Introduction
Deltochilum Eschscholtz, 1822 is one of the most diverse New World genera of dung beetles, with more than 100 species from the United States to Argentina (Halffter, 2003; Schoolmeesters, 2019). Deltochilum are roller beetles typical of tropical, subtropical and some temperate habitats (Génier, 2012; Halffter, 2003). Species in Deltochilum have a wide variety of trophic habits as coprophagy and necrophagy, with some species attracted to rotting fruits and fungi, or specialized to prey on diplopods (Cano, 1998; Capello & Halffter, 2019; Halffter, 2003; Halffter & Halffter, 2009; Larsen et al., 2009; Silva et al., 2012, 2015, 2017).
Deltochilum mexicanum Burmeister, 1848 was described from Mexico, without specific locality. Subsequently, the closely related D. burmeisteri Harold, 1867 was described from Ecuador. Paulian (1939) decided to consider D. burmeisteri as a junior subjective synonym of D. mexicanum. This synonymy was maintained in subsequent works (Chamorro et al., 2018, 2019; González et al., 2009; Howden, 1966; Howden & Young, 1981; Pereira & D’Andretta, 1955), despite that earlier taxonomists had recognized differences in body color between northern Mesoamerican and Costa Rican-South American specimens (Bates, 1887; Howden, 1966). The aim of this work is to revalidate and redescribe D. burmeisteri. The external and genital morphological characters that differ from D. mexicanum are highlighted and illustrated. An updated key is provided to ease the determination of closely related species from Mexico to Colombia.
Materials and methods
The phylogenetic species concept was used for this work (Wheeler & Platnick, 2000). It defines the species as the smallest aggregation of (sexual) populations or (asexual) lineages that is diagnosable by a unique combination of character states. For nomenclature of morphological structures Cristóvão and Vaz-de-Mello (2020), Génier (2019), González-Alvarado and Vaz-de-Mello (2014), and Harris (1979), were followed. Label data are given verbatim. The aedeagi were prepared with a 10% KOH solution, soaked during 24 hours at room temperature, rinsed with 96% ethanol and then with water, and stored in microvials (BioQuip Products, Inc., Rancho Dominguez, California, USA) with glycerol. Glycerol-stored genitalia is recommended to allow preservation and ease future studies (Cristóvão & Vaz-de-Mello, 2020). Microvials were pinned under the dissected specimens.
Measurements and pictures were taken using a Leica Z16APOA stereomicroscope with Leica Smart Touch and Leica DMC2900 camera (Leica, Wetzlar, Germany) using the manufacturer´s software and the z-stack image capture method (except for Figure 13). The stereomicroscope lightning was substituted (except for the endophallites) to obtain adequate color images as follows: a cylinder made of matte drafting acetate functioned as a light diffuser, while a cylinder made of a rolled LED light strip (300 LEDs / 5 m, 12 Vcc, white 6,000-7,000 k, LED 3,528, 13 W/h; Steren, Mexico City, Mexico) was used as light source. Final multistack images were edited using a Wacom Intuos PEN tablet CTL-6100WL (Wacom Co., Ltd., Toyonodai Kazo-shi, Saitama, Japan) with Adobe Photoshop CC v. 2015 (Adobe Systems Incorporated, San José, California, USA) and CorelDRAW X7 v. 17.0.0.491 (Corel Corporation, Ottawa, Canada).
Distributional data were taken from specimen labels, databases (GBIF Secretariat, 2019) and literature (Alvarado et al., 2014; Barretto et al., 2018; Bates, 1887; Chamorro et al., 2018, 2019; Creedy & Mann, 2011; Escobar & Chacón-de Ulloa, 2000; González et al., 2009; Howden, 1966; Howden & Young, 1981; Huerta et al., 2016; Inward et al., 2011; Medina et al., 2001; Morón & Terrón, 1984; Paulian, 1939; Pereira & D’Andretta, 1955; Ratcliffe et al., 2015; Rös et al., 2012; Solís & Kohlmann, 2012).
Specimens revised for this work are deposited in the following collections: Seção de Entomologia da Coleção Zoológica, Departamento de Biologia e Zoologia, Universidade Federal de Mato Grosso, Cuiabá, Brazil (CEMT); Colección Gonzalo Halffter, Instituto de Ecología A.C., Xalapa, Veracruz, Mexico (GHC); Colección Entomológica Dr. Miguel Ángel Morón Ríos, Instituto de Ecología A.C., Xalapa, Veracruz, Mexico (IEXA); Colección José Luis Sánchez Huerta, Xalapa, Veracruz, Mexico (JLSHC); Muséum National d’Histoire Naturelle, Paris, France (MNHN); Texas A&M University Insect Collection, College Station, Texas, United States of America (TAMU); and Colección Víctor Moctezuma, Puebla, Puebla, Mexico (VMC).
