Introduction
The commonly named “mojarras” of the family Gerreidae comprise a group of fishes distributed along the subtropical and tropical coastal areas of the world. In America, the family is represented by 22 valid species, belonging to 4 genera (Nelson et al., 2004): Gerres Quoy et Gaimard; Diapterus Ranzani; Eucinostomus Baird et Girard; and Eugerres Jordan et Evermann. Most species inhabit coastal lagoons and estuaries with sandy or muddy bottoms bordered by mangroves; however, they occasionally enter river mouths. It has been thought that Eugerres mexicanus (Steindachner) is the only gerreid species confined to freshwater habitats in southeastern Mexico and northern Guatemala (Castro-Aguirre et al., 1999; Deckert & Greenfield 1987; Miller et al., 2006). Herein, as part of a biological inventory of the Biosphere Reserve Montes Azules (BRMA), fish from the main canal of the Lacantún River were sampled for helminth parasites and a new nematode species of Rhabdochona Railliet, 1916 was found in the intestine of E. mexicanus, which is described below.
Materials and methods
In January 2013, 7 individuals of E. mexicanus were caught with the use of a gillnet in the main canal of the Lacantún River, Chiapas, next to the Chajul Station (16°06’38.4’’ N, 90°56’23.6’’ W). The Lacantún River is the main body of water of the Biosphere Reserve Montes Azules and drains to the Usumacinta River basin near the Mexico and Guatemala borders. Fish were transported alive to the Estación de Biología Tropical Chajul and examined immediately. The recovered nematodes (n = 28) were fixed in hot 4% formalin and some individuals in 100% ethanol for molecular analysis; subsequently, they were cleared with glycerine for light microscopy study. Drawings were made with the aid of a Nikon drawing attachment. After examination, the nematode specimens were stored in vials with 70% ethanol. Six specimens were dried by critical point method to further study with a scanning electron microscope Hitachi S-2460N. All measurements are given in micrometres unless otherwise stated. Type and paratypes specimens of the new species are deposited in the Colección Nacional de Helmintos, Instituto de Biología, Universidad Nacional Autónoma de México (CNHE), Mexico City.
Total DNA was purified from vouchered nematodes using the Quick-DNA™ Universal Kit from Zymo Research according to the tissue samples protocol. A partial fragment of cytochrome c oxidase subunit I (cox1) gene was amplified with the forward primer COIintF (5´-TGATTGGTGGTTTTGGTAA-3´) and the reverse primer COIintR (5´-ATAAGTACGAGTATCAATATC-3´) (Casiraghi et al., 2001), with the following conditions: initial denaturation at 94 °C for 3 min and 40 cycles of 94 °C for 45 sec, 57 °C for 45 sec, 72 °C for 90 sec, and a final extension for 5 min at 72 °C. PCR reactions (50 μl) consisted of 20 pmol of each primer, 5 μl of buffer (10X), 3 μl of MgCl2 (25mM), 4 μl of dNTPs mixture (2mM), 2 U of Taq DNA polymerase and 5 μl of DNA. The PCR products were verified on 1% agarose gels stained with ethidium bromide (Sigma-Aldrich, Toluca, Mexico) and purified with the QIAEX II Gel Extraction Kit (QIAGEN). The PCR products were sequenced at the Instituto de Biotecnología, Universidad Nacional Autónoma de México, Mexico City.
