Introduction
Dwarf crayfish are found in the genus Cambarellus Ortmann, 1905. Species included in the group are characterized by a series of traits, such as the hooks on the coxae of the second and third pereiopods of the form I male, a first pleopod with distal portion tightly folded, and distal end of sperm groove opening on 1 (central projection) of 3 terminal elements (mesial and caudal processes and central projection) (Hobbs, 1974). Species described from Mexico have been grouped in the subgenus Cambarellus by Fitzpatrick (1983), defined, among other characteristics, by a longitudinal groove in the mesial process of gonopod of the form I male, which also has a rounded or truncate tip.
Ten species from Mexico are currently considered valid (Crandall & De Grave, 2017). Cambarellus (Cambarellus) montezumae was the first species described by Saussure (1858) from the Valley of Mexico. Later, Faxon (1885) described C. (C.) areolatus from the basin of Parras, Coahuila, C. (C.) occidentalis from the western-most population for the genus at Mazatlán, Sinaloa, and C. (C.) chapalanus from Lake Chapala (Faxon, 1898). Villalobos (1943) described C. (C.) patzcuarensis from Lake Pátzcuaro, Michoacán, C. (C.) zampoalensis from Zempoala Lagoons, Morelos, and C. (C.) alvarezi from El Potosí, Nuevo León (Villalobos, 1951). Later, C. (C.) chihuahuae from Chihuahua (Hobbs, 1980) and C. (C.) prolixus from Lake Chapala were described (Villalobos-Figueroa & Hobbs, 1981). More recently, C. (C.) zacapuensis was described from the Zacapu Lagoon Michoacán (Pedraza-Lara & Doadrio, 2015), which elevated to 10 the number of recognized species from Mexico, most of them inhabiting distinct localities along the Trans-Mexican Volcanic Belt (TMVB). Although recognized as valid nowadays, several species of the subgenus Cambarellus were originally described as subspecies, forms or varieties. That is the case of C. areolatus (Faxon, 1885), C. occidentalis (Faxon, 1898), C. patzcuarensisVillalobos, 1943 and C. zempoalensisVillalobos, 1943. A formal nomenclatural act is in preparation to fullfil their definite taxonomic formality. Additionally, that work should include the definition for “C. montezumae var. tridens” (Von Martens, 1872), “C. montezumae dugesi” (Faxon, 1898), and “C. montezumae forma lermensis” Villalobos, 1943.
A phylogenetic hypothesis for the genus Cambarellus was postulated (Pedraza-Lara et al., 2012), in which all extant species and topotypes for most of them were included, except for C. chihuahuae, then believed to be extinct (Carson et al., 2015). For that work, sampling from El Vegil Lagoon was conducted following previous records which documented the occurrence of crayfish in that locality (Nuevo Vegil) and identified them as C. montezumae (Gutiérrez-Yurrita & Morales-Ortíz, 2002; Gutiérrez-Yurrita et al., 2002). Together with other lineages, Pedraza-Lara et al. (2012) found that specimens from El Vegil Lagoon and Miguel Allende Dam formed a monophyletic clade, which was mentioned as a possible undescribed species, and was left for further research. This lineage corresponds to populations inhabiting La Laja river sub-basin, part of the Lerma watershed that is the major drainage basin along the TMVB. The most recent common ancestor for this lineage was dated to have split from the rest of Cambarellus around 8.4 Mya (7.5-9.5 Mya, 95% HPD; Pedraza-Lara et al., 2012), providing additional evidence for its possible recognition as a new species. Because gonopod variation in Mexican species of Cambarellus is of reduced utility for species discrimination (Pedraza-Lara et al., 2012), an analysis of the morphological variability from populations assigned to this lineage was conducted, combined with additional genetic data, to determine species status for this population.
Materials and methods
Field sampling was made in 2 localities from La Laja River basin, the first from El Vegil Lagoon, Municipality of Huimilpan, Querétaro, and the second one at the reservoir Miguel Allende, San Miguel de Allende, Guanajuato, Mexico. In order to evaluate the hypothesis of being assignable to a new species, we conducted morphological and molecular analyses of these 2 populations and compared results with other species of the subgenus Cambarellus from the TMVB. Sampling included specimens from 2 field trips: the first one, in September 2006 which was included in the study by Pedraza-Lara et al. (2012) and the second one, in October 2015. Crayfishes were collected by dip-netting at the shore of both water reservoirs. Individuals were captured until obtaining several form I males and mature females. Data from most of the valid species inhabiting the TMVB were also included in the analysis, which comprised C. chapalanus from the Camecuaro River and C. zacapuensis from the type locality, both in Michoacán (Table 1; Fig. 1). Data were also taken from the type series of C. patzcuarensis and C. zempoalensis, deposited at the National Crustacean Collection (CNCR), Instituto de Biología, Universidad Nacional Autónoma de México, Mexico, as well as from individuals collected from the type locality of C. montezumae (Chapultepec Lake in the Valley of Mexico) (Table 1). Also, individuals from the locality of Lerma, Estado de México were included, which are the specimens possibly used by Villalobos (1943) to describe the form ‘lermensis’ of C. montezumae, which was further synonymized by Hobbs (1972) with C. montezumae. Additional specimens coming from recent collections from the region of Lerma were also included (Table 1). All specimens were identified using the available keys, and the respective taxonomic descriptions (Hobbs, 1972). Measurements were made on standard morphological characters used in crayfish taxonomy (Pedraza-Lara & Doadrio, 2015). Other traits more specific to the subgenus Cambarellus were also used as shown to be useful to differentiate species (Pedraza-Lara & Doadrio, 2015). Fifteen measures were taken from all individuals of both sexes (Table 2; Fig. 2). For paired characters, measures were taken from the left side of the specimen. Before taking definite measurements, a validation procedure was carried on by measuring 10 individuals chosen at random from El Vegil Lagoon and repeating the procedure 3 times for each specimen. Posteriorly, a Pearson correlation between replicates was done. Pearson correlation values above 0.8 were considered as indication of low measurement error. This was verified and all measurements aforementioned showed values above 0.8, which allowed us to measure individuals for further analyses. All species described from the TMVB were included except for C. prolixus and C. occidentalis, which are among the most divergent species of the genus, morphologically. A digital caliper (Mitutoyo’s Absolute Series 500, resolution: 0.01 mm) and a stereoscopic microscope (Leica M60 APO) at different magnifications were used to take measurements. To evaluate the influence of sexual dimorphism on the data, a two-way analysis of variance (ANOVA) was carried out considering sex and population as factors, as well as their interaction. The presence of allometry in the data was assessed by using a principal component analysis (PCA) of all variables and evaluating the scores of the variables from principal component 1, in which all coefficients were positive (Creighton & Strauss, 1986; Klingenberg, 1996). Additionally, a high coefficient of regression was observed between such scores and total length. Consequently, we carried out a standardization by calculating the ratio between all traits and each of the measurements. Later, data were log-transformed to overcome their non-normal distribution and to reduce the range in scales of values. PCA was performed on standardized and log-transformed variables to obtain the principal component scores (PCs). The statistics package PAST was used in the aforementioned statistical analyses (Hammer et al., 2001).
