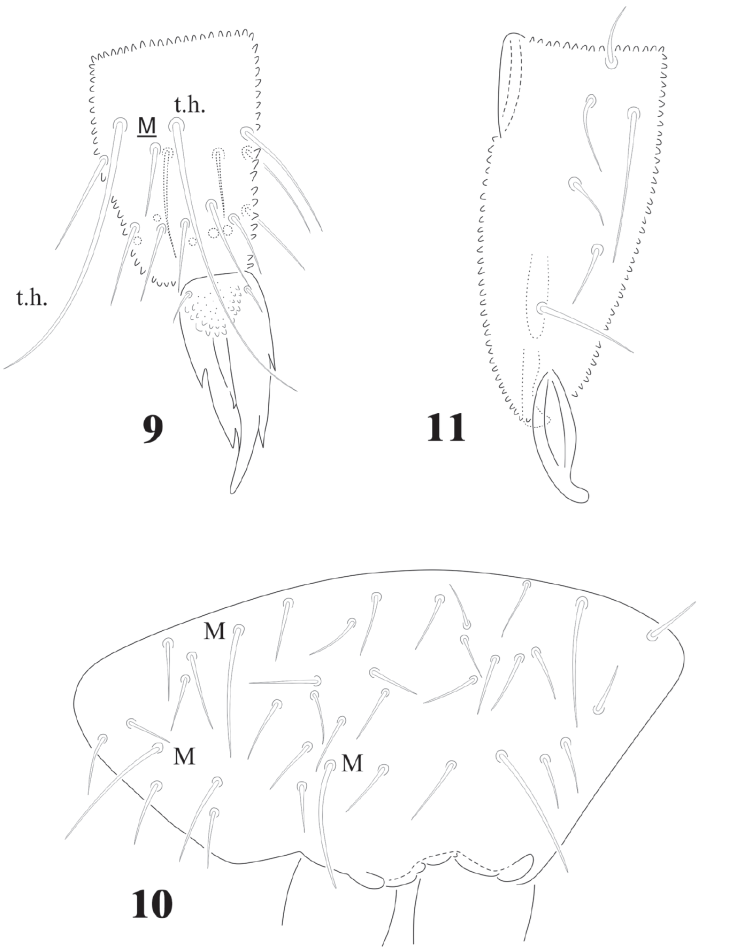
Figures 9-11 Cassagnaurida dentata. 9, Tita. I and unguis in ventral position; 10, manubrial chaetotaxy, ventro-lateral view; 11, dens with mucro, posterior view.
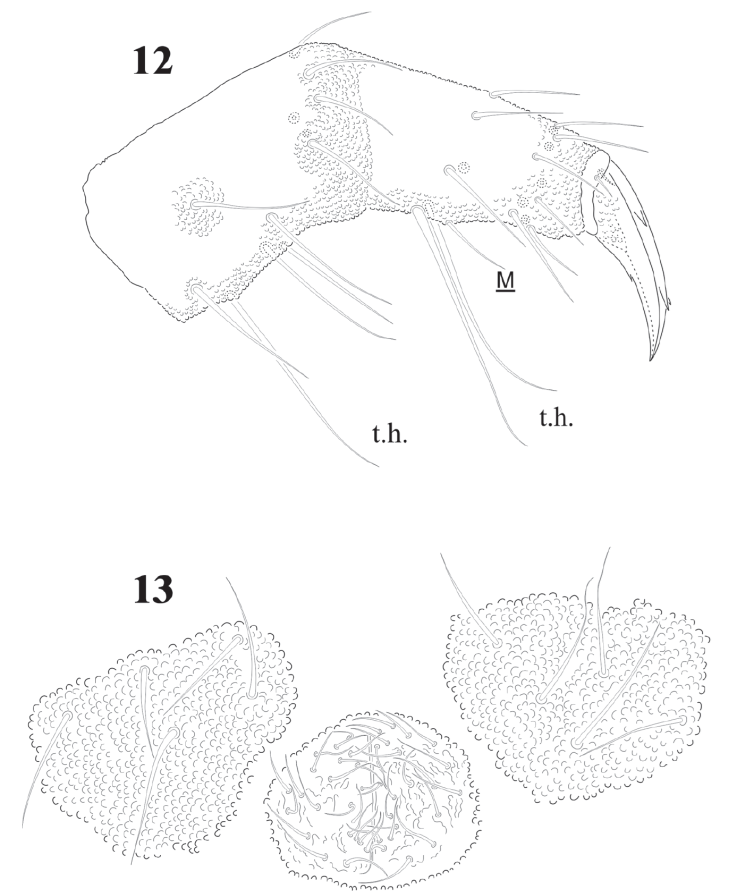
Figures 12-13 Cassagnaurida dentata. 12, Femur, Tita. and foot complex III, lateral view; 13, male genital plate.
Abdominal chaetotaxy in figure 8, intertergal areas from Abd. I-III with 6-7 + 6-7 setae. Macrosetae close to midline, with 4 + 4 microsetae between them on Abd. I and Abd. II; 2 + 2 on Abd. III and IV and 3 + 3 on Abb. V; Abd. VI with about 28 dorsal setae. VT with 4 setae on each side; tenaculum with 3 + 3 teeth. Well developed furcula; manubrium with 23 pairs of setae, 3 of them macrosetae (Fig. 10); dens with fine granulations on posterior surface and 6 setae, proximal external one longer than the others (Fig. 11), dens anterior surface with 2 smooth surfaces. Mucro about 1/3 of dens, with rounded apex, curved and lateral lamellae ending before the apex. Male genital plate with 5 + 5 pregenital setae, 34 circumgenital setae and not modified 5 + 5 eugenital setae (Fig. 13).
Variation. One specimen from Lago Chaiquenes, Chile has PAO with 4 vesicles on one side and 6 on the other, while that from Río de Las Minas, Magallanes Province, Chile has 8 and 6. The type material has 8 + 8 vesicles. Juveniles measures 2.35 mm. The juvenile had 125 modified setae on one Ant. IV sensorial file and 162 on the other antenna. Tibiotarsi with 2 ventral acuminate tenent hairs in proximal verticil, femora with 1, but one specimen had femur on leg II with 2 tenent hairs. Adult and juvenile present small morphological variations, nevertheless, they are very few, so they belong to the same species.
The type specimen used in the original description is from Argentina. This was collected in Los Cántaros, Futalaufquen, Department of Futaleufú, Province of Chubut in the Parque Nacional Los Alerces (42°49’ S, 71°43’ W), Argentina. Our redescription is based on 2 specimens from Chile: one from Province of Llanquihue in the National Park Alerce Andino (350-650 m snm) in Laguna Chaiquenes, Chile (41°40’ S, 73°35’ W) that is close to type locality (Andean-Patagonian region) and the other from the Reserva Nacional Magallanes, Río de las Minas, 5 Km from Punta Arenas, Chile (53°10’01” S, 70°56’01” W), which is more than 2,200 km south of the type locality.
Remarks
According to Massoud (1967), Cassagnaurida is related to Ceratrimeria, but this last genus lacks a ventral sensorial file on Ant. IV and the maxilla does not have serrate lamellae. The author also considered its close position to Delamarellina due to several common characters, but the last genus has a reduced furcula, only 5 + 5 eyes, no postantennal organ, and different body chaetotaxy with a very strong hyperchaetosis. C. dentata is easy to recognize due to moderate plurichaetosis and the presence of 1 pair of macrosetae on each of the segments from Th. II to Abd. V, close to midline. It has 8 + 8 eyes and a very small postantennal organ. Based on head and body chaetotaxy, intertergal areas and the number of setae (mainly on abdominal noto), and mouthparts morphology, it is clearly more related to Notachorudina.