The partial sequence of the cox1 gene was aligned with default parameters implemented in MAFFT with sequences of nematodes of Rhabdochona available in the GenBank database (Table 1): R. ahuehuellensis (MK353475, MK353476, MK353477); R. lichtenfelsi (DQ990982, DQ990984, DQ990987 ); R. xiphophori (MK353483); R. salgadoi (MK341632,); R. ictaluri (MK353479, MK353480, MK353482); R. acuminata (MK341634, MK341635, MK341636); R. osorioi (MK341623, MK341625, 341626), and R. mexicana (MK341611, MK353470). The sequences of R. juliacarabiasae sp. n. obtained in the present study were deposited in the GenBank with accession number MF405199. On the other hand, other Rhabdochona species deposited there were used in this study to compare with the new one, R. canadensis (MH778489), R. kidderi (MH778490), R. mexicana (MH778491), R. salgadoi (MH778492), R. xiphophori (MH778493, MN592670), and R. guerreroensis (MN592669). Spinitectus osorioi (MN592671) and Spinitectus humbertoi (MH778494) were used as outgroup. The best-fitting nucleotide substitution models (GTR+I+G) were estimated with the Akaike Information Criterion (AIC) implemented in jmodeltest2 (Posada, 2008). Maximum likelihood (ML) and Bayesian Inference (BI) were used to estimate the phylogenies for the data set. Maximum likelihood analysis (Felsenstein, 1973) was carried out in the RAxML (Stamatakis, 2006) program version 0.9.0 (Koslov et al., 2019). A GTRGAMMAI substitution model was used, and 10,000 bootstrap replicates were run to assess nodal support. Bayesian analysis was conducted using MrBayes version 3.2.6 (Altekar et al., 2004; Ronquist & Huelsenbeck, 2003). The analysis was run with 4 million generations and sampling tree topologies every 1,000 generations and a Burning of 25%. Posterior probabilities of clades were obtained from 50% majority rule consensus of sample trees after excluding the initial 25% as “burn-in”. The generated trees were visualized in FigTree vl.4.3. To assess the level of genetic distances among isolates, the uncorrected genetic distance was calculated in MEGA v7 for the cox1 datasets (Kumar et al., 2016; Table 2).
Table 1 Nematode species analyzed in this study, including host species, localities, and GenBank accession numbers to sequences generated in this work.
| Parasite/host (Family) | Localities (geographical coordinates) | GenBank accession number |