Table 1 Species of Cambarellus, localities, voucher codes, and number of males and females included in the morphometric analysis.
| Species | Locality | Voucher or batch code | Male (n) | Female (n) | Morfotype (n) |
|---|---|---|---|---|---|
| C. moi sp. nov. | El Vegil Lagoon, Querétaro | CNCR 35714 - CNCR 35716, CARF-CPL2808 - CARF-CPL2838 | 17* | 18* | 1 |
| C. moi sp. nov. | Miguel Allende Dam, Guanajuato | CARF-CPL 2839 - CARF-CPL 2856 | 11 | 7 | |
| C. chapalanus | Camecuaro River, Michoacán | CARF-CPL2857 - CARF-CPL2883 | 15 | 12 | |
| C. montezumae | Lago Mayor, Chapultepec, Mexico City | CNCR-1867 | 13** | 6** | |
| C. montezumae | Lerma, Estado de Mexico*** | CNCR-1515 | 6 | 6 | |
| C. patzcuarensis | Patzcuaro Lake, Michoacán | CNCR-1829 | 2* | 1* | 1 |
| C. zacapuensis | Zacapu Lagoon, Michoacán | CARF-CPL2884 - CARF-CPL2913 | 15* | 15 | 1 |
| C. zempoalensis | Zempoala Lagoons, Morelos | CNCR-1861 | 7* | 2* | 1 |
* Includes the specie’s type specimens (holotype, allotype). ** Topotypes. *** The locality of Lerma include specimens from the sampling used by Villalobos (1943) to describe the form lermensis, considered a junior synonym of C. montezumae.

Figure 1 Map of localities of Cambarellus species from central Mexico included in this study. 1) C. moi sp. nov. from El Vegil Lagoon, Querétaro, and 2)Miguel Allende Dam, Guanajuato; 3) C. zacapuensis from Zacapu Lake, Michoacán ; 4) C. prolixus from Ajijic, Jalisco; 5) C. chapalanus from Camécuaro, Michoacán; 6) Zapotlán Lagoon, Jalisco, and 7) San Juanico Lagoon, Jalisco; 8) C. montezumae from Xochimilco, Mexico City; 9) C. patzcuarensis from Pátzcuaro Lake, and 10) Chapultepec spring, Michoacán; 11) C. zempoalensis from Tepuxtepec Dam, Michoacán, and 12) Zempoala Lagoons, Morelos; 13) C. occidentalis from Tepic, Nayarit.
Table 2 Morphometric measurements (mm) of holotype, allotype and morphotype of Cambarellus moi sp. nov.
| Holotype | Allotype | Morphotype | * | |
|---|---|---|---|---|
| Total length (TL) | 40.28 | 41.51 | 31.41 | 29.83 |
| Cephalothorax | ||||
| Length (CL) | 18.28 | 19.02 | 14.29 | 14.08 |
| Height (CH) | 8.95 | 9.13 | 7.36 | 6.69 |
| Width (CW) | 8.97 | 9.45 | 6.81 | 6.62 |
| Cephalon length (CEL) | 12.44 | 12.95 | 9.96 | 9.88 |
| Abdomen width (AW) | 7.63 | 9.40 | 6.45 | 6.07 |
| Rostrum | ||||
| Length (RL) | 6.67 | 6.23 | 4.69 | 5.04 |
| Width (RW) | 2.95 | 3.28 | 2.12 | 2.17 |
| Acumen length (AL) | 1.87 | 1.81 | 1.42 | 1.61 |
| Antennal scale length (ASL) | 4.96 | 4.34 | 4.69 | 3.75 |
| Cheliped | ||||
| Chela length (CHL) | 12 | 9.55 | 9.69 | 8.71 |
| Chela width (CHW) | 3.9 | 3.71 | 2.69 | 2.56 |
| Dactyl length (DL) | 5.39 | 5.08 | 4.83 | 4.34 |
| Merus length (ML) | 7.38 | 5.88 | 5.89 | 5.45 |
| Areola | ||||
| Areola width (ARW)** | 5.7 | 5.70 | 3.87 | 4.20 |
| Areola length (ARL) | 3.06 | 1.77 | 2.77 | 1.73 |
* Average from all form I males. ** Not included in statistical analyses.