DelamarellinaRapoport et Rubio, 1963
Diagnosis (modified fromMassoud, 1967). Large body with paratergal and well developed intertergal areas, with complex mouthparts. Buccal cone forming a small beak. Ant. III and IV dorsally fused. Ant. IV with trilobed apical bulb, dorsally 1 microsensillum, 1 spherical organ and 16-18 small apical cylindrical sensilla. Strong plurichaetosis on head, body dorsal and ventral side, no real ventral sensorial file, but more than 100 small acuminate setae. Ant. III displaced forward, with 2 small microsensilla in a single groove but with no cuticular fold. Sgv in normal position close to ventral m’. Eyes 5 + 5, PAO absent. Moderately elongated labium, with setae A-G and a modified seta L and some small x labial organs. Mandible with 10 teeth, basal one stronger without ventral denticles; well developed maxilla head, with 3 lamellae, 2 elongated and 1 short with a strong basal tooth. Tibiotarsi with 2 acuminate ventral t.h. in proximal verticil, unguis with 1 internal tooth and 2 pairs of strong lateral teeth. VT with many setae. Tenaculum with 3 + 3 teeth. Furcula reduced, manubrium and dens with plurichaetosis, mucro triangular, reduced and sometimes absent. Dorsal and ventral body with strong plurichaetosis and macrosetae, each intertergal area from Th. I to Abd. II with several irregular rows of setae. Abd. VI completely hidden under Abd. V. Type species: Delamarellina globulosa (Cassagnau et Rapoport, 1962).
Delamarellina globulosa (Cassagnau et Rapoport, 1962).
Arlesia globulosaCassagnau et Rapoport, 1962
New synonymy Delamarellina guilleniRapoport et Rubio, 1963. (Figs. 14-32)
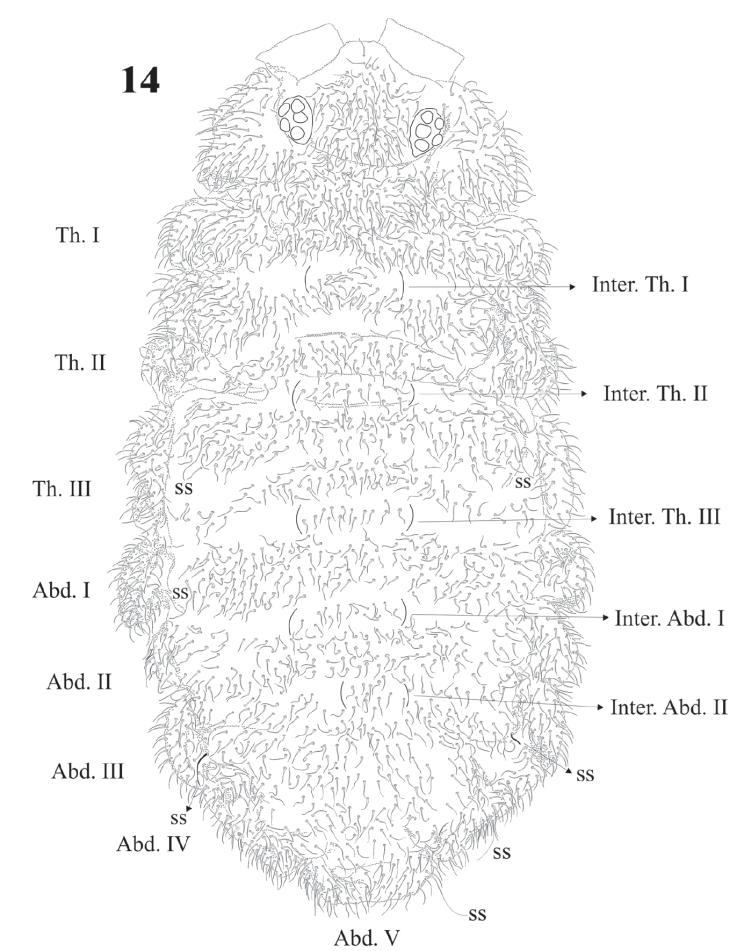
Figure 14 Delamarellina globulosa. Habitus, and body segmentation and chaetotaxy with intertergal areas marked.
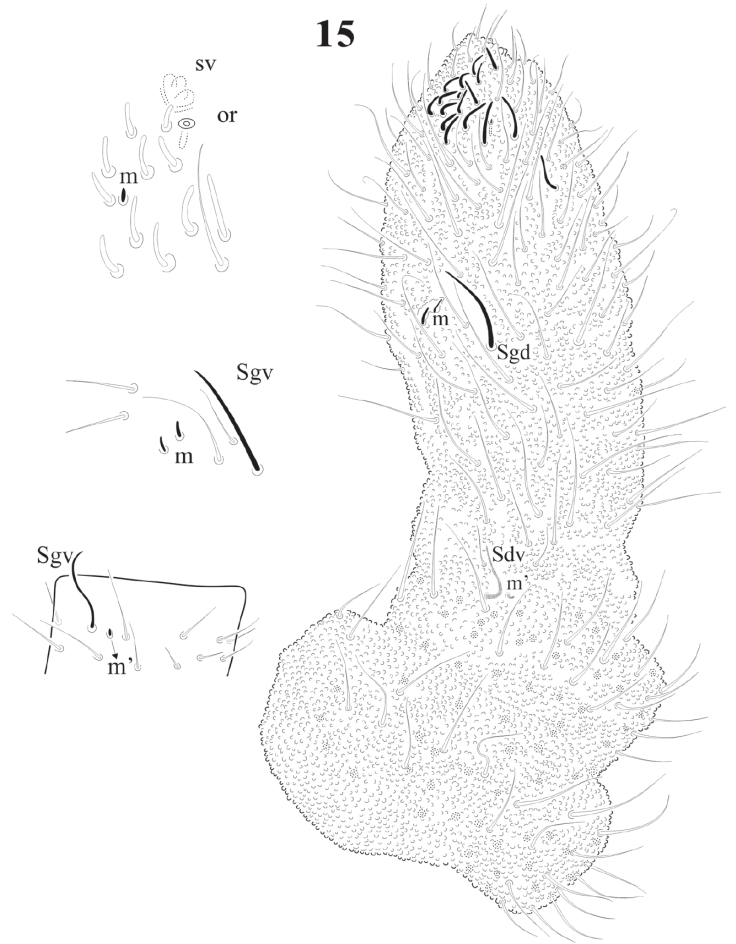
Figure 15 Delamarellina globulosa. Ant. I-IV, dorsal view, with magnification of sensorial structures.
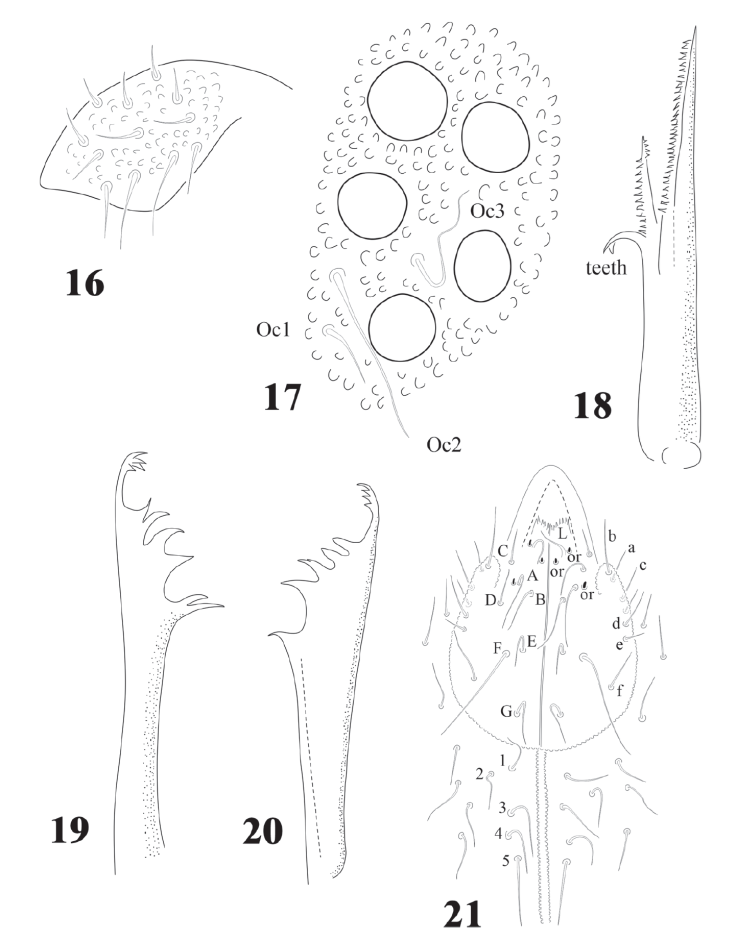
Figures 16-21 Delamarellina globulosa. 16, Labrum; 17, eyes and ocular setae; 18, maxilla; 19, mandible; 20, variation of mandible; 21, labial and postlabial chaetotaxy.