Reference |
| Rhabdochona acuminata Brycon guatemalensis (Characidae) | El Mangal, Tenosique, Tabasco (17°38’24” N, 91°22’48” W) Río Pichucalco, Chiapas (17°30’ N, 93°06’36” W) | MK341634 - 36 | Santacruz et al., 2019 |
| Rhabdochona adentata Profundulus oaxacae (Profundulidae) | Río Grande, Oaxaca (16°55’38.12” N, 96°19’42.58” W) | MN927199 - 201 | Caspeta-Mandujano et al., 2020 |
| Rhabdochona ahuehuellensis Ilyodon whitei (Goodeidae) | Huaquechula, Puebla (18°46’1” N, 98°32’09” W) | MK353475 - 77 | Lagunas-Calvo et al., 2019 |
| Rhabdochona canadensis Notropis boucardi (Cyprinidae) | Barranca la Cuera, Tlayecac, Morelos (18°45’28” N, 98°52’54” W) | MH778489 | Present study |
| Rhabdochona guerreroensis Sicydium multipunctatum (Gobiidae) | Villa Purificación, Jalisco (19°42’02” N, 104°35’53” W) | MN592669 | Present study |
| Rhabdochona ictaluri Ictalurus pricei (Ictaluridae) | Nombre de Dios, Durango (23°47’22” N, 104°18’09” W) | MK353479 MK353480 MK353482 | Lagunas-Calvo et al., 2019 |
| Rhabdochona juliacarabiasae Eugerres mexicanus (Gerreidae) | Río Lacantún, Chiapas (16°06’38.4” N, 90°56’23.6” W) | MF405199 | Present study |
| Rhabdochona kidderi Cichlasoma istlanum (Cichlidae) | Barranca la Cuera, Tlayecac, Morelos (18°45’28” N, 98°52’54” W) | MH778490 | Present study |
| Rhabdochona lichtenfelsi Goodea atripinis (Goodeidae) | Pátzcuaro, Michoacán (19°36’05” N, 101°39’13” W) La Minzita, Michoacán (19°38’4” N, 101°16’28”) | DQ990982 DQ990984 DQ990987 | Mejía-Madrid et al., 2007a |
| Rhabdochona mexicana Astyanax mexicanus (Characidae) | Barranca la Cuera, Tlayecac, Morelos (18°45’28” N, 98°52’54” W) Río Jalpan, Querétaro (21°12’14.76” N, 99°28’28.2” W) Río Amacuzac, El Chisco, Morelos (18.4°80’73” N, 99.19°04’02” W) | MH778491 MK353470 MK341611 | Present study Santacruz et al., 2019 |
| Rhabdochona osorioi Astyanax mexicanus (Characidae) | Río Paso de Ovejas, Pueblo el Crucero, Veracruz (19°18’36” N, 96°31’48” W) | MK341623 MK341625 MK341626 | Santacruz et al., 2019 |
| Rhabdochona salgadoi Profundulus labialis (Profundulidae) | Pluma Hidalgo, Oaxaca (15°50’25” N, 96°21’26” W) Nacimiento del Río Tehuantepec, Oaxaca (16° 53’ 24” N, 99° 09’ 36” W) | MH778492 MK341632 | Present study Santacruz et al., 2019 |