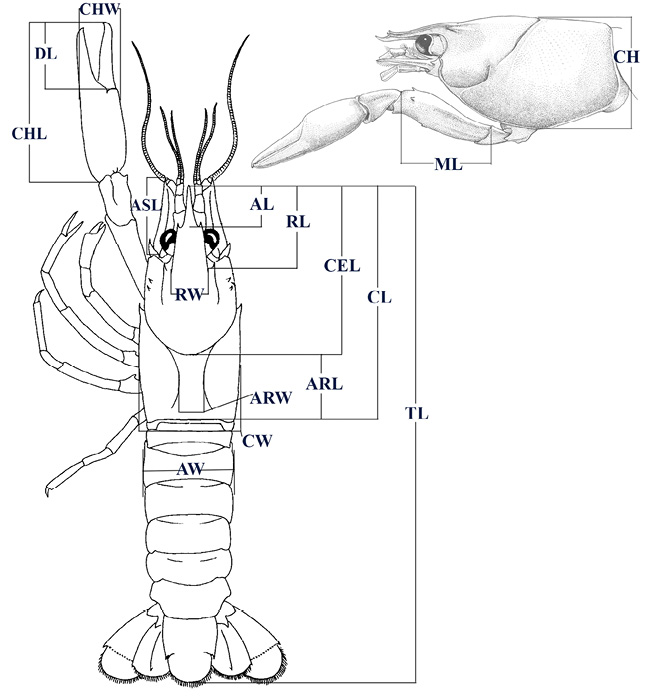
Figure 2 Morphological traits measured, modified from Hobbs (1972), and Pedraza-Lara & Doadrio (2015). Acumen length (AL), areola length (ARL), areola width (ARW), antennal scale length (ASL), abdomen width (AW), cephalon length (CEL), cephalothorax height (CH), chela length (CHL), chela width (CHW), cephalothorax length (CL), cephalothorax width (CW), dactyl length (DL), merus length (ML), rostrum length (RL), rostrum width (RW), and total length (TL).
In order to test the results from PCA, a multivariate analysis of covariance (MANCOVA) was used. In this analysis, standardized and log-transformed measures were considered as dependent variables, the assigned species as a factor, in which the specimens from El Vegil Lagoon and Miguel Allende Dam were categorized as one entity, and log-transformed TL as co-variable. The inclusion of TL as co-variable was necessary as certain amount of size information is usually expected (Garita-Alvarado et al., 2018; Klingenberg, 2016). Pairwise comparisons were performed for each variable and species by generating 1,000 bootstrap replicates, in which significance was set to p < 0.05 and corrected for multiple tests effects by Bonferroni correction. MANCOVA was conducted using the statistical package SPSS v.22.0.0 (IBM, 2013). Individuals coming from the population of Pátzcuaro were removed due to our low sampling size of this locality (n = 2).
Sequences from 3 mitochondrial (COI with 1526 bp, 16S rDNA with 495 bp and 12S rDNA with 356 bp) and 2 nuclear genes (28S rDNA with 992 bp and H3 with 322 bp) were obtained from 5 individuals from El Vegil Lagoon. These were added to 2 sequences already available from that locality and 3 from Miguel Allende Dam from the work of Pedraza-Lara et al. (2012) (Table 3). DNA extraction and amplifications were carried out following conditions previously specified for the group (Pedraza-Lara et al., 2010). Fragments were sequenced on an ABI 3730XL DNA Analyzer. Sequences of the different gene fragments were aligned using MUSCLE (Edgar, 2004). In the case of mitochondrial fragments, recommendations to detect the occurrence of possible numts (nuclear mitochondrial pseudogenes) were carried out for each sequence (Buhay, 2009; Song et al., 2008). Phylogenetic placement of the new species was corroborated by the inclusion of all extant species of the genus Cambarellus from México (except for C. chihuahuae) downloading the corresponding data from Genbank (Table 3) (Pedraza-Lara et al., 2012). C. texanus Albaugh and Black, 1973 and C. diminutus Hobbs, 1945 (previously identified as members of the sister clade to all Cambarellus species from México) as well as Procambarus clarkii (Girard, 1852) were included as outgroups. Phylogenies were estimated using maximum likelihood (ML) and Bayesian inference (BI) approaches. To identify the most appropriate evolutionary model of nucleotide substitution, we considered the Akaike corrected information criterion (AICc) (Akaike, 1974), and the Bayesian information criterion (BIC) (Schwarz, 1978) as estimated using jModeltest (Darriba et al., 2012) for each gene fragment. A phylogenetic tree was constructed under ML using PHYML 3.0 (Guindon et al., 2010) and AICc-selected parameters for the concatenated matrix. Confidence in branches was assessed using 1,000 nonparametric bootstrap replicates under the best-fit evolutionary model (Felsenstein, 1985). Bayesian inference of phylogeny was implemented in MrBayes v. 3.2, following the BIC-selected parameters and applying a Monte Carlo Markov Chain (MCMC) search procedure for 10 million generations (Ronquist et al., 2012). Sequences were partitioned by codon position for COI and by gene for the rest of fragments, using the parameters found by BIC as priors and unlinking the run parameters. Convergence was supervised between the different run parameters in paired simultaneous runs (4 chains by run), trees were sampled every 100 generations and run length was adjusted considering an adequate sampling based on average standard deviation of split frequencies being < 0.01 (Huelsenbeck & Ronquist, 2005) . Confidence in nodes was assessed from the posterior probabilities along the MCMC run. Highly supported nodes are termed herein as those with a value of 95% or more in posterior probabilities and 90% or more in bootstrap values. ML-distances of the COI fragment between species were estimated using MEGA (Kumar et al., 2018) and the parameters of the best substitution model inferred for that fragment in jModeltest. Variances in estimated distances were calculated using bootstrap (Tamura & Kumar, 2002).
Table 3 Cambarellus species, localities, and Genbank accession numbers used in this study.
