Introduction
Neuroptera is an endopterygote order of insects; adults are characterized by having 2 pairs of membranous wings with numerous ribs that form a reticulum. The order is distributed world-wide and comprises about 6,000 species in 17 families (Aspöck et al., 2015). Neuropterans are among the most beneficial insects (Penny et al., 2007); they are important predators in aquatic and terrestrial ecosystems. The terrestrial Neuroptera, mainly those of the Chrysopidae, Hemerobiidae and Coniopterygidae families are considered of economic importance, because their larvae are predators of agricultural pests (aphids, whiteflies and scale insects) (Fathipour & Maleknia, 2016). Some species are reared and sold commercially as bio-control agents.
In citrus, around 76 species of Chrysopidae (green lacewings), 18 species of Hemerobiidae (brown lacewings) (Carnard, 2001; Freitas & Penny, 2001; Kondo et al., 2015; Monserrat, 1990; Szentkirályi, 2001), and 11 species of Coniopterygidae (dustywings) (Badgley et al., 1955; Monserrat, 1984, 1994, 2002; Quayle, 1912) are recognized worldwide. Although there are relatively numerous published data on lacewings living on citrus (Alvis et al., 2003; DeBach et al., 1950; Freitas & Penny, 2001; Leon & Garcia-Marí, 2005; Lozano-Contreras & Jasso-Argumedo, 2012), their seasonal activity (Duelli, 2001; Penny et al., 2007; Ripolles & Melia, 1980) and synchrony with the temporal distribution pattern of pests have been reported only in few cases (Soler et al., 2002; Szentkirályi, 2001).
In Mexico, recent studies of natural enemies of citrus orchards pests mention the presence of the family Chrysopidae, known as efficient predators because of the ability of their larvae in the search for food, for being generalists and for having a high survival rate in agroecosystems (Freitas & Penny, 2001). In contrast, information about Coniopterygidae and Hemerobiidae in this agroecosystem is scarce. In most of the studies, only the presence of the species has been registered (Alvis & Garcia, 2006; Cortez-Mondaca et al., 2011, 2016; Elekçioğlu & Şenal, 2007; Kondo et al., 2015; Miranda-Salcedo, 2018; Ruíz et al., 2006). Sampling has not been performed for a full year, and its phenology has not been recorded. In fact, 20 species of chrysopids associated to citrus orchards, 3 species of Coniopterygidae and just 1 species of Hemerobiidae have been reported in Mexico (Table 1), of them only 4 species of chrysopids are reproduced in Mexican laboratories [Chrysoperla carnea s. l. (Stephens, 1836) against whitefly and aphids, Chrysoperla comanche (Banks, 1938) and Ceraeochrysa valida (Banks, 1895) against Asian citrus psyllid, and Chrysoperla rufilabris (Burmeister, 1839) against citrus pests] and none of Hemerobiidae or Coniopterygidae. So, it is evident the lack of information for these families although they are natural enemies of citrus orchards pests. The present study was aimed to provide information on the temporal assemblage (abundance, richness, and diversity) of the neuropterans associated with Mexican lime trees [Citrus aurantifolia (Christm.) Swingle] in Tecomán, Colima. It is expected that the maximum values of Neuroptera diversity will correspond with the periods of vegetative sprouting in lime trees.
Table 1 Chrysopidae, Coniopterygidae and Hemerobiidae species associated to citrus orchards in Mexico.
Materials and methods
The study was conducted in a 10-ha Mexican lime orchard, untreated chemically. The orchard is located in Tecolapa (18°58’45.98” N, 103°50’27.14” W, 81 m asl), municipality of Tecomán, Colima. The climate is very warm semi-dry of the type Aw0(w)ig, according to Koppën’s classification as modified by García (1988)), with an annual mean temperature of 26.5 °C and a rainfall regime mainly in the summer (annual precipitation of 810.6 mm) (Sedesol, 2012).