| Rhabdochona xiphophori Xiphophorus hellerii (Poeciliidae) Poeciliopsis gracilis (Poeciliidae) | Pluma Hidalgo, Oaxaca (15°50’25” N, 96°21’26” W) Camino a Hierve el Agua, Oaxaca (16°54’23” N, 96°19’55” W) | MH778493 MN592670 MK353483 | Present study Lagunas-Calvo et al., 2019 |
| Spinitectus osorioi Atherinella osorioi (Atherinopsidae) | Arroyo San José, Chiapas (16°06‘50” N, 90°56‘03.3” W) | MN592671 | Present study |
| Spinitectus humbertoi Profundulus labialis (Profundulidae) | Río Papagayo, Guerrero (16°47’01” N, 99°36’10” W) | MH778494 | Present study |
Table 2 Genetic distance among Rhabdochona spp. calculated with the cox1 matrix.
| Species | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 |
| 1 R. acuminata | 0.00 | ||||||||||||||
| 2 R. adentata | 10.40 | 0.00 | |||||||||||||
| 3 R. ahuehuellensis | 8.61 | 8.30 | 0.00 | ||||||||||||
| 4 R. canadensis | 9.84 | 8.67 | 8.97 | 0.00 | |||||||||||
| 5 R. guerreroensis | 10.28 | 8.19 | 5.02 | 9.43 | 0.00 | ||||||||||
| 6 R. ictaluri | 11.44 | 11.00 | 11.90 | 11.61 | 13.03 | 0.00 | |||||||||
| 7 R. juliacarabiasae | 12.95 | 11.75 | 13.00 | 12.11 | 14.09 | 13.00 | 0.00 | ||||||||
| 8 R. kidderi | 9.67 | 9.06 | 8.61 | 9.75 | 10.24 | 9.59 | 12.31 | 0.00 | |||||||
| 9 R. lichtenfelsi | 8.33 | 7.45 | 7.00 | 9.00 | 9.35 | 10.53 | 12.94 | 8.77 | 0.00 | ||||||
| 10 R. mexicana | 12.95 | 11.75 | 11.00 | 13.21 | 13.21 | 14.15 | 14.89 | 12.01 | 11.40 | 0.00 | |||||
| 11 R. osorioi | 8.52 | 11.95 | 10.26 | 11.70 | 11.39 | 10.86 | 12.22 | 9.97 | 10.96 | 11.74 | 0.00 | ||||
| 12 R. salgadoi | 7.38 | 8.86 | 7.69 | 7.39 | 9.43 | 11.06 | 11.63 | 8.66 | 8.55 | 11.40 | 9.97 | 0.00 | |||
| 13 R. xiphophori | 8.20 | 7.71 | 6.90 | 7.98 | 8.63 | 9.71 | 11.18 | 7.45 | 7.24 | 11.40 | 9.62 | 7.10 | 0.00 | ||
| 14 S. osorioi | 13.00 | 12.14 | 13.19 | 13.29 | 13.41 | 14.71 | 15.40 | 13.29 | 13.82 | 14.26 | 13.45 | 10.86 | 12.16 | 0.00 | |
| 15 S. humbertoi | 13.44 | 12.33 | 12.82 | 12.11 | 14.01 | 12.75 | 15.89 | 12.16 | 11.84 | 15.20 | 14.00 | 11.04 | 11.04 | 10.86 | 0.00 |
Description
Family Rhabdochonidae Travassos, Artigas and Pereira, 1928
Rhabdochona juliacarabiasae sp. n. Figs. 1-19 http://zoobank.org/urn:lsid:zoobank.org:act:72BF6143-6156-4C29-A819-2F1AAD3AB068
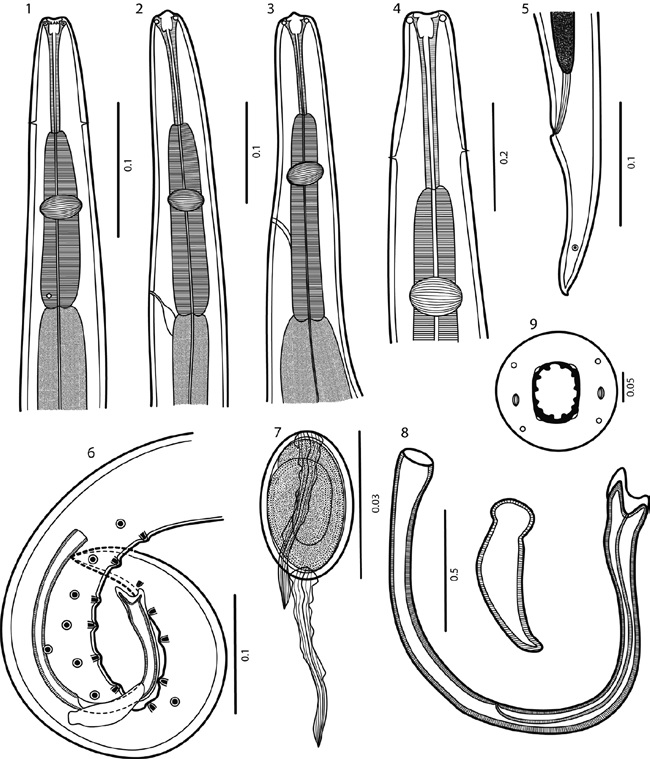
Figures 1-9 Rhabdochona juliacarabiasae sp. n. 1) Anterior end of male, ventral view; 2, 3) anterior end of female, lateral views; 4) anterior end of male, ventral view, larger magnification; 5) posterior end of female, lateral view; 6) posterior end of male, lateral view; 7) mature egg; 8) spicules; 9) cephalic end of female, apical view. Scale bar in mm.