| Species | Locality | Voucher/ code† | Accesion numbers | |||||
|---|---|---|---|---|---|---|---|---|
| 16S rDNA | 12S rDNA | COI | 28S rDNA | H3 | ||||
| 1 | C. moi sp. nov. | El Vegil, Querétaro * | CPL2559 | MT891130 | MT891135 | MT891125 | MT891140 | MT891145 |
| 1 | C. moi sp. nov. | El Vegil, Querétaro * | CPL2560 | MT891131 | MT891136 | MT891126 | MT891141 | MT891146 |
| 1 | C. moi sp. nov. | El Vegil, Querétaro * | CPL2561 | MT891133 | MT891138 | MT891128 | MT891143 | MT891148 |
| 1 | C. moi sp. nov. | El Vegil, Querétaro * | CPL2562 | MT891132 | MT891137 | MT891127 | MT891142 | MT891147 |
| 1 | C. moi sp. nov. | El Vegil, Querétaro * | CPL2563 | MT891134 | MT891139 | MT891129 | MT891144 | MT891149 |
| 1 | C. moi sp. nov. | El Vegil, Querétaro** | 57-1 | JX127727 | JX127601 | JX127870 | JX127462 | JX127322 |
| 1 | C. moi sp. nov. | El Vegil, Querétaro** | 57-2 | JX127767 | JX127641 | JX127910 | JX127502 | JX127362 |
| 2 | C. moi sp. nov. | Miguel Allende Dam, Guanajuato** | 41-3 | JX127768 | JX127642 | JX127911 | JX127503 | JX127363 |
| 2 | C. moi sp. nov. | Miguel Allende Dam, Guanajuato** | 41-4 | JX127792 | JX127666 | JX127935 | JX127527 | JX127387 |
| 2 | C. moi sp. nov. | Miguel Allende Dam, Guanajuato** | 41-5 | JX127797 | JX127671 | JX127940 | JX127532 | JX127392 |
| 3 | C. zacapuensis | Zacapu Lake, Michoacán*** | 1 | KP712910 | KP712905 | KP71290 | KP712915 | KP712920 |
| 3 | C. zacapuensis | Zacapu Lake, Michoacán*** | 2 | KP712914 | KP712909 | KP712904 | KP712919 | KP712924 |
| 4 | C. prolixus | Ajijic, Jalisco** | 43-1 | JX127729 | JX127603 | JX127872 | JX127464 | JX127324 |
| 5 | C. chapalanus | Camécuaro, Michoacán** | 44-1 | JX127735 | JX127609 | JX127878 | JX127470 | JX127330 |
| 6 | C. chapalanus | Zapotlán Lagoon, Jalisco** | 39-1 | JX127742 | JX127616 | JX127885 | JX127477 | JX127337 |
| 7 | C. chapalanus | San Juanico Lagoon, Michoacán** | 37-1 | JX127733 | JX127607 | JX127876 | JX127468 | JX127328 |
| 8 | C. montezumae | Xochimilco, Mexico City** | 56-1 | JX127776 | JX127650 | JX127919 | JX127511 | JX127371 |
| 8 | C. montezumae | Xochimilco, Mexico City** | 56-2 | JX127777 | JX127651 | JX127920 | JX127512 | JX127372 |
| 9 | C. patzcuarensis | Pátzcuaro Lake, Michoacán** | 31-1 | JX127728 | JX127602 | JX127871 | JX127463 | JX127323 |
| 10 | C. patzcuarensis | Chapultepec Spring, Michoacán** | 33-1 | JX127741 | JX127615 | JX127884 | JX127476 | JX127336 |
| 11 | C. zempoalensis | Tepuxtepec Dam, Michoacán** | 28-1 | JX127771 | JX127645 | JX127914 | JX127506 | JX127366 |
| 12 | C. zempoalensis | Zempoala Lagoons, Morelos** | 23-1 | JX127750 | JX127624 | JX127893 | JX127485 | JX127345 |
| 13 | C. occidentalis | Tepic, Nayarit** | 59 | JX127813 | - | JX127956 | JX127548 | JX127408 |
| - | C. texanus | Fort Bend County., Texas** | 13-1 | JX127819 | JX127683 | JX127962 | JX127554 | JX127414 |
| - | C. diminutus | Mobile County, Alabama** | 2-1 | JX127810 | - | JX127953 | JX127545 | JX127405 |
| - | P. clarkii | Muzquiz, Coahuila** | - | JX127829 | JX127692 | JX127971 | JX127563 | JX127424 |
* Sequences generated in this study. ** From Pedraza-Lara et al., 2012. *** From Pedraza-Lara & Doadrio, 2015. † Specimen code assigned in this study or from previous works.
Description
Cambarellus (Cambarellus) moi Pedraza-Lara, Ortíz-Herrera & Jones 2021 sp. nov.
http://zoobank.org/urn:lsid:zoobank.org:act:594C4779-D25B-41FA-A81C-D9BC0CB07378
Diagnosis. Eyes pigmented, well developed. Rostrum with lateral margins subparallel to convergent, bearing terminal spines. Acumen 0.4-0.75 as long as rostrum width and at most 0.35 times rostrum length. Rostrum length 2.3 times rostrum width on average (1.9-2.6). Carapace without cervical spine. Branchiostegal spine absent. Postorbital ridge ending in spine overreaching posterior margin of orbit. Antennal scale approximately 3 times as long as wide, broadest to midlength. Merus of cheliped 1.1 times in rostrum length on average (0.7-1.4). Hooks on ischia of second and third pereiopod of male form I, simple and not overreaching basioischial articulation nor opposed by tubercle on corresponding basis; cephalomesial and caudomesial bosses on coxa of fourth pereiopod, both sublaminated in shape, the former subacute, cephalically directed, the latter thin, rounded, caudally directed. First pleopods of male form I symmetrical, caudodistally oriented at distal end, lacking subapical setae and shoulder on cephalic surface; terminal elements corneous, directed at approximately 45 degree angle to main appendage axis; mesial surface of mesial process forming groove directed caudodistally; central projection acute, oriented caudally; slender caudal process extending caudally beyond rest of elements. Annulus ventralis about 1.5 times as broad as long, caudal face with median concavity at base receiving postannular sclerite when under flexion; ondulated sinus directed sinistrally; outline of postanular sclerite campanulate, 1.1-1.2 times as broad as long. Maximum abdomen width 0.2 times in total length (0.19-0.22).
Holotypic male, form I. Cephalothorax (Fig. 3A) subovate, it´s width approximately half it´s length (0.49). Areola length 0.14 times in total length. Surface of carapace slightly punctuated. Rostrum (Fig. 3B) with slender lateral carinae terminating in spines extending beyond basal segment of antennule; acumen long, well overreaching antennular peduncle slightly beyond antennal scale; dorsal surface with submarginal rows of setiferous punctuations; rostral dorsal surface concave, subrostral margins subparallel, slightly convergent. Suborbital angle well defined, forming obtuse angle. Branchiostegal spine lacking, cephalic point of branchiostegite rounded. Cervical spine absent.