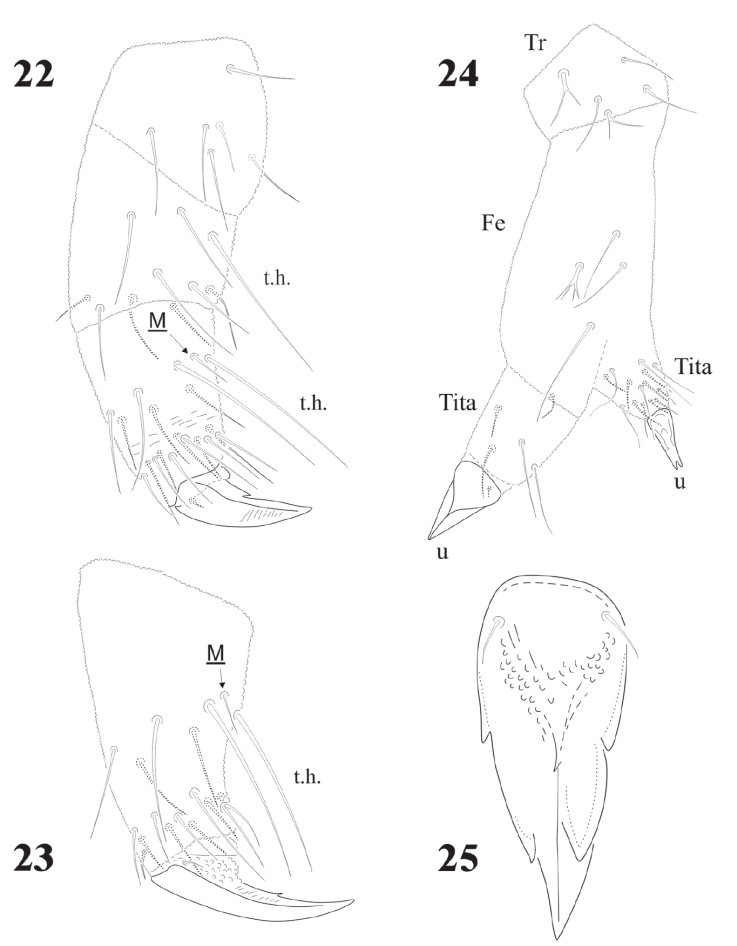
Figures 22-25 Delamarellina globulosa. 22, Leg I; 23 Tita. and foot complex III; 24, teratological leg; 25, unguis, ventral view.
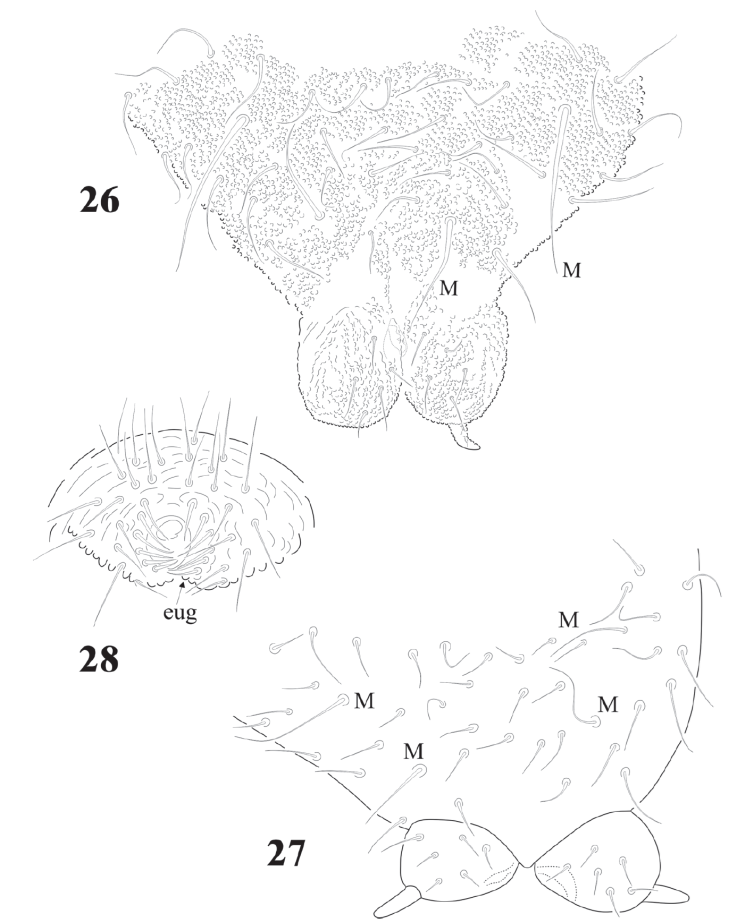
Figures 26-28 Delamarellina globulosa. 26, Asymmetric furcula with manubrial area; 27, normal furcula with manubrial area; 28, male genital plate with modified setae.
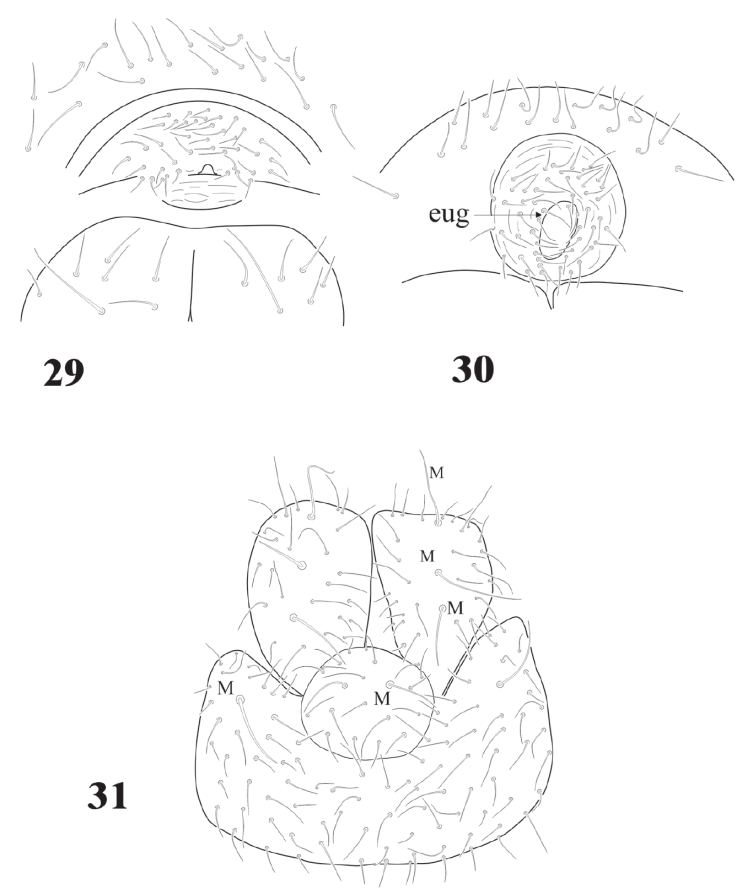
Figures 29-31 Delamarellina globulosa. 29, Female genital plate; 30, male genital plate; 31, anal valves and Abd. V, ventral view.