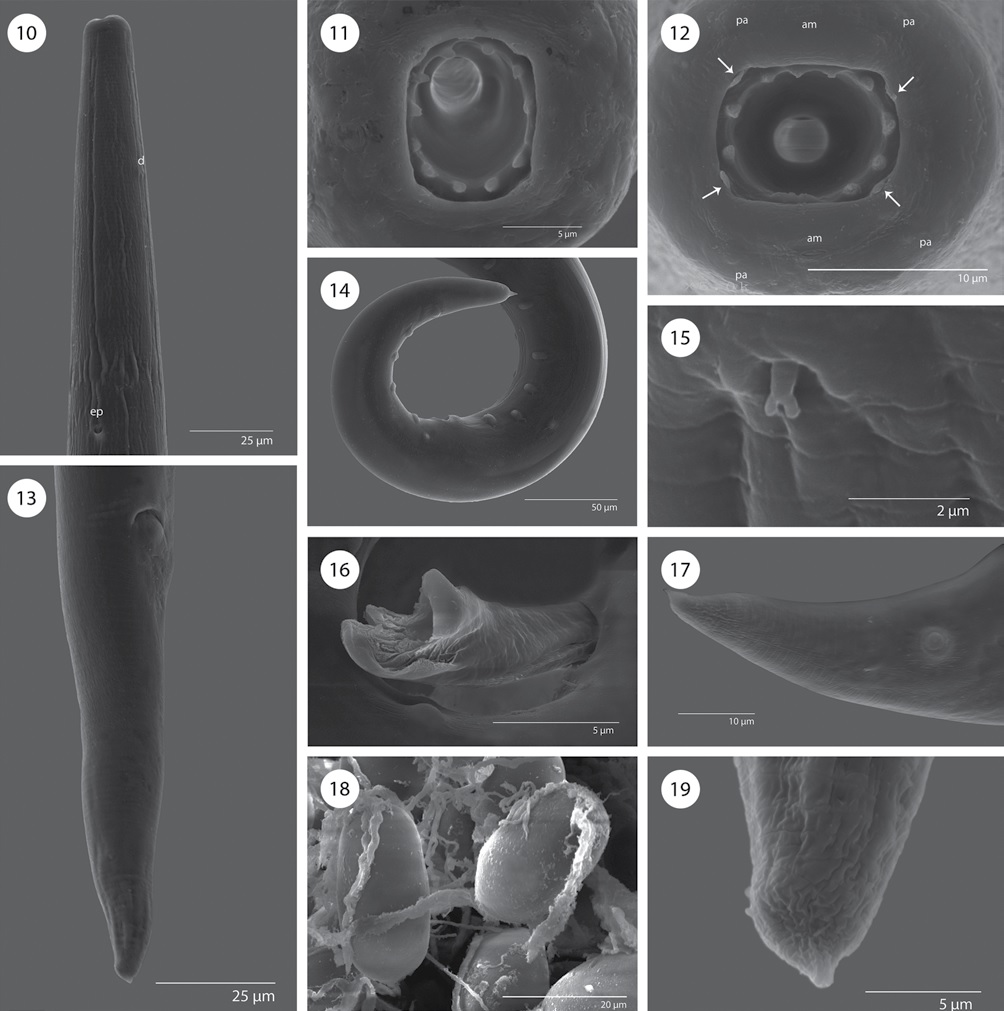
Figures 10-19 Rhabdochona juliacarabiasae sp. n., scanning electron micrographs. 10) Anterior end of male, ventral view; 11, 12) cephalic end of female and male, apical views (arrows indicate sublabia); 13) posterior end of female, subterminal view; 14) posterior end of male, lateral view; 15) deirid; 16) distal tip of larger spicule; 17) tail tip of male; 18) eggs; 19) tail tip of female. am = amphid, pa = papilla.
General. Medium sized nematodes with a transversely striated cuticle. Oral aperture oval, dorso-ventrally elongated, surrounded by 4 small submedian plates (sublabia), 4 submedian cephalic papillae and pair of lateral amphids (Figs. 11, 12). Prostom funnel-shaped, wide, with basal teeth; anterior margin of prostom armed internally with 12 teeth (exceptionally 11) (2 dorsal, 2 ventral and 4 lateral on each side forming pairs) (Figs. 9, 11, 12). Vestibule relatively long (Figs. 1-4). Deirids small, bifurcated (Fig. 15), somewhat asymmetrical, located short distance posterior to prostom. Tail of both sexes conical, with sharply pointed tip (Figs. 5, 6, 13, 14, 17, 19).
Male (10 specimens; measurements of holotype in parentheses). Length of body 3.53-4.72 (4.17) mm, maximum width 75-133 (75). Prostom 10-12 (12) long and 7-8 (7) wide. Length of vestibule including prostom 75-87 (83), muscular esophagus 112-143 (125) long, glandular esophagus 937-1.35 mm (1.10 mm) long. Nerve ring, excretory pore and deirids 106-131 (118), 181-231 (206) and 56-62 (60), respectively, from anterior end. Precloacal papillae: 9-10 pairs of subventrals and 1 lateral pair, situated between second and third subventral pairs (counting from cloacal opening) (Figs. 6, 14). Postcloacal papillae: 6 pairs; second pair lateral, remaining pairs subventral (Figs. 6, 14). Ventral precloacal cuticular ridges (area rugosa) absent. Larger (left) spicule 287-324 (312) long, length of shaft 137-162 (162) (representing 48-52% of spicule length); distal tip tetrafurcated (Figs. 8, 16). Smaller (right) spicule 72-75 (75) long, without dorsal barb (Fig. 8). Length ratio of spicules 1:3.8-4.5 (4.2). Tail conical, 187-275 (225) long (Figs. 14, 17).