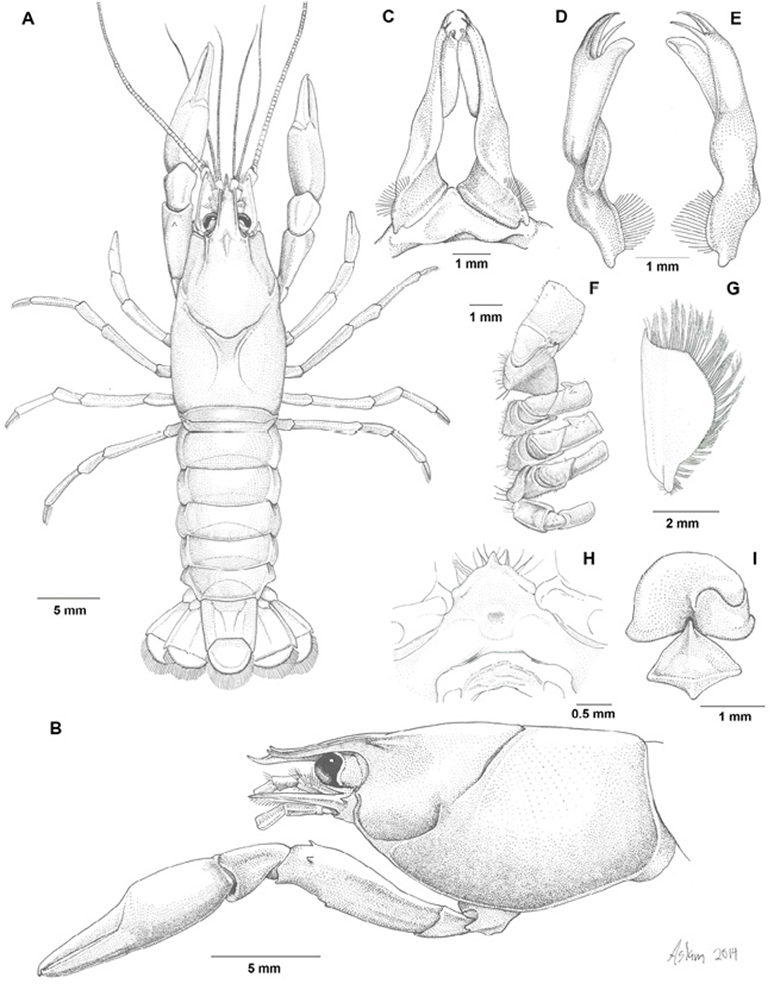
Figure 3 Cambarellus moi sp. nov. A-H From the holotype, I from the allotype: A, dorsal view; B, lateral view; C, caudal view of pair of first pleopods; D, mesial view of left first pleopod; E, lateral view of left first pleopod; F, basal podomeres of second to fourth pereiopods; G, antennal scale; H, epistome; I, annulus ventralis.
Cephalic lobe of epistome joined to main body (Fig. 3H), subtriangular, apex of anterior margin produced; main body with distinct fovea, epistomal zygoma strongly arched. Proximal podomere of antennule with conspicuous spine on ventromesial border at about midlength. Antennal peduncle with spine well developed on distolateral surface of basis; ischium with small ventral projection; flagellum extending back to almost midlength of telson. Antennal scale (Fig. 3G) 2 times as long as width, widest about midlength; lateral spine overreaching antennular peduncle but slightly shorter than tip of acumen.
Cephalic portion of third maxilliped extending slightly beyond basal segment of antennular peduncle; mesial half of ischium with broad row of simple setae, single row of plumose setae flanking ventromesial side of lateral costa, distolateral angle not produced; exopod reaching midlength of propodus.
Right chela (Fig. 3A, B) subovate in cross-section, somewhat depressed; surface lacking spines and tubercles, provided with few small punctuations bearing small short setae, more numerous along opposable sides of fingers; dactyl with corneous tip slightly beyond distal end of fixed finger.
Carpus of cheliped about 1.2 times as long as wide, bearing setiferous punctuations; distal ventrolateral area slightly produced. Merus of cheliped with setiferous punctuations, 1 preterminal spine along dorsal margin and one more on dorsolateral margin, ventral surface with 1 spine about distal third. Ischium also with setiferous punctuations, devoid of spines or tubercles.
Hooks on ischia of second and third pereiopods simple (Fig. 3F), the former overreaching basioischial articulation, not opposed by tubercle on corresponding basis; that on third more tapering. Coxa of fourth pereiopod with cephalomesial and caudomesial bosses on coxae of fourth pereiopod, both sublaminated in shape, the former subacute and cephalically directed, the latter thin, rounded, caudally directed; coxa of fifth pereiopod devoid of caudomesial boss. Sternum between second, third and fourth pereiopods deep; lateral margins not strongly produced ventrally, with setae born on them.
First pleopods as described in diagnosis (Fig. 3C-E). Abdomen slightly narrower than carapace (Fig. 3A). Pleura of third through fifth segments truncate ventrally and lacking angles. Lateral and mesial lobes of proximal podomeres of uropod pointed, distolateral spines on mesial and lateral ramus.
Allotypic female. Apart from sexual features, differing from holotypic male in the following: abdomen distinctly wider than carapace. See table 2 for differences in body proportions.
Annulus ventralis as described in Diagnosis (Fig. 3I). First pleopods absent. Basal podomere of uropod as in holotype.
Morphotypic male, form II. Differing from holotype in the following aspects: rostrum shorter than in holotype, acumen not overreaching antennal scale; epistome triangular, cephalic side not produced; flagellum of antenna broken; hooks on ischia of second and third pereiopods much reduced in size; cephalomesial surface of boss on fourth pereiopod not defined; first pleopod more straight, mesial process shorter and slender, caudal process and central projection as in holotype but shorter, less developed, none of terminal element corneous.
Taxonomic summary
Type material. Type material specimens were deposited at the National Crustacean Collection of the Instituto de Biología, Universidad Nacional Autónoma de México (CNCR): holotype (CNCR 35714), allotype (CNCR 35716), morphotype (CNCR 35715), and paratypes (4 males and 4 females, CNCR 35717). Additional paratype series were deposited at the Collection of Arthropods of Forensic Reference, at the Licenciatura en Ciencia Forense, Universidad Nacional Autónoma de México (CARF), CARF-CPL 2808-2815, ♂I and CARF-CPL 2816-2827 ♀.
Type locality. El Vegil Lagoon, a small dam in the northern side of the town El Vegil (20°26’8.54” N, 100°20’36.79” W; 2,052 m), Municipality of Huimilpan, Querétaro, Mexico.