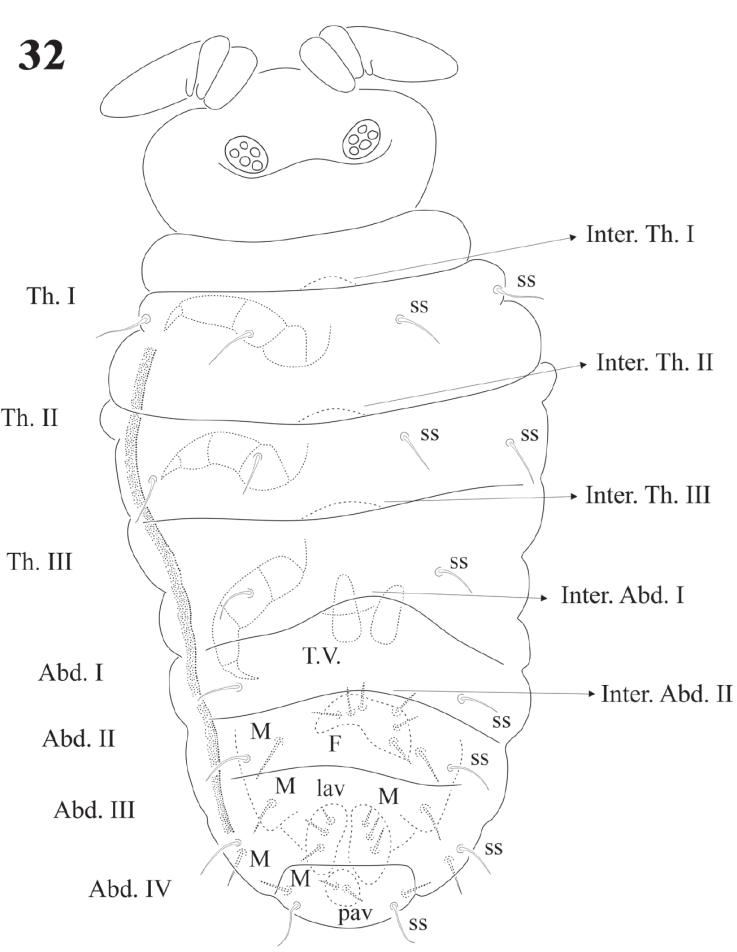
Figure 32 Delamarellina globulosa. Juvenile specimen with dorsal and ventral distribution of sensorial setae and macrosetae (microsetae omitted).
Diagnosis. Body length (n = 25): 2.9 mm (range 2.0-4.0 mm). Juveniles about 1.7 mm. Color from light blue with sparse pigment to very dark blue. Ventral side paler and distal half of the antenna paler than dorsum. Legs without pigment. Strong hyperchaetosis over the body (Fig. 14), antenna (Fig. 15), ventral tube, dens, anal valves (Fig. 31), and ventral abdominal segments, but not on ocular area (Fig. 17), labium, or legs (Figs. 22, 23). Chaetotaxy with many abundant microsetae about 20-50 µm long, macrosetae 120 µm, sensilla difficult to distinguish in adults but easy in juveniles. Macrosetae with a constant pattern on body ventral side. Depressed body, well delimited paratergal areas; cuticular granulation medium size and uniform, more developed in juveniles. Abd. VI completely hidden under Abd. V (like in Denisimeria longilobata and other members of the Uchidanurinae).
Antenna shorter than cephalic diagonal (ratio: 0.9: 1) with many setae (Fig. 15). Ant. IV dorsally fused to III; ventrally clearly separate. Dorsal apex with 8 -18 small, geniculate, cylindrical sensilla. Apical bulb very small and trilobed, 1 microsensillum and 1 subapical spherical organ. Ventrally there are about 110 short and acuminate small setae but no real file. Ant. III short with about 42 setae, sensorial organ with 2 short microsensilla not under a cuticular fold (about the same size of sensilla of Ant. IV). Sgd curved, long and acuminate (as ordinary seta) located dorsally in the middle part of fused dorsal Ant. III and IV; Sgv, long and sinuous, about half the length of Sgd, and close to ventral m’ (Fig. 15). Ant. II with about 50 setae, 15-17 dorsal and 29 ventral setae. Ant. I with about 55 setae, 20-22 dorsal and 33-35 ventral setae (Fig. 15).
Head with strong hypertrichosis. Labrum with 2/2, 3, 3, 2, 2 (Fig.16). 5 + 5 eyes, ocular area with 3 setae, Oc2 the longest (Fig.17), no postantennal organ. Capitulum of maxilla with 3 lamellae, of which 2 fused and strongly serrated, the longest with about 30 serrations, the smallest with 15 and 1 elongated and curved basal tooth (Fig. 18). Buccal cone short, labium with characteristic setae for the family, including the tuberculate setae L and 3 sensorial x organs per side (Fig. 21). Mandible with 10 teeth, 4 apical, 4 strong median teeth, and 1 basal (Fig. 20) or 2 teeth (Fig. 19). Postlabial setae from 3 to 5 pairs (Fig. 21), often with asymmetries.
Dorsal sensilla formula by demitergite: 0, 2, 2/1, 1, 1, 1, 1. Dorsally with very abundant small setae, intertergal areas with few setae. Inter. Th. I, II, III and Inter. Abd. I and II usually with 8 + 8 setae (Fig. 14).
Legs chaetotaxy from I to III from precoxae to tibiotarsi is 2, 6, 6,11-12,19; 10-16, 7, 6, 11-12,19 and 14-16, 6, 6, 12,18 (Figs. 22, 23). Foot complex with no empodial appendix, and Tita. with 2 acuminate ventral t.h. on proximal verticil. Seta M between t.h. Femora with 1 ventral t.h. (Fig. 22). VT with variable and usually asymmetrical number of setae, from 9 to 26 on each side. Tenaculum with 3 + 3 teeth; in 30 specimens 21 had 3 teeth, 9 had 2 teeth, sometimes asymmetric (2 + 3), and one specimen had no tenaculum. Ventral Abd. I with no setae; Abd. II with 10 microsetae on each side; Abd. III with 36; Abd. IV with 20-24 plus 1 macroseta per side; Abd. V with 9-10 plus 3 pairs of macrosetae per side (Fig. 32).
Furcula short; manubrium with variable number of microsetae from 15 + 15 to 20 + 20 and always with 2 pairs of smooth macrosetae (Figs. 26, 27). Dens globular (Figs. 26, 27), almost spherical, with 4 to 14 short setae asymmetrical (average of 21 specimens = 7 + 7). In parenthesis the number of cases: 1 + 0 (1); 4 + 5 (1); 5+5(2);6+5(1);5+7(1);6+7(4);7+7(2);6+ 8 (1); 7 + 8 (1), 11 + 12 (1); 11 + 13 (1); 13 + 14 (1). Mucro short, triangular with an internal lamella difficult to see in dorsal view; one juvenile had 1 mucro reduced to triangular spine. Out of 20 specimens: 13 had mucro, in some of them they are vestigial, 6 had no mucro and in one case 1 specimen had mucro only on one side (Fig. 26).
Genital plates of females and males with 12-32 pregenital setae, 26-56 circumgenital setae, females with 1 + 1 eugenital (Fig. 29), males 6 + 6 eugenital setae shorter and thicker than circumgenital (Figs. 28, 30). Abd. VI completely hidden under Abd. V. Ventral side of Abd. V with about 100 setae and 1 pair of macrosetae (Fig. 31). Lav with 24-36 setae of different sizes and 3 macrosetae each. Pav with 1 pair of macrosetae and about 20 setae. None of them as h.r setae. Abd. V with 1 pair of dorsal sensilla (Fig. 32) at the hind edge of body.
Variation. Sensorial organ of Ant. III with 2 short curved sensilla, after original description without guard sensilla, but they are thin and difficult to see, dorsal apex with 8-16 sensilla very geniculate. Very strong plurichaetosis on Ant. IV.
Distribution of ventral macrosetae very constant although sometimes those setae are not very thick. Postlabial setae 5 + 5, but some asymmetrical specimens were found. Tita. with 2 acuminate ventral t.h. on proximal verticil, sometimes other setae also long (thus they seem to have 3 or 4 ventral t.h.), 1 specimen with only 1 tenent hair (from 24 examined). One specimen has 1 leg teratologic, with 2 abnormal tibiotarsi (Fig. 24). This kind of teratological abnormality in Collembola has been mentioned by Najt and Massoud (1976) and Najt and Dalens (1979), interestingly found in another member of the Pseudachorutinae. Adult males have 6 pairs of eugenital setae, very often similar to regular setae, but sometimes modified, spiniform, probably due to an epitoky (occurring during reproductive period) as seen in 2 specimens collected in December 1992.