Collecting was carried out during 13 months, from May 2013 to June 2014. Sampling was carried out monthly by 5 collecting methods (Malaise trap, sweeping net, aerial net, yellow pan traps, and canopy fogging) as follows: collecting with sweeping and aerial nets was carried out once per month, giving 100 strokes on the surrounding flora and 100 strokes on the canopy of the lime trees; the collected insects were placed in plastic bags with ethanol at 70%. The Malaise trap was placed among the trees and operated for 7 days in the same place. Ten yellow pan traps were placed on the ground, under the trees, and operated for 24 h during each sampling period. For canopy fogging, a random tree was chosen monthly for fumigation with Cypermethrin (3ml per liter of water); captured specimens were placed in plastic containers with ethanol at 70%.
Most Neuroptera specimens were stored in containers with ethanol at 80%, and only some specimens of Chrysopidae and Hemerobiidae were subjected to critical point drying (Noyes, 2020). To determine the species, genitalia extraction was performed with the technique of Freitas et al. (2009), Meinander (1972), and Oswald (1993). The taxonomic keys used for identification were Brooks (1994), Brooks and Barnard (1990), Freitas et al. (2009), Monserrat (2002)), Oswald (1993), Penny (2002), Sziráki (2011), Tauber (2004), and Tauber and De Léon (2001 ). Furthermore, the species were compared with those of the CNIN at IB-UNAM.
The values of abundance, species richness, estimated species richness and diversity were analyzed for the 3 families as a whole and per family. These values were analyzed by month and by sprouting periods of the Mexican lime trees. To analyze the total expected species richness in this agroecosystem, the abundance-based coverage estimator (ACE) was used, considering as a sampling unit the number of species collected per month; ACE was obtained through the program SPADE (Chao & Shen, 2010). Diversity was evaluated using effective species number (true diversity) (Jost, 2006).
All specimens are deposited in the Entomophagous Insect Collection of the National Center of Biological Control Reference (CIE-CNRCB) in Tecomán, Colima.
Results
A total of 508 specimens were collected, which belong to 3 families and 21 species (Table 2). Of the total number of specimens collected, 4 (0.78%) were in poor condition, so it was not possible to identify them, 35 females of Chrysopidae (6.8%) and 13 of Coniopterygidae (2.5%) were identified only at the genus level.
Table 2 Species of Chrysopidae, Coniopterygidae, and Hemerobiidae (Neuroptera) present in a Mexican lime orchard in Tecomán, Colima (bold typeface = new record for citrus worldwide; * = new record for the state of Colima).
| Family | Species | Abundance |
|---|---|---|
| Chrysopidae | Ceraeochrysa cincta (Schneider, 1851) | 75 |
| Ceraeochrysa claveri (Navás, 1911) | 15 | |
| Ceraeochrysa cubana (Hagen, 1861) | 169 | |
| Ceraeochrysa valida (Banks, 1895) | 21 | |
| Ceraeochrysa sp. nr. smithi (Navás, 1914) | 3 | |
| Ceraeochrysa sp. | 35 | |
| Chrysoperla comanche (Banks, 1938) | 1 | |
| Chrysoperla externa (Hagen, 1861) | 85 | |
| Chrysoperla rufilabris (Burmeister, 1839) | 1 | |
| Chrysopodes (Neosuarius) sp. nov. | 1 | |
| Leucochrysa (Nodita) americana Banks, 1897 * | 5 | |
| Plesiochrysa brasiliensis (Schneider, 1851) | 6 | |
| Plesiochrysa sp. nov. | 6 | |
| Damaged unidentified specimens | 4 | |
| Coniopterygidae | Coniopteryx (Coniopteryx) minuta Meinander 1972 * | 2 |
| Coniopteryx sp. nr. delta Johnson 1980 * | 17 | |
| Coniopteryx (Scotoconiopteryx) josephus Sarmiento-Cordero & Contreras-Ramos, 2019 | 4 | |
| Coniopteryx sp. | 13 | |
| Neoconis inexpectata Meinander, 1972 * | 1 | |
| Neoconis szirakii Sarmiento-Cordero & Contreras-Ramos, 2019 | 1 | |
| Semidalis hidalgoana Meinander 1975 * | 37 | |
| Hemerobiidae | Megalomus minor Banks, 1905 | 3 |
| Notiobiella mexicana Banks, 1913 * | 1 | |
| Sympherobius subcostalis Monserrat, 1990 * | 2 |
Chrysopidae was the most abundant family (427 ind.), followed by Coniopterygidae (75), and finally the Hemerobiidae (6). The genus Ceraeochrysa was the best represented with 66.2% of the total collected chrysopids, followed by Chrysoperla with 20.3%, while the genus Chrysopodes presented only 1 individual.