Range and specimens examined. The species is known from the type locality and from the Miguel Allende Dam in Guanajuato (20°54’18.07” N, 100°47’51.43” W; 1,840 m), although its occurrence among the numerous permanent water bodies in the vicinity is possible. Crayfishes from the type locality were first collected by Patricia Ornelas and Carlos Pedraza on September 2006, along the northern side of the water body using seine nets. The reservoir is formed by the retention of a small stream which is active during the rainy season.
Specimens of the type series were collected at the type locality, September 2006, Collectors: CPL, P. Ornelas-García; additionally, a total of 29 specimens were examined from 4 sampling trips: CARF-CPL 2828-2834, ♂ and CARF-CPL 2835-2838, ♀: same data as type locality, September 2006, Collectors: CPL, P. Ornelas-García; CARF-CPL 2839-2844, ♂I and CARF-CPL 2845-2848, ♀: Miguel Allende Dam, San Miguel de Allende, Guanajuato, September 2006, Collectors: CPL, P. Ornelas-García; CARF-CPL 2849-2853, ♂I and CARF-CPL 2854-2856, ♀: Miguel Allende Dam, San Miguel de Allende, Guanajuato, September 2006, Collectors: CPL, HSOH, J. Contreras, M. Torres; P. Ornelas-García.
Etymology. The specific epithet of Cambarellus moi sp. nov. is derived from the term used by Ñañu people, the inhabitants of the region of the type locality, to designate crayfish.
Remarks
Differences in morphological measurements between sexes were significant, so all subsequent analysis were separated by sex (Fig. 4). PCA from males and females showed separation of specimens from El Vegil Lagoon and Miguel Allende Dam from all other species (Fig. 4). For males, PCI explained 42.5% of the variance (Table 4); the variables contributing the most to ordination were acumen length and rostrum width. PCII explained 22.5% of variance and most relevant variables were rostrum width and acumen and chela length, while PCIII explained 14.7% of variance, and the most important variables were rostrum, acumen, antennal scale and chela lengths. Accumulated variance for the 3 first components was 79.7%. Results from the PCA for females revealed that all cephalic variables found for males were also the most determinant for its ordination, plus chela width instead of its length. Accumulated variance by the first 3 components was 77.03%. Pairwise comparisons from MANCOVA revealed significant differences between individuals from El Vegil Lagoon and Miguel Allende Dam when compared with all other species (Table 5). Cephalic variables were significantly different between C. moi sp. nov. and the rest of analized Cambarellus species (i.e., those related to rostrum, acumen and antennal scale). Measurements were significant for males, as well as females (p < 0.05).
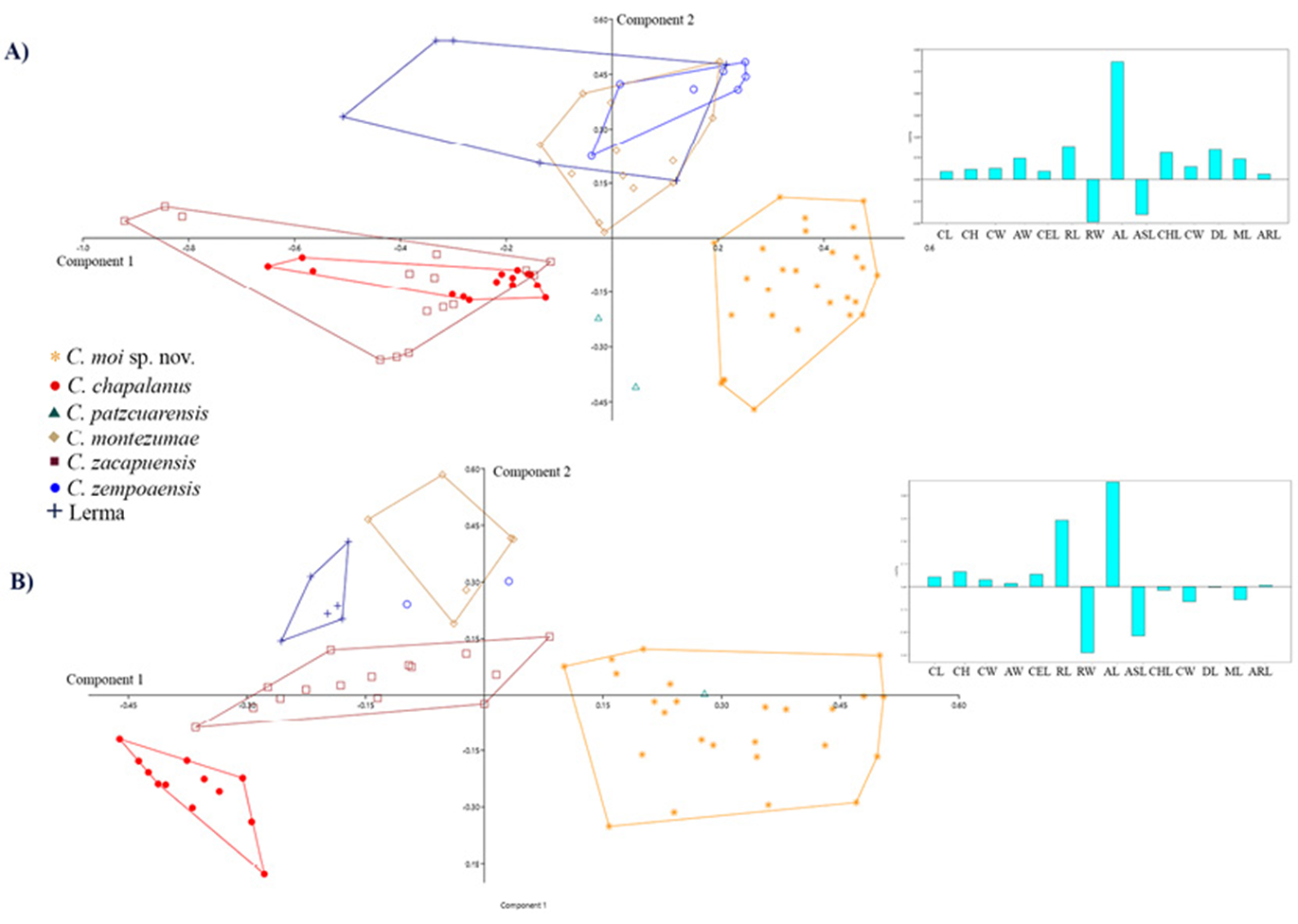
Figure 4 Principal component analysis of morphometric variables from males (A) and females (B) of Cambarellus species from central Mexico. To the right a histogram of PC1 loadings.