Ontogenetical development. In 7 juveniles 12 pregenital setae were observed with no circumgenital or eugenital setae, but in adults from 12 to 32.
Remarks
Weiner and Najt (1997) studied 3 specimens of this species from Brunswick Peninsula, Laguna Parillar (Punta Arena) from a Nothofagus forest, identified as D. guilleni. The female was 4.5 mm and juveniles 1.6 mm long. In alcohol, their color was dark grey-blue. The specimens had 17 short, thick subcylindrical sensilla on Ant. IV with a large “sensory file”, but those were probably only the unmodified ventral setae. Claw with 1 inner basal tooth, ventral tube with about 30 + 30 setae, tenaculum with 2 + 2 teeth. Dens reduced, globular, with 11-30 setae and mucro absent. The authors stated that D. guilleni differs from D. globulosa by the shape of maxillary head, absence of mucro, and tenaculum with only 2 + 2 teeth (3+3 in N. globulosa), but all those characters can vary as shown in the previous redescription.
Delamarellina globulosa (Cassagnau et Rapoport, 1962) was described thanks to findings in Puerto Best and Lago Frías, Argentina (2 specimens at each site, only 4 km distant from each other). Rapoport and Rubio (1963) formally described the genus and a presumed second species (11 specimens from Cerro El Roble, Valparaíso, Chile, in a Nothofagus obliqua forest), however, they suggested conducting a more precise study based on a larger number of specimens to analyze the morphological variation. In the original description of D. globulosa, the authors affirm that the antennal organ III does not have guard sensilla, because it is difficult to distinguish them since they are thin and acuminate; however, they are indeed present. According to Massoud (1967), this genus is related to other genera who have complex mouthparts, such as Pseudachorudina, Notachorudina and Quatacanthella, but the latter genus has 8 + 8 eyes, lacks furcula and the Abd. VI is not completely hidden under the Abd. V. When they are compared with members of other subfamilies, they appears more similar to Morulina (Morulininae) and Denisimeria (Uchidanurinae), because they share with them the presence of body intersegments with many setae, strong antennae, body hypertrichosis, cryptogypy, and the lack of a true sensory organ in Ant. IV.
According to Massoud (1967), Delamarellina belongs to genera with complicated mouthparts such as Pseudachorudina, Notachorudina, and Quatacanthella but he classifies it closer to Arlesia because of the absence of postantennal organ and the reduction in the number of eyes; however, this should be an object of further discussion because of the differences of mouth parts and body chaetotaxy and many other characters. Some members of Aethiopella are similar in having reduced furcula.
It is interesting that the members of Morulina (Neanuridae: Morulininae), a subfamily restricted to Northern Hemisphere with clear body tubercles, have a strong plurichaetosis on antennae, dorsal and ventral body, ventral tube, pregenital setae, and anal valves; and often have Abd. VI completely hidden under Abd. V. So, if those characters are compared, there are more similarities of Delamarellina with members of Morulina than with other genera of Pseudachorutinae and some differences like the furcula reduction (with small dens and few setae and total lack of mucro) and the mouth parts, besides the dorsal tuberculations.
New records: Delamarellina globulosa (Cassagnau et Rapoport, 1962). 6 slides from Chile, X Region 4-I-1993, D. Burckhandt col. Deposited at the Genève Museum of Natural History on September 8, 2010.
Material deposited at Facultad de Ciencias, UNAM: 25 slides.
NotachorudinaCassagnau et Rapoport, 1962.
Diagnosis. Aspects similar to genera Pseudachorutes and Pseudachorudina. Paratergites absent. Ant. III and IV dorsally fused. Ant. IV with apical vesicle trilobed, sensilla simple, and 1 long ventral sensorial file with many modified setae. Ant. III organ with 2 microsensilla in 1 groove, under a big cuticular fold. The mouth beak similar to Pseudachorudina. Maxillae complicated with several lamellae. Mandibles with many teeth. 8 eyes per side on a very dark patch. PAO absent. Unguis with internal and lateral teeth. Tenaculum and furcula present. Abd. VI clearly visible in dorsal view.
Type species: Notachorudina patagonicaCassagnau et Rapoport, 1962.
Remarks
The ventral sensorial field on Ant. IV and the apical displacement of Ant. III microsensilla are similar to Neotropiella, but this genus has only 5 eyes per side (Palacios-Vargas & Callohuari, 2020); the mandible of Notachorudina has more teeth and serrate lamellae on maxillae, the smallest lamella with a big tooth, like the members of Delamarellina and Cassagnaurida. The absence of PAO and presence of 8 + 8 eyes, well-developed furcula, manubrium with 19 + 19 setae and 3 pairs are macroseta, and mucro without lamella differentiate Notachorudina very clearly from Neotropiella. Notachorudina patagonicaCassagnau et Rapoport, 1962.
Nov. Syn. Notachorudina rapoportiQueiroz et Zeppelini, 2017 (Figs. 33-41)
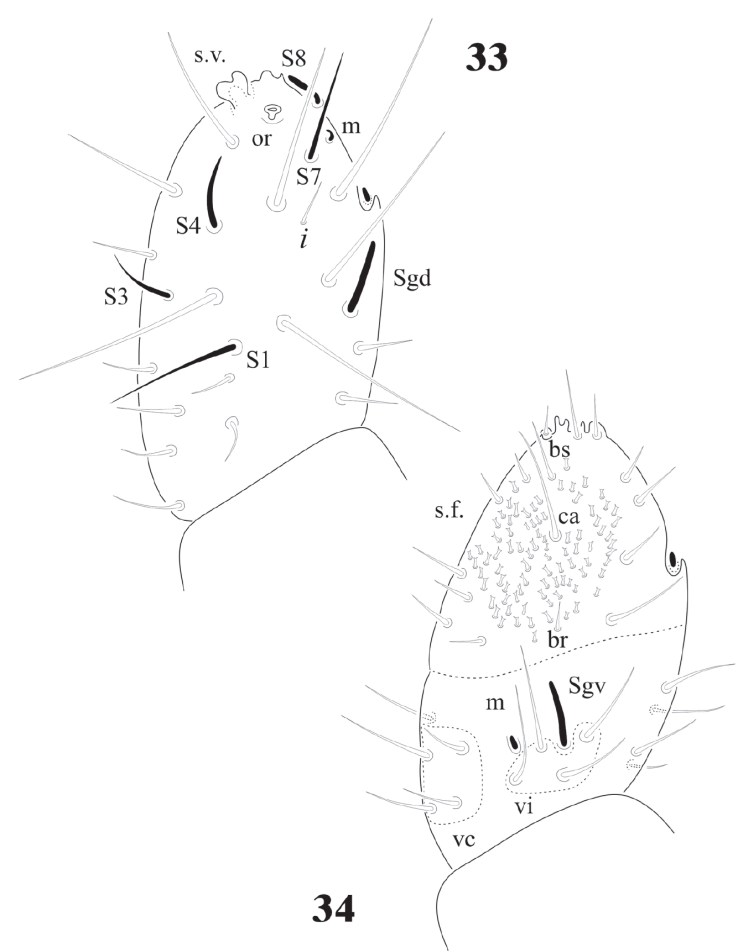
Figures 33-34 Notachorudina patagonica. 33, Ant. III-IV of juvenile, dorsal view; 34, Ant. III- IV of juvenile, ventral view.