Ceraeochrysa cubana was undoubtedly the most abundant species of Chrysopidae, reaching 39.5% of the total, and was collected during the entire sampling period, except for July and August, followed by Chrysoperla externa (19.9%), which was collected for 9 months and Ceraeochrysa cincta (17.56%), which was collected during the 13 months of sampling. Fifty-eight percent of the chrysopids species were represented by less than 10 individuals.
A total of 75 dustywing individuals were collected (Table 2); the genus Coniopteryx included 48% of the collected specimens, while Semidalis hidalgoana represented 49.3% of them. Only 1 individual of Neoconis inexpectata and 1 of N. szirakii were collected. Hemerobiidae was undoubtedly the least abundant family, which represent 1.18% of the total abundance of the 3 families of Neuroptera. Chrysopidae was the best represented family, with 5 genera and 12 species, all included in the subfamily Chrysopinae. The richest genus was Ceraeochrysa with 5 species, followed by Chrysoperla with 3 species (Table 2). For the genus Leucochrysa, 5 individuals in a single species, L. (Nodita) americana, were collected, this represents a new record for Colima; and Plesiochrysa brasiliensis represents a new record for citrus orchard in Mexico, however, it has been already registered for other countries worldwide (Freitas & Penny, 2001).
According to the ACE richness estimator, it would be expected to find 26 species of Neuroptera in the study area, of which 80.7% were collected (Table 3). Coniopterygidae presented 50% of species of Chrysopidae; 4 of them are both, new records for citrus orchards and for the state of Colima. In the study area, 69.7% of the expected species were recorded (Table 3).
Table 3 Diversity analysis of Chrysopidae, Coniopterygidae, and Hemerobiidae in a Mexican lime orchard in Tecomán, Colima.
| Family | Abundance | 0D | Expected richness | 1D | 2D |
|---|---|---|---|---|---|
| Chrysopidae | 388 | 12 | 14.3 | 4.7 | 3.5 |
| Coniopterygidae | 63 | 6 | 8.6 | 3.0 | 2.3 |
| Hemerobiidae | 6 | 3 | 3.6 | 2.7 | 2.5 |
| Assemblage of Neuroptera | 457 | 21 | 26.3 | 7.1 | 4.7 |
The hemerobiids presented 3 species (Table 3). Megalomus minor was already reported for Colima, however, it is a new record for a citrus orchard (Oswald et al., 2002); Notiobiella mexicana is a new record for both citrus and for the state of Colima, and Sympherobius subcostalis had already been recorded in citrus but it is a new record for the state of Colima (Monserrat, 1990). Species richness of 3.6 was estimated for this family, of which 83% of the species were collected.
The diversity analysis included only individuals identified at the species level; although several specimens were identified to genus, it was not possible to determine whether 1 or more species were involved. By including the species of the 3 families and their relative abundance in the measure of the diversity of order 1 (q = 1), this group presented a diversity of 7.1 effective species (Table 3). With the measure of the diversity of order 2 (q = 2), it presented 4.7 effective species. Chrysopidae was clearly the most diverse family because it presented a diversity (1D) value equal to that of a theoretical community of 4.7 species, where they all had the same abundance (Table 3), and 3.5 species according to the diversity of order 2 (2D).
The dustywings presented a measure of 1D of 3.0 effective species, which means that the chrysopids are 1.5 times more diverse than the dustywings in the study area. With the measure of 2D, it was observed that the common species of chrysopids are 1.5 times more diverse than dustywings (Table 3). The hemerobiids presented a 1D of 2.7 effective species, which means that Chrysopidae is 1.7 times more diverse than Hemerobiidae. Coniopterygidae is only 1.10 times more diverse than hemerobiids. According to the diversity of order 2, it was observed that the common species of chrysopids are 1.3 times more diverse than hemerobiids; however, Hemerobiidae is 0.9 times more diverse than Coniopterygidae, although the diversity of order 1 is smaller (Table 3). However, it should be considered that collected hemerobiids were very few.