Female (10 specimens, measurements of the allotype in parentheses). Length of body of gravid specimens 9.48-10.66 (10.66) mm, maximum width 100-200 (170). Prostom 13-18 (16) long and 12-13 (13) wide. Length of vestibule including prostom 100-110 (100), muscular esophagus 131-200 (181) long, glandular esophagus 1.301.78 (1.38) mm long. Nerve ring, excretory pore and deirids 137-168 (168), 212-250 (250), and 93-100 (100), respectively, from anterior end. Vulva preequatorial, 3.124.56 (4.55) mm from anterior extremity. Muscular vagina directed anteriorly. Uterus below level of posterior end of esophagus. Size of mature (embryonated) eggs 30-32 x 18-20; surface of eggs with 1 thick polar filament on each pole (Figs. 7, 18). Tail conical, 125-143 (138) long (Figs. 5, 13).
Taxonomic sumary
Type host: Eugerres mexicanus (Steindachner) (Gerreidae).
Site of infection: intestine.
Type locality: Chajul Station (16°06’38.4” N, 90°56’23.6” W), Lacantún River, Chiapas, Mexico (collected January 2013).
Prevalence and intensity of infection: 7 fish infected/7 examined; 3-40 nematodes per fish.
Type material: holotype, CNHE-8052, allotype CNHE-8053, and paratypes CNHE-8054 at Instituto de Biología, UNAM, Mexico City.
Etymology: this species has been named in honor of Prof. Julia Carabias Lillo from the Universidad Nacional Autónoma de Mexico (UNAM), acknowledging her contributions to environmental restoration and management of natural resources in the Biosphere Reserve of Montes Azules in the state of Chiapas.
Sequences: sequences obtained from 1 specimen of the new species and other specimens: R. canadensis (MH778489), R. kidderi kidderi (MH778490), R. mexicana (MH778491), R. salgadoi (MH778492), R. xiphophori (MH778493, MN592670), R. guerreroensis (MN592669), and sequences from S. osorioi (MN592671) and S. humbertoi (MH778494), were deposited in GenBank.
Remarks
The number of prostomal teeth, the shape of deirids, the presence or absence of superficial formations on eggs, the shape of the distal tip of the larger spicule and the number of postcloacal papillae in males, have been shown to be the most important interspecific characteristics that help in the discrimination of species of Rhabdochona (Caspeta-Mandujano & Moravec, 2000; Caspeta-Mandujano et al., 2000, 2001, 2002; Moravec, 1968, 1972, 1975, 1978, 2010; Moravec et al., 2008, 2009, 2013; Moravec, Bilal et al., 2012).
Rhabdochona includes more than 100 valid species parasitizing freshwater fish in all zoogeographical regions (Caspeta-Mandujano et al., 2020; Moravec et al., 2013; Santacruz et al., 2019). Currently, 23 valid species and 2 subspecies of Rhabdochona have been reported in America (Caspeta-Mandujano, 2010; Caspeta-Mandujano et al., 2020; Moravec et al., 2011; Moravec, Salgado-Maldonado et al., 2012; Santacruz et al., 2019), 12 of which are registered as parasites of freshwater fishes in Mexico: R. acuminata Molin, 1860; R. adentataCaspeta-Mandujano, Salinas-Ocampo, Calixto-Rojas, Rubio-Godoy et Pinacho-Pinacho, 2020; R. ahuhuellensis Mejía-Madrid et Pérez-Ponce de León, 2003; R. canadensis Moravec et Arai, 1971; R. cascadilla Wingdor, 1918; R. guerreroensis Caspeta-Mandujano, Aguilar-Aguilar et Salgado-Maldonado, 2000; R. ictaluri Aguilar-Aguilar, Rosas-Valdez and Pérez-Ponce de León, 2010; R. kidderi Pearse, 1930; R. lichtenfelsi Sánchez-Álvarez, García-Prieto et Pérez-Ponce de León, 1998; R. mexicana Caspeta-Mandujano, Moravec et Salgado-Maldonado, 2000; R. ovifilamenta Weller, 1938; R. salgadoi CaspetaMandujano et Moravec, 2000; R. xiphophori CaspetaMandujano, Moravec et Salgado-Maldonado, 2001 (Moravec, Salgado-Maldonado et al., 2012).