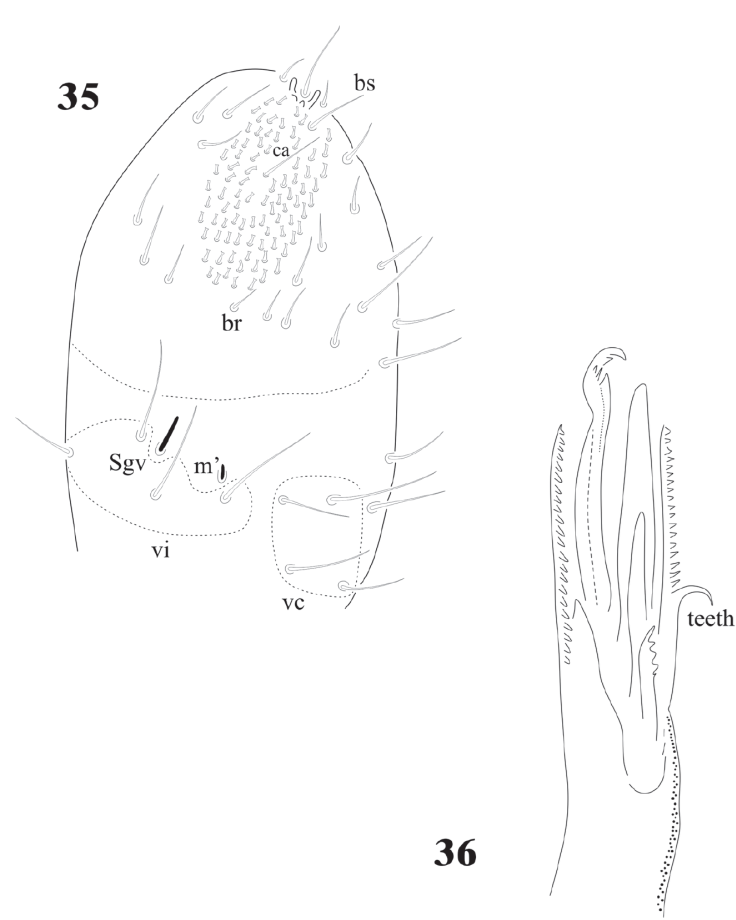
Figures 35-36 Notachorudina patagonica. 35, Ant. III- IV of adult, ventral view; 36, maxilla in frontal view.
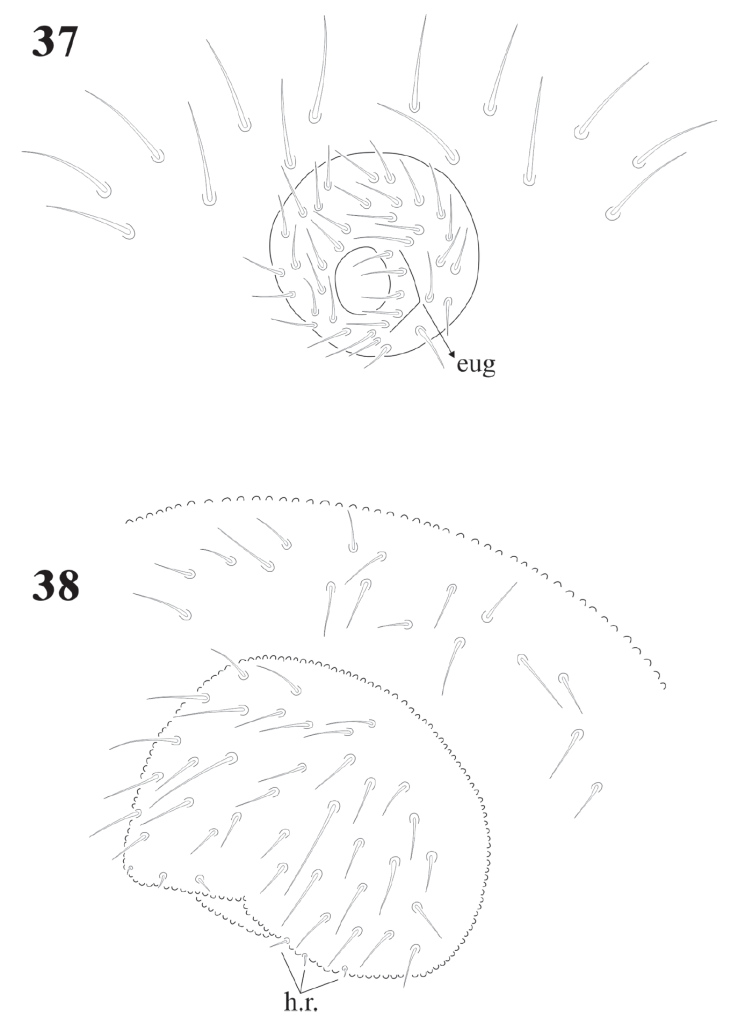
Figures 37-38 Notachorudina patagonica. 37, Genital plate of male; 38, ventral view of Abd. V and VI of juvenile.
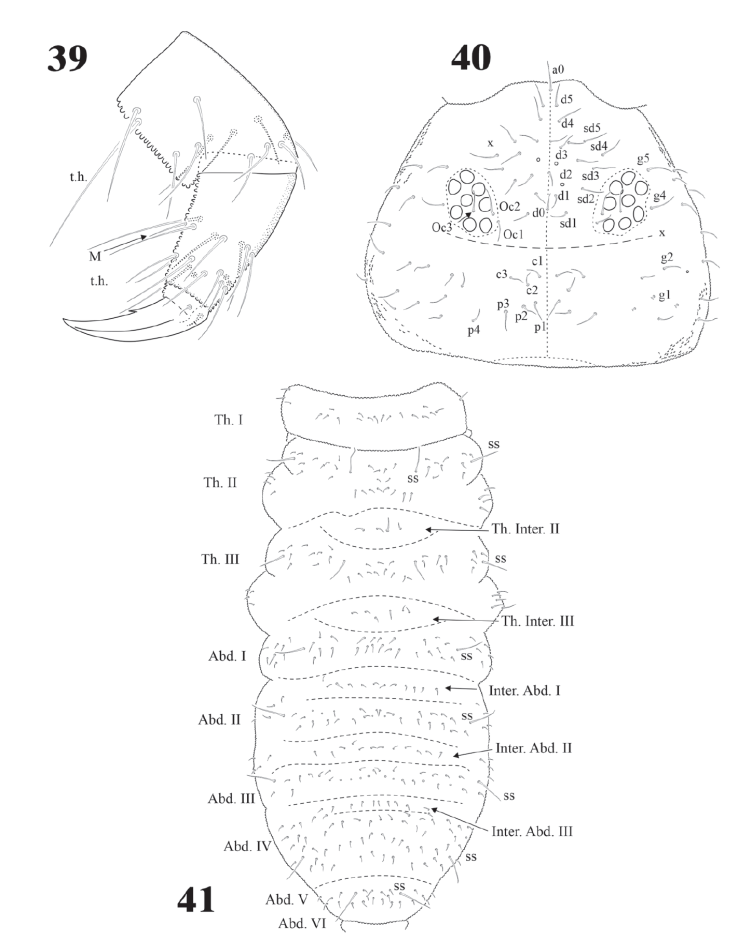
Figures 39-41 Notachorudina patagonica. 39, Femur, Tita. and foot complex I, lateral view; 40, head chaetotaxy, modified from Queiroz and Zeppelini (2017); 41, body chaetotaxy, modified from Queiroz and Zeppelini (2017) with intertergal areas notation.
Complement to original description. Length female: (n = 25) 2.7 mm; male (n = 12) 2.5 mm; juveniles (n = 6) 1.5 mm. Body color, dark blue; white legs and ventral abdomen. Ratio antenna: head = 1: 1.3. Ant. IV with trilobed apical bulb (rarely bilobed). Six sensilla (S1-S4, S7 and S8), only S4 and S8 thick, others thin, 12 ordinary setae (Fig. 33); ventral file from 60 to more than 90 small setae with truncate apex (Fig. 34), no difference between juveniles and adults (Fig. 35). Ant. III organ with microsetae apically displaced, at level of the small seta i, under a big cuticular fold; Sgd and Sgv not apically displaced. Ant. I and II with 7 and 13 setae respectively; incorrectly mentioned as 13 and 7 by Queiroz and Zeppelini (2017) who made an excellent description of the supposed N. rapoporti; therefore, only additional information is provided in this paper with the new synonymy of this species with N. patagonica.