Table 4 Factor scores from PCA analyses of form I males and females of species of Cambarellus included in this study. Values in bold are those contributing the most to ordination.
| Factor/males | Factor/females | |||||
|---|---|---|---|---|---|---|
| Standarized measure* | I | II | III | I | II | III |
| Cephalothorax length | 0.0536 | 0.0469 | 0.1076 | 0.0642 | 0.0402 | 0.1629 |
| Cephalothorax height | 0.0689 | 0.0256 | 0.1291 | 0.0980 | -0.0457 | 0.2620 |
| Cephalothorax width | 0.0756 | 0.1667 | 0.0510 | 0.0452 | 0.1816 | 0.2186 |
| Cephalon length | 0.0553 | -0.0421 | 0.0965 | 0.0813 | -0.0263 | 0.1560 |
| Abdomen width | 0.1455 | 0.1590 | 0.0256 | 0.0212 | 0.1694 | 0.2092 |
| Rostrum length | 0.2232 | -0.2425 | 0.3334 | 0.4370 | -0.2977 | 0.2752 |
| Rostrum width | -0.2920 | 0.3874 | -0.5418 | -0.4345 | 0.4102 | -0.0742 |
| Acumen length | 0.8155 | -0.1023 | -0.5590 | 0.6894 | 0.6099 | -0.3191 |
| Antennal scale length | -0.2404 | 0.1380 | -0.3475 | -0.3235 | 0.2700 | -0.2499 |
| Chela length | 0.1851 | 0.3251 | 0.2097 | -0.0234 | 0.1256 | 0.2653 |
| Chela width | 0.0871 | 0.3412 | 0.0925 | -0.0986 | 0.2983 | 0.4519 |
| Dactyl length | 0.2044 | 0.4229 | 0.1964 | -0.0036 | 0.1735 | 0.3408 |
| Merus length | 0.1404 | 0.4000 | 0.1696 | -0.0850 | 0.0250 | 0.3101 |
| Areola length | 0.0360 | 0.3781 | 0.0371 | 0.0081 | 0.3109 | 0.2376 |
*All values were previously standarized by TL and log transformed.
Table 5 P-values of the pairwise comparisons between Cambarellus moi sp. nov. and the rest of species included. Significant p-values after Bonferroni correction are shown in bold. Acumen length (AL), areola length (ARL), areola width (ARW), antennal scale length (ASL), abdomen width (AW), cephalon length (CEL), cephalothorax height (CH), chela length (CHL), chela width (CHW), cephalothorax length (CL), cephalothorax width (CW), dactyl length (DL), merus length (ML), rostrum length (RL), rostrum width (RW).
| Males | C. chapalanus | C. montezumae | C. zacapuensis | C. zempoalensis | Lerma |
|---|---|---|---|---|---|
| CL | 0.000 | 0.003 | 0.000 | 1.000 | 0.591 |
| CH | 0.001 | 0.000 | 0.001 | 1.000 | 1.000 |
| CW | 0.000 | 1.000 | 0.110 | 1.000 | 1.000 |
| AW | 0.000 | 1.000 | 0.000 | 1.000 | 1.000 |
| CEL | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| RL | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| RW | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| AL | 0.000 | 0.317 | 0.000 | 1.000 | 0.000 |
| ASL | 0.000 | 0.000 | 0.000 | 0.008 | 0.002 |
| CHL | 1.000 | 1.000 | 0.000 | 0.001 | 0.577 |
| CHW | 0.001 | 1.000 | 1.000 | 1.000 | 0.148 |
| DL | 0.003 | 0.361 | 0.007 | 0.000 | 0.154 |
| ML | 1.000 | 1.000 | 0.001 | 0.000 | 0.120 |
| ARL | 0.090 | 0.000 | 1.000 | 0.000 | 0.000 |
| Females | C. chapalanus | C. montezumae | C. zacapuensis | C. zempoalensis | Lerma |
| CL | 0.000 | 0.021 | 0.000 | 1.000 | 1.000 |
| CH | 0.074 | 0.007 | 1.000 | 1.000 | 0.000 |
| CW | 0.004 | 0.000 | 0.156 | 1.000 | 0.126 |
| AW | 1.000 | 0.001 | 0.041 | 1.000 | 0.176 |
| CEL | 0.000 | 0.000 | 0.000 | 1.000 | 0.000 |
| RL | 0.000 | 0.000 | 0.000 | 0.001 | 0.000 |
| RW | 0.000 | 0.000 | 0.002 | 0.006 | 0.000 |
| AL | 0.000 | 1.000 | 0.000 | 1.000 | 0.001 |
| ASL | 0.000 | 0.000 | 0.000 | 0.377 | 0.000 |
| CHL | 1.000 | 1.000 | 1.000 | 0.017 | 0.002 |
| CHW | 1.000 | 0.001 | 0.001 | 0.000 | 0.000 |
| DL | 1.000 | 1.000 | 1.000 | 0.215 | 0.184 |
| ML | 0.360 | 1.000 | 1.000 | 0.000 | 1.000 |
| ARL | 0.076 | 0.001 | 1.000 | 0.000 | 0.000 |
The phylogenetic analyses placed all samples from El Vegil Lagoon and Miguel Allende Dam in one clade with high nodal support (Fig. 5), including the individuals collected at different sampling dates (2006 and 2015). This clade lies within the Mexican group (sensu Pedraza-Lara et al., 2012), but the resultant phylogenetic structure places the new species as a notably divergent basal clade within the Mexican group. As such, this does not allow a sister species relationship to be postulated for this new species within the group. Considering variable sites (V) and parsimony informative sites (PI) of COI, the aforementioned populations differentiated, respectively, by 122 and 91 sites from C. chapalanus, 105 and 94 sites from C. zacapuensis, 111 and 76 sites from C. zempoalensis and 221 and 119 from C. montezumae. ML distances from the same fragment showed values above 8% for all comparisons, which, in congruence with phylogenetic structure does not allow the proposal of any species as closest to the new species (Table 6). Such genetic distance with the rest of species from the Mexican group (DML 8.4-14.0%) strongly supports the designation of these populations as a new species, as it is well above the common recorded values for between species distance in crayfish and crustaceans, in general (Fetzner & Crandall, 2002; Matzen da Silva et al., 2011).