Redescription
Family Scarabaeidae Latreille, 1802
Subfamily Scarabaeinae Latreille, 1802
Genus Deltochilum Eschscholtz, 1822
Deltochilum (Calhyboma) burmeisteri Harold, 1867, valid species
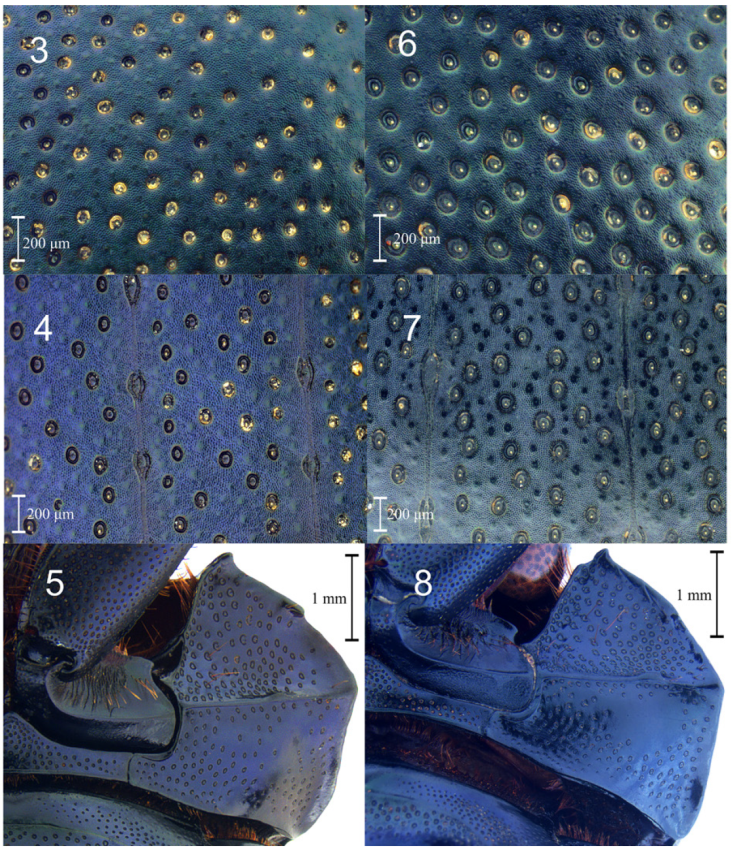
Figures 3-8 Deltochilum burmeisteri. 3, Pronotal integument; 4, elytral integument; 5, hypomeron. Deltochilum mexicanum. 6, Pronotal integument; 7, elytral integument; 8, hypomeron.
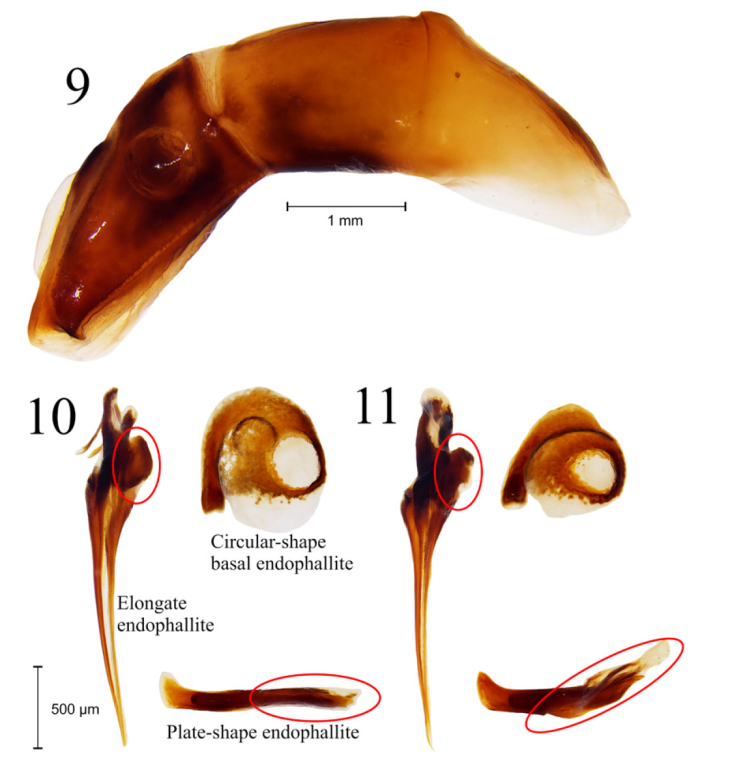
Figures 9-11 9, Aedeagus of Deltochilum burmeisteri. 10, Endophallites of Deltochilum burmeisteri and 11, Deltochilum mexicanum.