The 3 families share maximum values during the first period of sprouting (November - March) of Mexican lime trees. However, the values of abundance and diversity of order 2 were higher in April and May for the Coniopterygidae, yet, no specimens of Hemerobiidae were collected in that period. During the second sprouting period (June - September), low values for abundance, richness, and diversity were recorded for the 3 families (Fig.1a-c).
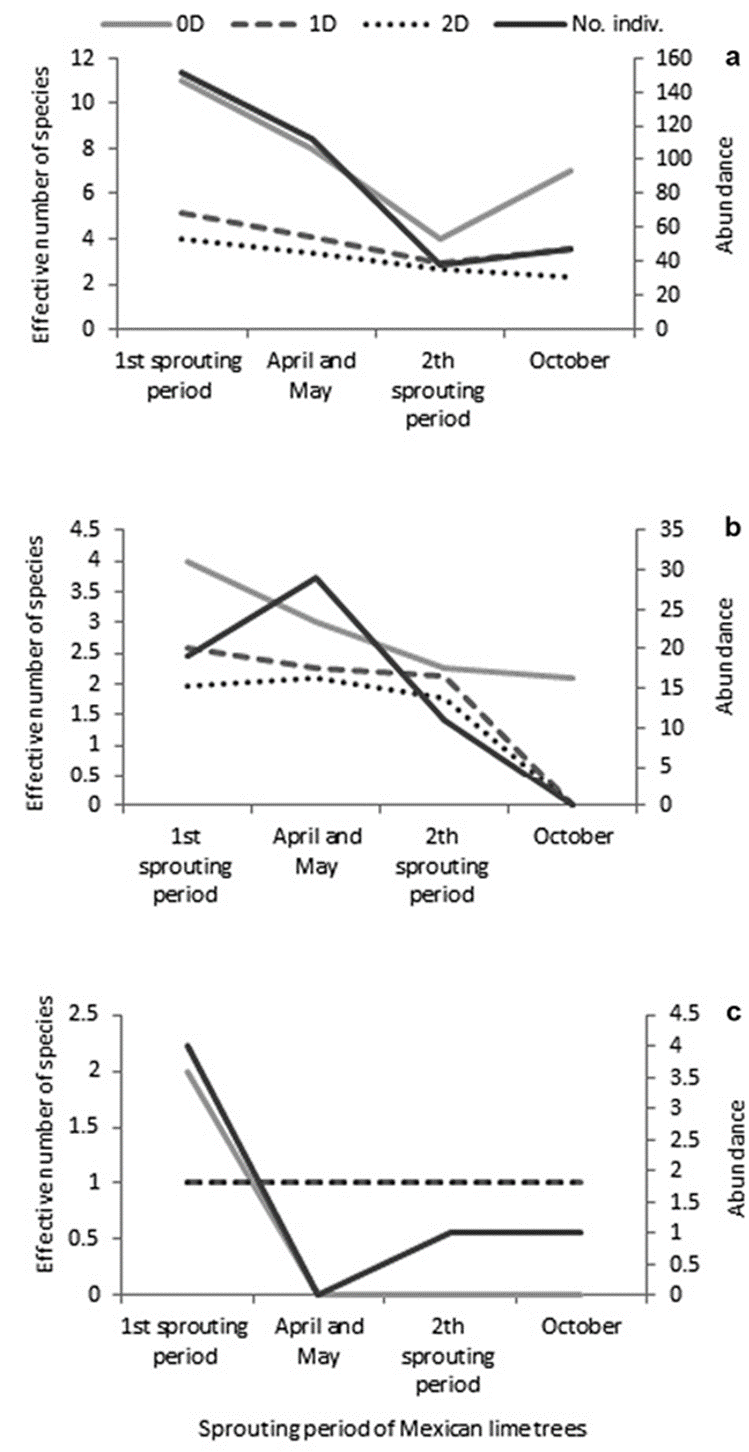
Figure 1 The observed diversity of orders 0 (0D), 1 (1D), and 2 (2D), and abundance of the Chrysopidae (a), Coniopterygidae (b), and Hemerobiidae (c) throughout the sampling period in Mexican lime orchard in Tecomán, Colima. First sprouting period (Nov. - Mar.), second sprouting period (Jun. - Sep.).