Eyes 8 + 8, PAO absent. Dorsal head chaetotaxy with small plurichaetosis: a0 present and other medial setae present; rows d1-5 and sd1-5 normal; rows c and p with 3 setae per side (Fig. 40) like Cassagnaurida dentata. Mandible with 9 teeth, basal one with many small denticles basally. Maxillary head with 2 strong teeth, 4 serrate lamellae (one of them with a big basal tooth), and one long and thin lamella with several small apical teeth and one short with a few teeth (Fig. 36). Labium truncate with setae A and C longer than B and D with papillated seta L and x organs absent. Thorax and abdomen with moderate pluritrichoses (Figs. 40, 41) very similar to C. dentata with several small microsetae and long sensilla with intertergal areas from Th. II to Abd. III. Th. I with 8 + 8 setae in 2 rows and 3 laterals. Interseg. Th. II with 2 + 2 setae; interseg. Th. III with 3 + 3; interseg. Abd. I with 6 + 6 setae and interseg. III with 5 + 5 setae. Sensillar formula by demitergite: 022/11111. Abd. V with 5 + 5 setae between sensilla. Tita. with 2 acuminate t.h. and seda M between them. Lateral anal valves not clearly separated with 3 h.r. each (Fig. 38) since the first instar to adults.
Female genital plate with 16 pregenital, 11 circumgenital, and 1+1 eugenital setae; male plate with 14 pregenital, about 30 circumgenital, and 5 + 5 eugenital setae (Fig. 37). Juveniles have the same number of pregenital setae, but shorter (Fig. 38).
Variation. Juveniles from 1.1 mm to 2.25 mm long. 1.16 mm in average. The number of r.h. setae does not vary during the development, but the ventral setae on Abd. V changes during the development and continues after the specimens are adults. During the growth of juveniles some of the circumgenital setae appear, preadults can have from 4 + 4 setae but eugenital setae are absent. Adults have 18 pregenital, up to 56 circumgenital, and 5 + 5 eugenital setae in males and 1 +1 seta in females. One adult male had only 1 dens and mucro, on the other side they had none. In 2 males there were 6 + 5 and 5 + 4 eugenital setae, respectively.
Remarks
Notachorudina rapoporti after Queiroz and Zeppelini (2017) is supposed to differ from N. patagonica by only a few differences: 1) sensorial field of N. patagonica with 50 modified setae and N. rapoporti with 90, which are in the range of variation; 2) the sensorial setae formula by demitergite in N. patagonica 22/22222 and in N. rapoporti 22/11111, which is a wrong interpretation; even Queiroz and Zeppelini noted that the first formula is quite unusual for Pseudachorutinae. In the original description, Cassagnau and Rapoport (1962) wrote on page 172: “on trouve sur chaque segment, de Th. II à Abd. V, une paire de longues soies dorsales sur le tergite et un pair de longer soies latérales”, which does not imply that formula and they must have seen a specimen of Cassagnaurida dentata, which had similar appearance and apparently that formula arises when macrosetae are wrongly recognized as sensorial setae (figures 7 and 8 in this contribution). Unguis of N. patagonica is supposed to have a strong basal inner tooth, 2 baso-lateral teeth, and sometimes 2 latero-distal teeth, while N. rapoporti is supposed to have an inner basal tooth, not so strong, inconspicuous and apically baso-lateral teeth with a strong unpaired latero-distal tooth in adults. One subadult male from Osorno municipality presents only a small and inconspicuous latero-distal tooth. But this character varies within the species. Ventral tibiotarsal setae B4 and 5 are very long in some specimens from the same locality, as noted in the original description and by Izarra (1972), who illustrated the tibiotarsus and setae of a specimen from Isla Victoria, National Park, where the species was first found. Our specimens show M seta as in Fig. 39, similar to the supposed Notachorudina rapoporti. Body plurichaetosis from head to Abd. VI (including intertergal areas) is very little compared with the strong in Delamarellina. In Cassagnaurida it is moderate and Notachorudina has a very few setae and most of segments only have 2 rows of setae.
Discussion
The sensilla on Ant. IV of juveniles and adults C. dentata are difficult to distinguish, except for S7 and S8 (Fig. 1), others are very similar to the ordinary setae. In D. globulosa there are several very short and small apical sensilla (12 or more) and a few other (about 4) longer (Fig. 15). Notachorudina patagonica has 6 dorsal sensilla, as illustrated in figure 12 by Queiroz and Zeppelini (2017), but juveniles lack some of them (Fig. 33), but the antennal organ III (Fig. 34) is hidden behind a big cuticular fold and strongly displaced forwards as it happens in members of Neotropiella. For the ventral chaetotaxy of Ant. III in C. dentata (Fig. 2) there are 3 groups of setae (vi, vc, and ve) as described by Smolis (2008) in Endonura (Neanurinae: Neanurini), but in D. globulosa and N. patagonica only groups vi and vc are easy to distinguish and they have 4 setae in both cases (Figs. 15, 35). On the ventral side of Ant. IV of C. dentata (Fig. 2) and N. patagonica (Fig. 34) there is a well-defined ventral file and the setae groups br, ca, and bs also defined by Smolis (2008) but in D. globulosa there are many small acuminate setae not modified in the form of a sensorial file.
Cassagnaurida dentata has labial setae A-G and the papillate setae L, but no x organ, and it has 2 pairs of postlabial setae (Fig. 6), identical to N. patagonica. The labium of Delamarellina globulosa has setae from A to G, a very modified seta L without tubercle and 3 sensorial x organs on each side (Fig. 21). This is somewhat unique in genera from Patagonia, but such organs are present in other Neanuridae like Morulina (Morulininae), which is only distributed in the Holarctic region. Delamarellina has 5 + 5 postlabial setae but sometimes there are some asymmetries. The labial illustration of Notachorudina patagonica represented by Queiroz and Zeppelini (2017) shows setae A-G, papillate setae L but no sensorial x organ. As mentioned by Fjellberg (1999), members of Pseudachorutinae display a variety of labial types, but only a few genera have been surveyed. The lack of x organ in C. dentata and N. patagonica is similar to Micranurida pygmaea, another member of the same subfamily, but Delamarellina globulosa has 3 labial x organs, something seen in Pseudachorutes sp. (Deharveng, 1983), but Fjellberg (1999) did not find any x organ in Pseudachorutes boerneri, P. parvulus, or P. dubius. Such labial organs are present in other Pseudachorutinae: Pseudachorutes plurichaetosus (1 x organ), Anurida granaria and A. tullbergi (2 x organs), and Gastranurida denisi (1 x organ) according to the descripitons of Jordana et al. (1997). So, it seems that this character is very variable within genera of the subfamily and among the members of the same genus and then of little use for phylogenetic research.
The mandible in the 3 genera is similar with many teeth, except D. globulosa (Figs. 19, 20). The other 2 species have ventral denticles in the basal tooth (Fig. 5). The maxillae are also very alike with the internal lamella serrate and with a big basal tooth (Figs. 4, 18, 36). This also happens in Tasmanura as described by Greenslade (2020) and in Platanurida marplesioides. Among the 3 genera, Notachorudina seems to have the most plesiomorphic maxilla because it has 2 big teeth and 4 lamella (one of them also with a big basal tooth), which is shared with those previously mentioned genera from New Zealand.