Diagnosis. Deltochilum burmeisteri is easily recognized among others within the subgenus Calhyboma by the following character combination (some characters are shared with closely related species, but these characters conform altogether a unique character combination within Calhyboma): discal elytral tubercles obsolete in males (those are however humped), elytra without distinctly impressed sculpture, glossy metallic blue-green color, yellow antennae, border of pronotum posteriorly rounded, and frontolateral angles of pronotum distinctly notched. Deltochilum burmeisteri and D. mexicanum are closely related and similar in morphology (Figs. 1, 2). Both species are blue-green dorsally, but D. mexicanum is dull. Moreover, D. burmeisteri differs by the pronotal and elytral punctures less dense and more reduced in size, and integument lightly granulate (D. mexicanum with pronotal tegument micropunctate and elytra distinctly granulate), and the frontolateral margin of the hypomeron less densely punctate (Figs. 3-8). The aedeagi of D. burmeisteri and D. mexicanum are similar in shape (Fig. 9), but the superior right portion of the elongate endophallite of D. burmeisteri is lobed (notched in D. mexicanum), the circular-shape endophallite is less compact, and the plate-shape endophallite is not cristate as in D. mexicanum (Figs. 10, 11). Detailed images for D. carinatum (Westwood, 1837); D. cristinae Martínez, 1991; D. hypponum (Buquet, 1844); D. luederwaldtiPereira and D’Andretta, 1955; D. robustus Molano and González, 2009; and D. tessellatum Bates, 1870; are available from González et al. (2009).
Male. Habitus: glossy metallic blue-green dorsally; integument lightly granulate, scabriculous (Fig. 1). Head: punctation deeply impressed, ocellate, reduced in size toward the clypeus, rounded. Each clypeal puncture bearing an inconspicuous seta. Clypeus bidentate. Genal suture distinctly impressed. Yellow antennae. Pronotum: glabrous dorsally. Pronotal punctation deeply impressed, ocellate, rounded, variable in size, becoming smaller in the anteromedial portion of disk (Fig. 3). Pronotal anterior angle acute; pronotal margin between anterior and medial lateral angles notched; pronotal margin between medial lateral and posterior angles straight; pronotal medial lateral angle rounded. Hypomeron carinate medially. Hypomeral carina inconspicuously indentate medially. Hypomeral punctures deeply impressed, ocellate, rounded, variable in size (Fig. 5). Elytra: elytral disk with inconspicuous humps. Striae fine, superficially impressed, without internal line. Strial punctures ocellate, deeply impressed, obtusely oval to round. Interstrial punctures ocellate, deeply impressed, variable in size. Strial punctures deeply impressed, ocellate, rounded (Fig. 4). Base of the interstriae 6 with carina short, strongly developed. Basal margin of the interstriae 3 and 5 bearing a tubercle inconspicuous, rounded. Apex of the interstriae 3-7 with carina or rounded tubercle. Tergite 8: punctation obtusely oval to rounded, ocellate, deeply impressed. Metasternum. Almost impunctate medially, with anterior and posterior fossae. Legs: protibiae tridentate, with apical projection short, tick. Profemur with indentation in frontolateral margin. Mesotibia with indentation in posteromedial margin. Metatibiae with indentation in posterolateral margin. Mesotrochanter with indentation strongly developed, projected posteriorly. Genitalia: apex of parameres rounded, indentate ventrally (Fig. 9). Endophallites as in Fig. 10.
Female. Similar to the male except for the elytral humps obsolete and last abdominal sternite (ventrite) not concave medially (Fig. 13).
Variation. Mean length 22.8 mm (19.4-25.8 mm). The development of the elytral humps varies with the body size, being almost completely reduced in the smallest males.
Taxonomic summary
Type locality. Ecuador, Quito.