Most of the species of the family Chrysopidae were shared among the months, except for Chrysopodes sp. nov. which was present in November, and C. comanche and C. rufilabris which were present in March. Six species of Coniopterygidae were recorded; 2 of them were exclusive, C. rufilabris for July and N. szirakii for March. Of the 3 species recorded for Hemerobiidae, 2 were exclusive, N. mexicana for July and S. subcostalis for November.
Forty percent of the total abundance and 91% of Chrysopidae species were recorded during the first period of vegetative sprouting of Mexican lime trees (Fig. 1a); in counterpart, only 10% of the abundance and 25% of Chrysopidae species were recorded in the second period of sprouting. C. cincta, C. cubana and C. externa were the 3 species recorded in the second sprouting period, which were also present during the first period. These species were the most abundant in the family.
The same case is for Coniopterygidae, during the first sprouting period, 32% of the total abundance and 80% of the species collected were recorded (Fig.1b); during the second sprouting period, 18% of the abundance and 60% of the collected species were recorded. However, it was during April and May, intermediate months between the 2 periods of sprouting when the greatest abundance (43%) and 60% of richness were recorded. Two species, Coniopteryx josephus and S. hidalgoana were present in both periods of vegetative sprouting.
Of the 3 recorded species of Hemerobiidae, definitely the first period was the most active with 4 individuals and 2 species (Fig.1c); 1 species was present in the second sprouting period.
Discussion
Chrysopidae was the most abundant (427 ind.) and richest family, and Hemerobiidae the poorest, a total of 6 individuals were collected for this family during the year; usually the population density of most species of Neuropterida is very low, especially when compared with other insect orders, this is probably due to collection difficulties and, in general, to the small number of specimens that usually constitute their populations, so greater effort is necessary in front of other orders of insects to obtain a greater number of species and individuals (Marín & Monserrat, 1987). Twenty species of Chrysopidae were registered in citrus orchards in Mexico before this study (Table 1) (Cortez-Mondaca et al., 2011, 2016; López-Arroyo et al., 2010; Lozano-Contreras & Jasso-Argumedo, 2012; Ramírez, 2007; Tauber, 2004), of those, 9 were collected during this work, and 2 new species were registered; so there are currently 23 species registered in this agroecosystem, of which 52% are present in the study area. One Coniopterygidae species was registered in citrus in Mexico before this study (Monserrat, 1984), Semidalis boliviensis, which was not recorded in this research. Of the 6 collected species 2 were new for science, those are described by Sarmiento-Cordero and Contreras-Ramos (2019); the rest of the species are new records for citrus worldwide. For the family Hemerobiidae, 1 species was registered in citrus in Mexico before this research (Table 1), therefore 2 of the 3 species collected in this study, are new records for citrus in Mexico (Table 2), and 2 (M. minor and N. mexicana) are new records for citrus worldwide. In general, with this study the number of species collected in citrus from these 3 families was increased by 33%.
The small number of hemerobiids collected is possibly due to their nocturnal activity, thus decreasing their detectability and underestimating their presence in the studied areas (Michaud, 2002; Szentkirályi, 2001). In addition, it is known that the Hemerobiidae are collected to a lesser extent in orchards than in forests or wild environments (Duelli, 2001; Gonçalves, 2011; Vas et al., 2001). The most frequent collected genus in citrus crops in Argentina is Hemerobius (Reguilón, 2002); however, in the present study, it was not collected, even when it has been recorded for the state of Colima (Oswald et al., 2002). According to the ACE richness estimator, 79.84% were collected, so the sampling effort could be considered representative (Soberón & Llorente, 1993). The true alpha diversity presented a value of 7.12 effective species during the sampling year, that is, it has many rare and few dominant species. Sixty percent of the species were represented by 5 or fewer individuals.
No study has been carried out in Mexico to analyze the diversity of these 3 families in citrus. Most of the researches where these species have been recorded have focused on the family Chrysopidae. For example, Valencia-Luna et al. (2006) carried out collections in different locations in the state of Morelos between 1982 and 1986; in citrus they registered C. cincta, C. cubana, C. everes, C. sanchezi, C. valida, C. comanche, and Leucochrysa (Nodita) texana Banks 1939. Four of these species were collected in this study. Ceraeochrysa cincta, C. cubana, and C. valida were among the most abundant species for the state of Morelos and for those registered in this study, unlike C. comanche, which was the most abundant species for the genus in Morelos, in this study only 1 individual was recorded.