Figure 5 Phylogenetic tree of Cambarellus species from central Mexico including the 5 genetic markers analyzed, 3,691 bp in total: COI with 1526 bp, 16S rDNA with 495 bp, 12S rDNA with 356 bp, 28S rDNA with 992 bp, and H3 with 322 bp (Table 3). Bootstrap support values above nodes (maximum likelihood, only values above 85 are depicted) and posterior probabilities are shown below nodes (bayesian inference, only values above 0.90 are depicted).
Table 6 Maximum likelihood (ML) distances for the COI fragment between the Cambarellus species included in this study (bellow diagonal) and standard error (above diagonal). A COI fragment of Procambarus clarkii is included as external group.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| C. moi sp. nov. | 0.014 | 0.015 | 0.016 | 0.015 | 0.014 | 0.024 | 0.017 | 0.045 | 0.080 | 0.066 | |
| C. prolixus | 0.084 | 0.002 | 0.005 | 0.010 | 0.009 | 0.019 | 0.009 | 0.031 | 0.046 | 0.037 | |
| C. chapalanus | 0.094 | 0.011 | 0.006 | 0.010 | 0.009 | 0.021 | 0.010 | 0.033 | 0.049 | 0.039 | |
| C. zacapuensis | 0.096 | 0.026 | 0.033 | 0.010 | 0.010 | 0.021 | 0.009 | 0.038 | 0.053 | 0.041 | |
| C. patzcuarensis | 0.087 | 0.062 | 0.068 | 0.068 | 0.006 | 0.023 | 0.009 | 0.034 | 0.058 | 0.036 | |
| C. zempoalensis | 0.084 | 0.059 | 0.065 | 0.066 | 0.035 | 0.023 | 0.009 | 0.034 | 0.056 | 0.038 | |
| C. montezumae | 0.140 | 0.115 | 0.128 | 0.128 | 0.140 | 0.144 | 0.022 | 0.066 | 0.104 | 0.083 | |
| C. occidentalis | 0.094 | 0.059 | 0.063 | 0.057 | 0.060 | 0.056 | 0.142 | 0.038 | 0.052 | 0.038 | |
| C. texanus | 0.220 | 0.183 | 0.190 | 0.206 | 0.196 | 0.195 | 0.324 | 0.208 | 0.028 | 0.040 | |
| C. diminutus | 0.325 | 0.243 | 0.256 | 0.265 | 0.289 | 0.282 | 0.435 | 0.259 | 0.159 | 0.048 | |
| P. clarkii | 0.293 | 0.202 | 0.212 | 0.218 | 0.199 | 0.206 | 0.375 | 0.204 | 0.210 | 0.251 |
The mesial process of the gonopod ending in a longitudinal groove with rounded tip, undoubtedly identifies C. moi sp. nov. as part of the subgenus Cambarellus (Fitzpatrick, 1983). Phylogenetic information places the new species in the Mexican group (sensu Pedraza-Lara et al. 2012), together with the rest of species of the genus from the TMVB. However, based on distance analyses of COI considering the likelihood model, this is a divergent species regarding the rest of the Cambarellus inhabiting Mexico and no closer relative can be proposed based on the phylogenetic cladogram (Fig. 5; Table 6). Two other species seem to be in similar situation, C. montezumae and C. occidentalis, which are morphologically and genetically divergent from the rest of the species. Current geographic distribution of clades recovered by phylogenetic reconstruction could reflect biogeographic history of the genus in the TMVB (Pedraza-Lara et al., 2012), as well as recent modifications to river basins induced by human activity, as documented for other freshwater organisms in the region (e.g., Domínguez-Domínguez et al., 2012; Ornelas-García et al., 2012).
Morphological analyses here employed support the recognition of several morphological traits to recognize the new species from the rest of Cambarellus from the TMVB (Table 5). This species has a longer cephalon (CE/TL
To our knowledge, this study reports for the first time the analysis of female morphology for species discrimination in Cambarellus. Specifically, female body morphology was useful to separate C. moi sp. nov from other species. Females of the new species were significantly distinct from the other 4 species here included and the population from Lerma by having a longer and slender rostrum, with a different width-length proportion (RW/RL
Also, these findings allow us to conclude that evaluation of body shape variation is of great value for species discrimination in this group of crayfish, in which interspecific variation in gonopod structure is not so evident as in other groups (Ornelas-García et al., 2014; Pedraza-Lara & Doadrio, 2015). Although variation is observed, most individuals collected have a characteristic pale coloration, possibly related to the low transparency of the water in the locality. Studies by the authors are in progress to review the taxonomy of the genus Cambarellus using morphological and molecular variation.
The type locality is found in the headwaters of the river El Pueblito (Fig. 6), part of the Laja river and possibly isolated from the downstream section due to strong habitat perturbations such as intermittent obstruction of the river course and pollution from industrial, agriculture and urban activities. The Miguel Allende Dam is a major reservoir and is fed by the La Laja River, which eventually joins the large Lerma watershed, inhabited by other Cambarellus species. In different localities from the same dam, specimens identified as C. chapalanus have also been found, so a possible contact among species can be found here (Pedraza-Lara et al., 2012). Cambarellus moi sp. nov. has only been collected in the 2 localities aforementioned, although a number of reservoirs in the area and in the El Pueblito River harbor similar habitats and could be inhabited by the species. Considering this, the new species is proposed to be considered as critically endangered under the IUCN criteria (CR B-1 a I,iii,iv). Following criteria for national protection in México, C. moi sp. nov. should be considered as in danger of extinction (14A II, BI, CII, DI).











 nova página do texto(beta)
nova página do texto(beta)