Type material examined. Lectotype female, present designation (Fig. 13). Ecuador. Pichincha. Labeled: “Quito”. The lectotype is designated herein in order to fix the name of the species on a single specimen and to provide accurate future identifications. The specimen in question is the only one that could be unambiguously traced in the MNHN to the original type-series (unknown number of specimens, but at least 2 because of the body size variation mentioned in the original description).
Non-type material examined. Costa Rica. Cartago. 4 males, 1 female: “Sierra de Talamanca, Empalme. 23-IX-69. G. Halffter y P. Reyes C. col. Selva húmeda de montaña. Alt. 1,800 m. Cebo cadáver.” (GHC: 4 males, 1 female, IEXA: 1 male). San José. 2 males: “San Isidro del General. 23-IX-69, G. Halffter y P. Reyes, cols. Selva tropical de montaña. Cebo excremento, noche/ día. Alt. 1,480 m” (VMC); 1 male: “21 km N. E. San Isidro del General. 21-IX-69. G. Halffter y P. Reyes col. Selva tropical de Montaña. Alt. 1,900 m. Cebo tlacuache. Noche/día” (JLSHC); 3 males: “21 km N. E. San Isidro del General. 20-IX-69. G. Halffter y P. Reyes col. Selva tropical de Montaña. Alt. 1,900 m. Cebo tlacuache. Día/ noche” (GHC); 1 male: “San Isidro del General. 23-IX-69.P. Reyes y G. Halffter col. Selva tropical de Montaña. Alt. 1,900 m. Cebo tlacuache” (TAMU); 1 male: “San Isidro del General. 21-IX-69. P. Reyes y G. Halffter col. Selva tropical de Montaña. Alt. 1,900 m. Cebo tlacuache. Noche/día” (CEMT); 1 male: “San Isidro del General. 22-IX-69. G. Halffter y P. Reyes col. Selva tropical de Montaña. Alt. 1,900 m. Cebo excremento. Noche/mañana” (CNMN); 3 unsexed specimens: Coto Brus. Est. Biol. Las Alturas. 08°57’N 82°50’ W. 1,550-1,600 m. FIT. III/IV-2002. Cline & Tishechkin” (CEMT). Colombia. Valle del Cauca. 2 unsexed specimens: “Calima. Río Bravo, La Palmera Est. Nov-Dic-94. Var. Col” (CEMT); 1 unsexed specimen: “Río Calima, Quebrada El Pital. Nov. 12. 80. R. Torres” (CEMT); 1 unsexed specimen: “Río Calima. 900 m. VII-1993. R. Aldana” (CEMT); “Calima. La Palmera. Julio 91. A. Papamija” (CEMT); “Queremal. 2,000 m. T. Exc. H. Bosque intervenido. Julio 14 1996. C. Medina” (CEMT); 1 unsexed specimen: “Yotoco. 1,600 m. Bosque. 27-29 mayo de 2003. C.A. Medina” (CEMT). Bolivar. 4 unsexed specimens: “Serranía de San Lucas. 8°02’15” N, 74°12’09” W. 1,550 m. T pitfall Ex. Human. Mar/2019. Jorge A. Noriega” (CEMT).
Distribution and ecology. Copronecrophage, nocturnal, roller species, that ranges from Costa Rica to Bolivia, typical of the tropical mountain cloud forests between 900-2,200 m. The closely related D. mexicanum is restricted to the tropical mountains of northern Mesoamerica from Mexico to Nicaragua. The records of D. burmeisteri for Brazil (González et al., 2009) and D. mexicanum for Durango (Paulian, 1939) are doubtful and were omitted for this work (Fig. 12).
Discussion
Despite D. burmeisteri and D. mexicanum have been previously considered as synonyms (González et al., 2009; Howden, 1966; Howden & Young, 1981; Paulian, 1939; Pereira & D’Andretta, 1955), differences in morphology between them have been highlighted before our work. The first author who highlighted the differences in body color was Bates (1887), asserting that Mexican specimens were “dull bluish-black” while the Costa Rican and Panamanian specimens were “rich dark blue”. Even Paulian (1939) recognized the blue mate color in D. mexicanum: “En Amérique du Sud, l’espèce est généralement noire, seul le type de Harold faisant exception à ma connaissance; en Amérique centrale et au Mexique, aue contraire, elle est généralement d’un bleu plus ou moins mat” - In South America, the species is generally black, to my knowledge only the Harold’s type is the exception; contrary to Central America and Mexico, it is generally blue mate. Moreover, Howden (1966) mentioned that “the species ranges from Mexico into South America, with Mexican specimens, in general, having the purple or bluish color more subdued”.