Ramírez (2007) reported 8 species of crisopids in citrus in 13 states, of which 3 are different from those we collected. Temporal abundance is similar to what we found. The most abundant species was C. nr. cincta (it was collected for 10 months), other important species were C. valida and C. rufilabris, which were collected for 9 months. Cortez-Mondaca et al. (2016) identified 5 species of Chrysopidae in Mexican lime and orange orchards in different regions of the state of Sinaloa; C. comanche, C. rufilabris, C. carnea s. l., C. valida and C. claveri. C. comanche and C. valida were the most abundant species unlike this study, where C. comanche was present only with 1 individual and C. valida was the fourth most abundant species.
The variation in the abundance that the different species of Neuroptera present throughout the year will depend of the climatic factors and availability of resources, as well as on the biology of each one (Díaz-Aranda et al., 1986; Penny et al., 2007). In the case of perennial trees such as the Mexican lime, and in tropical areas such as Colima, 2 seasons of vegetative sprouting are observed. The first begins in November, when the irrigation cycle begins, and ends in March; during this period the largest number of individuals, species, and diversity of the 3 families were recorded. These high values may be influenced by the 2 or 3 flows of vegetative sprouting that usually occur, which in addition to the low winter temperatures and the high availability of tender tissue favor the presence and increase in populations of some groups of phytophagous insects (COELIM-Col., 2002), which are part of the neuropterans’ diet. The second time of sprouting is between June and September, with 1 or several sprouting flows (COELIM-Col., 2002), being in summer when the number of new shoots tends to be higher than in winter-spring, also it has abundant presence of fruits, despite this, it was during this period that the lowest abundance, richness, and diversity were recorded; possibly the sprouting of this period is still tender and can be affected by rainfall (COELIM-Col., 2002), along with insect populations.
Ceraeochrysa cubana, C. cincta and C. externa are the most abundant species in the present study, these have been documented in a wide variety of crops and prey (Albuquerque et al., 2001). Chrysoperla externa has a wide distribution in Mexico, in addition, it reaches high densities in agricultural crops mainly in Central and South America (Albuquerque et al., 2001; Duelli, 2001); because it is frequently associated with short plants (Lomelí-Flores et al., 2013), this is not consistent with the findings in this study, where its incidence in Mexican lime was relatively low, compared to other crops, most of the specimens collected were on surrounding herbs (58.8%). In contrast, the genus Ceraeochrysa was the most commonly collected in the Neotropical region, many species presenting a wide distribution over a variety of wild-ecosystems and agro-ecosystems (Albuquerque et al., 2001). Ceraeochrysa cincta is more arboreal, often it has been found in olive and citrus trees (Duelli, 2001). Ceraeochrysa cubana does not present diapause, it is active during most of the year, its survival in agroecosystems could be influenced by the availability of food resources, as well as C. cincta (López-Arroyo et al., 1999).
The abundance of the species C. cubana, C. externa and S. hidalgoana in the Mexican lime orchard, indicates the potential that these predators have in the agroecosystem. For example, in a sampling carried out in northern Mexico, the species C. comanche and C. valida, in addition to being the most abundant, they were predators of the immature stages of Diaphorina citri Kuwayama, 1908 (Cortez-Mondaca et al., 2016). In contrast, in citrus crops in Brazil, the species C. cubana has been considered as a serious option for biological control programs against various pests such as mealybugs (Hemiptera, Pseudococcidae), whiteflies, black citrus fruit flies (Hemiptera, Aleyrodidae), leaf miners (Lepidoptera, Gracillariidae), and mites (Oliveira et al., 2016; Souza et al., 1996).
These results indicate the need to generate knowledge on the number of species of this order present in citrus orchards and other agroecosystems, and their specificity for this habitat and their distribution, as well as on their habits and the relationships they maintain with other insects, mainly in Neotropical areas, since most of the information available is from temperate regions. This information would be the starting point of many biological investigations and applied research projects, such as biological control programs for insect pests.











 text new page (beta)
text new page (beta)