Based on the number of prostomal teeth, species of Rhabdochona can be arranged into 7 groups: those possessing 6 teeth or those with 8, 10, 12, 14, 16 and 20-22. The American species of Rhabdochona have been reported to possess 6 (R. cubensis, R. longleyi, R. xiphophori), 10 (R. ahuhuellensis, R. kisutchi, R. lichtenfelsi, R. mexicana), 12 (R. guerreroensis), 14 (R. acuminata, R. canadensis, R. cascadilla, R. cotti, R. decaturensis, R. ictaluri, R. kidderi, R. osorioi, R. rotundicaudatum, R. uruyeni, R. zacconis), and 16 (R. fabianae, R. ovifilamenta, R. salgadoi). In addition, R. adentata has an unusual prostom lacking anterior teeth. The newly described species belongs to the group of species possessing 12 teeth. In the New World, only R. guerreroensis belongs to this group. Although both species are rather similar, they can be differentiated by: 1) the distribution of prostomal teeth (4 dorsal, 4 ventral and 2 lateral on each side vs. 2 dorsal , 2 ventral and 4 lateral on each side, respectively) (Figs. 20, 21); 2) shape of deirids (22, 23); 3) the distal tip of the larger (left) spicule (trifurcated vs. tetrafurcate in R. juliacarabiasae) (Figs. 24, 25); 4) the length of the small spicule (right) (92-140 vs. 72-75, being smaller in the new species; 5) the absence of dorsal barb in the small spicule, which is present in R. guerreroensis (Figs. 26, 27); 6) the absence of area rugosa which is present in R. guerreroensis (Figs. 28-29), and 7) the number of pre and postanal papillae (12-12, 13-13 and 5-8, 7-8, 7-9 vs. 9-9, 10-10 and 6-6, respectively; Figs. 30, 31). In addition, both species differ in the host species (Sycidium multipunctatum [Gobidae] vs. Eugerres mexicanus [Gerreidae]) and the drainage system (Balsas vs. Usumacinta rivers, respectively).
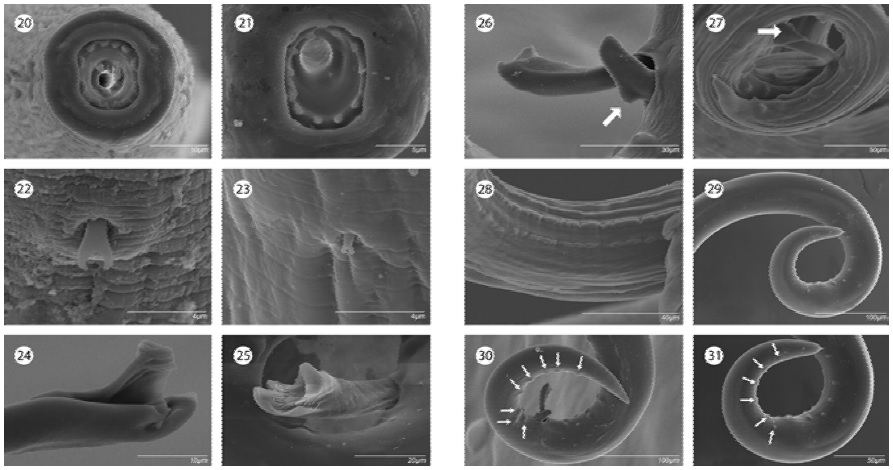
Figures 20-31 Rhabdochona guerreroensis vs. R. juliacarabiasae n. sp. 20) Anterior end of R. guerreroensis, apical view; 21) anterior end of R. juliacarabiasae, apical view; 22) deirid of R. guerreroensis; 23) deirid of R. juliacarabiasae; 24) distal tip of larger spicule of R. guerreroensis; 25) distal tip of larger spicule of R. juliacarabiasae; 26) distal tip of smaller spicule showing dorsal barb of R. guerreroensis; 27) distal tip of smaller spicule showing dorsal barb of R. juliacarabiasae; 28) area rugosa of R. guerreroensis; 29) area rugosa of R. juliacarabiasae; 30) postcloacal papillae of R. guerreroensis; 31) postcloacalpapillae of R. juliacarabiasae.