Differences in color between D. mexicanum and D. burmeisteri are also recognized by us, but differences in the pronotal and elytral integument, and the endophallites are reported herein for the first time. The endophallus of D. burmeisteri was previously illustrated by Pereira and D’Andretta (1955), but the endophallites were not dissected and compared with those of D. mexicanum. The endophallites of D. burmeisteri were illustrated by González et al. (2009) but not compared with those of D. mexicanum.
We hope that our contribution on the taxonomic knowledge of D. burmeisteri and D. mexicanum will be of great value for the understanding of ecology and conservation of Scarabaeinae beetles. Both copronecrophage and nocturnal roller species are almost exclusively associated with well-conserved mountain cloud forests (Alvarado et al., 2014; Creedy & Mann, 2011; Escobar & Chacón-de Ulloa, 2000; González et al., 2009; Howden & Young, 1981; Huerta et al., 2016; Inward et al., 2011; Medina et al., 2001; Morón & Terrón, 1984; Rös et al., 2012; Solís & Kohlmann, 2012), being appropriate subrogates to study the effects of the habitat modification and climate change as a consequence of their vulnerability to human disturbance (Barretto et al., 2018).
Key for the species of the subgenus Deltochilum (Calhyboma) occurring from Mexico to Colombia (modified from González et al., 2009).
1 Pronotum without notch between anterior and medial angles ..........................................................2
- Pronotum notched between anterior and medial angles ..................................................................3
2 Pronotum carinate, not iridescent, posterior angles of pronotum with spiniform protrusion, points of the elytral striae distinctly impressed, elytral disk with interstriae 3 and 4 carinate ………………………………………………………D. carinatum (Westwood, 1837)
- Pronotum not carinate, iridescent, posterior angles of pronotum sinuate without spiniform protrusion, points of the elytral interstriae with deeply impressed concavities, elytra disk with interstriae 3 and 4 not carinate………….............................……….D. hypponum (Buquet, 1844)
3 Elytral striae fine, lightly impressed, without uplifts or concavities.....................................................4
- Elytral striae distinctly impressed, with uplifts or concavities ............................................................5
4 Clypeal teeth separated by approximately 2 diameters; posterior angles of pronotum rounded; interstriae with relatively large concavities, each occupying about 1/5 of the distance between each striae………………………………………………7
- Clypeal teeth separated 1 diameter; posterior angles of pronotum spiniform; interstrial points small, each occupying about 1/13 of the distance between each striae……………….......D. robustus Molano and González, 2009
5 Red iridescent species with green sheen; medial angles of pronotum salient and slightly projected posteriorly; elytral interstriae with uplifted and bright irregular areas, salient punctures and points distinctly marked…………..D. luederwaldtiPereira and D’Andretta, 1955
- Dull or brilliant species, but never iridescent, and lacking the former combination of characters.............................................6
6 Bright blue or black species, clypeal teeth separated at most by 2 diameters, anterior and medial angles of pronotum distinctly notched; elytral striae with points longitudinally subovate, elytral striae with obliterate line. Tergite 8 with each point separated about 1 diameter……………….......D. tessellatum Bates, 1870
- Dull light blue species; clipeal teeth separated by more than 2 diameters, anterior and medial angles of pronotum slightly notched; elytral striae with depressed points; elytral interstriae with irregular and salient punctures and deeply impressed points. Tergite 8 with rounded points, each point separated more than 1 diameter.................................................................................................................... D. cristinae Martínez, 1991
7 Glossy species (Figs. 1, 13); pronotal and elytral punctures less dense and more reduced in size (Figs. 3-4); pronotal integument lightly granulate (Fig. 3); frontolateral margin of the hypomeron notched, with integument less densely punctate (Fig. 5), superior right portion of the elongate endophallite lobed; plate-shape endophallite cristate (Fig. 10). From Costa Rica to Bolivia (Fig. 12)........................D. burmeisteri Harold, 1867 valid species
- Dull species (Fig. 2); pronotal and elytral punctures more dense and larger in size (Figs. 6, 7); pronotal integument micropunctate (Fig. 6); frontolateral margin of the hypomeron not notched, with integument less densely punctate (Fig. 7); superior right portion of the elongate endophallite notched; plate-shape endophallite not cristate (Fig. 11). From Mexico to Nicaragua (Fig. 12).....................................................................D. mexicanum Burmeister, 1848











 text new page (beta)
text new page (beta)