The intertergal areas from Th. I to Abd. III are well developed and with setae in C. dentata (Figs. 7, 8) and N. patagonica (Figs. 40, 41), but in D. globulosa we were not able to detect the intertergal area Abd. III due to the strong plurichaetosis (Fig. 14), not even in the juveniles (Fig. 32); but there is a very clear presence of setae on the other intertergites. Intertergites are very common in many Poduromorpha, but not the presence of setae on them and when they are, usually only a very few as in Pseudachorutes polychaetosus Gao and Palacios-Vargas, 2008 (from China), with only 2 setae on intertergal thorax I (17 species from the genus in different continents lack such setae). Neotropiella arretada Bellini et al., 2019 (from Brazil) has only 2 setae on intertergal abdomen III, but N. peruanaPalacios-Vargas et Calloyuari, 2020 lacks them. Delamarellina is similar to Forsteramea, which is monospecific and endemic of New Zealand. F. megacephala needs to be redescribed for a better morphological comparison. The setae on ventral Abd. V of Delamarellina are extremely abundant when compared with other Pseudachorutinae (and most of Neanurinae) mainly on Abd. V but also on VI, being more similar to members of Morulininae or Uchidanurinae, which are distributed in the Holactic region and Melanesia and Micronesia areas, respectively. In many Pseudachorutinae there are usually only 3 + 3 pregenital setae. Cassagnaurida has 5 + 5 pregenital setae, Notachorudina has from 14 to 16 pregenital setae, even in juveniles, but Delamarellina has from 12 to 32. Even this is a plesiomorphic character (and the reduction an apomorphic character). It is difficult to find any pattern because other species like Honduranura centraliamericanaPalacios-Vargas, 2017 (Neanurinae: Sensillanurini) has 6 + 6 pregenital setae and belong to a tribe distributed only from the Southern USA to Colombia. The displacement of abdominal segment VI under the V, or cryptopygy, occurs in other Pseudachoutinae genera and even in other Neanuridae subfamilies and should be analyzed more carefully to verify if this is a convergence or has a meaningful relationship.
A remarkable feature of Notachorudina, pointed out by Queiroz and Zeppelini (2017), is the denticulate basal tooth on mandibles. Since some characters in Notachorudina, such as serrate lamellae of maxillae and labium with papillated chaeta L, are considered primitive character states, denticles of basal mandible tooth could also be interpreted as a plesiomorphic character, representing vestiges of a mandibular molar plate (Queiroz & Zeppelini, 2017). However, more research should be done to put more light on their phylogenetic importance. Besides, Notachorudina patagonica, the endemic Cassagnaurida dentata and Pseudachorutella castrii also present such type of mandibles. Other Pseudachorutinae with strong plurichaetosis and complex maxillae are Anurida bisetosa Bagnal, 1949 and A. maritima (Guérin-Méneville, 1837) but they lack intertergal areas with setae and cryptopygy. Anyway, the genus Anurida is cosmopolitan with about 80 species, which are mainly related to seashores (marine littoral habitats). The problem of dividing Pseudachorutinae intro tribes is complicated and comparison based on morphological characters and molecular data from different genera may reveal the relationships within this large subfamily and put more light on its phylogeny and evolution.
Zoogeographical remarks
Cassagnau and Rapoport (1962) described N. patagonica from Nahuel Huapi National Park in Argentina, at the border of the Subantarctic subregion of the Andean region. One specimen identified as N. patagonica was collected in the Nahuelbuta National Park in Chile, northern part of the Subantarctic subregion (Rapoport & Rubio, 1968) and 2 from Isla Victoria, close to the type locality, within Nahuel Huapi (Izarra, 1972).
Rapoport (1968) established the biogeographic bases of the Patagonian subregion, which shares fauna with southern Australia, Tasmania, and New Zealand. Massoud (1967) provided distribution maps of all known Neanuridae at that time and discussed them extensively; however, many taxa need revision. All the species of these 3 genera: Delamarellina, Pseudachorudina, and Notachorudina (except Delamarellina ubiquata which is endemic to New Zealand) are distributed only in the Subantarctic subregion, Maule province (Pehuén district), Selva Valdiviana province (districts of Llanquihue and Chiloé Sur) and the province of Bosque de Magallanes (Malleco); the district of the Cordillera de Chillán (Pehuén and Nahuelbuta) according to the provinces and districts suggested by Morrone (2015) for the Patagonia.
According to Massoud (1967), the ancestor of the Neanuridae might have lived before the Devonian period, and it evolved in many completely different ways. Two branches that separated very early from the ancestor, Odontellidae and Brachystomellidae, have completely lost their jaws: and have a wide distribution.
The Frieseinae have retained many of the characters of the hypogastrurids such as a slightly elongated maxilla, a short mouth cone, and often anal spines; they have acquired new characteristics such as the frequent reduction of the number of eyes and the furcula for euedaphic life or kept plesiomorphic characters for hemi edaphic environments and other adaptations to different environments.
Until 1955, many authors had separated the rest of the Neanuridae into Neanurinae and Pseudachorutinae (Anuridae included). These subfamilies were based on characters such as the number of eyes, presence or absence of the postanntenal organ, development of furcula, appearance of the body with tubercles, reticulation of the integument, and bilobed or entire form of the sixth abdominal segment. But many of those characters are present or absent in genera of closely related families like Brachystomellidae and Odontellidae.
Cassagnau (1955) proposed a classification for the Holarctic neanurids based solely on the mouthparts. He placed species that have a complicated maxilla type in Pseudachorutinae and those that have simple mouthparts in Neanurinae. Later, Cassagnau (1982, 1983) proposed a phylogenetic classification of the Neanurinae into tribes.
In South America, there are Anuridini without reduced eyes and according to Massoud (1967), close to the European Pseudachorudina: Notachorudina; without postantennal organ, with a typical appearance of Pseudachorutinae; with a characteristic sensorial file ventrally in antennal segment IV, with only 1 species N. patagonica (described from Nahuel Huapi, Argentina) and Cassagnaurida, with only C. dentata, with Pseudachorutes type and a “Ceratrimerian” appearance (originally from Los Cántaros in Patagonia). This is an erroneous assertion, since both genera have a similar appearance, with intertergites with setae and a very similar chaetotaxy if we compare drawings of chaetotaxy of their corresponding representatives in this contribution (Figs. 7, 8, 41).
There is also one single Anuridini genus with reduced eyes and complex maxilla: Delamarellina. It has 5 eyes per side and is characterized by the absence of postantennal organ. According to Massoud (1967), it is very close to Arlesia but with a complex maxilla. Delamarellina shows a very strong plurichaetosis and the presence of intertergites with setae that are absent in Arlesia, so they are very different to be considered closely related genera.
On the other hand, in Delamarellina there is a noticeable reduction of the furcula, and the abdominal segment VI is completely hidden under V. Furthermore, the only species that exists in Patagonia, D. globulosa, has great intraspecific variation as observed in the present study. We need a good study of the chaetotaxy of D. proprieta that lives in New Zealand and its comparison with the only species from Patagonia, because it has a well-developed furcula with 6 setae and a long mucro but other characters are still unknown. It should be noted that 14 of 57 genera of Pseudachorutinae: Acanthanura, Anuritelsa, Cassagnaurida, Delamarellina, Forsteremea, Holacanthella, Megalanura, Meganurida, Notachorudina, Platanurida, Quatacanthella, Tasmanura, Holocanthella, and Womersleymeria, have a distribution limited to the southernmost part of the world in Australia, Tasmania, and New Zealand, but only 3 of them in Patagonia (Fig. 42), which corresponds to the Austral kingdom (Morrone, 2015). Most of them are monospecific and it would be convenient to make a detailed study of their morphology in order to do a more precise phylogenetic analysis and propose their separation within the tribe Anuridini of the subfamily Pseudachorutinae. This aggrupation would exclude Grananurida, which is distributed only in the Sino-Japanese region and has many autopomorphies like the lack of eyes, furcula reduction, and needle-like maxilla.
An update of the original observations of Rapoport (1971) is necessary and the proposals about Collembola biogeography of Christiansen and Bellinger (1995) need to be reviewed. Most springtails closely related to Patagonian Pseudachorutinae are mainly distributed in Tasmania, Australia, and New Zealand, restricted to the Southern Hemisphere. A similar case is Spinothecidae (Fig. 42) which is represented by Adelphoderia regina in Australia and Tasmania, Spinotheca magnasetaceae in New Zealand, S. cyaneae and S. patagonica both in Nothofagus forest from Patagonia, and one endemic genus and species Troglospinotheca refsgaardiorum discovered in Caverna del Leon in an arid area (Las Lajas) in Neuquen, Argentina (Palacios-Vargas, 1999).











 text new page (beta)
text new page (beta)