Moravec, Salgado-Maldonado et al. (2012) reported another species of Rhabdochona, R. kidderi, parasitizing several species of fishes from the Lacantún River, as well as the presence of the fourth-stage larva of R. kidderi in E. mexicanus. However, all fourth-stage larvae of Rhabdochona spp. are rather similar morphologically, which preclude determining the specific identity. Nevertheless, it is possible to assume that the specimens of Moravec, Salgado-Maldonado et al. (2012), which share host species and locality with our material, belong to the new species.
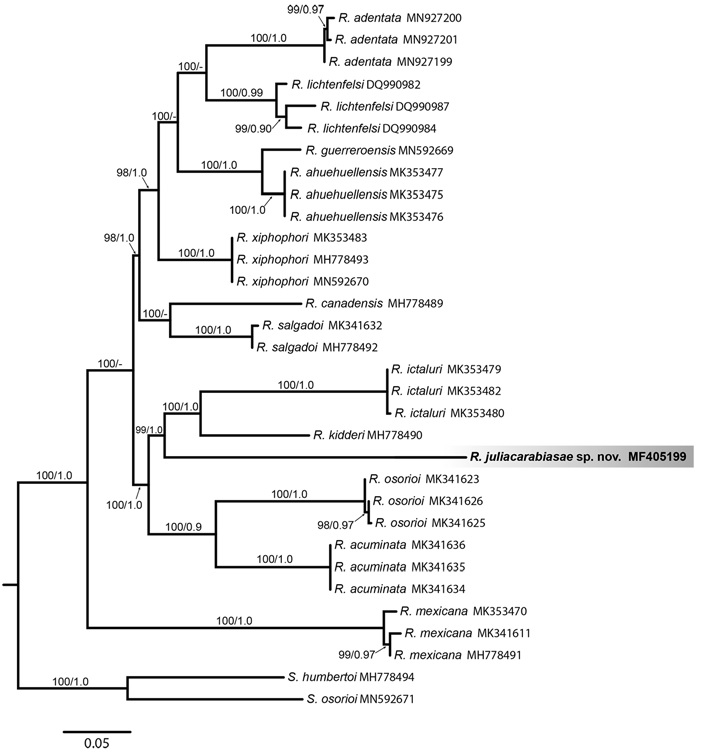
Furthermore, R. juliacarabiasae was recovered as sister of the clade form by R. ictaluri and R. kidder (Fig. 32). The genetic divergence between R. juliacarabiasae and it congeners ranged between 11.18-14.89%. These genetic distances with the cox1 gene are equal and sometimes higher than distances recovered from already described and valid species, indicating that R. juliacarabiasae is in fact a new taxon.
Discussion
Moravec (1975) based on a complex of morphological features, mainly number of teeth in the prostom and presence or absence of cervical alae, divided Rhabdochona into 4 subgenera: Rhabdochona, Globochona, Globochonoides, and Sinonema. In this respect, the general morphology of R. juliacarabiasae is typical of the subgenus Rhabdochona (12 prostomal teeth, eggs provided with polar filaments and absence of lateral alae). However, it is important to note that all species recorded in the New World, belong to the nominotypical subgenus Rhabdochona (see Moravec, 1975, 2010).
With the exception of R. acuminata, R. canadensis, R. cascadilla, R. kidder, and R. ovifilamenta, all remaining species from Mexico (7 including R. juliacarabiasae) appear to be endemic. This places Mexico as the country with the highest number of endemic species of Rhabdochona in the world.
Mitochondrial molecular markers are currently being used more frequently in helminth parasite research. Mitochondrial genes show higher substitution rates and have been shown that the use of these genes is more informative in molecular phylogenies (Blouin, 2002). In this work, the partial sequence of the subunit 1 gene of cytochrome c oxidase (cox1) was obtained from an organism of R. juliacarabiasae sp. n. Phylogenetic analyzes of the data set included sequences retrieved from GenBank and from organisms collected in this study. The ML and BI analyzes for the partial sequences of the cox1 gene show that R. juliacarabiase sp. n. is part of an independent clade with high nodal support. The fragment size of the cox1 of the new species was 662 bp, and has shown a high level of genetic divergence (11.18 - 14.89%). This result is consistent with those of Santacruz et al. (2019), which found high values of genetic divergence comparing Rhabdochona species reported in Mexico (10 - 12%).











 nova página do texto(beta)
nova página do texto(beta)