Introduction
Weeds are plants adapted to habitats modified by humans and that may interfere with their activities (Holzner, 1978). Their habitats range from home gardens to agricultural fields, and they establish self-sustained populations (Baker, 1974; Hanan et al., 2015). They are also evolutionarily interesting because of their adaptations to novel selective pressures associated with human-managed environments. As the concept of weeds is broad, Holzner (1982) divided them into several classes, of which the 2 most commonly used have been agrestal and ruderal weeds. Agrestals have been defined as weeds that grow associated with crops grown in tilled, arable land cropping. Although this concept usually refers to weeds of cereals or other annual crops, the definition can be broadened to include all weeds of cultivated lands, such as, for example, coffee plantations (Holzner, 1982). Ruderals, instead, are weeds that grow in highly disturbed, human-modified habitats, such as trash dumps, home gardens, city parks, roofs, roadsides, etc. For the purposes of this article, we will focus on agrestal weeds.
In general, the composition of a weed community is regulated as in natural ecosystems; it is also influenced by the regional species pool, distance from propagule sources, climatic constraints, and management practices (such as the use of herbicides, fertilizers, etc.), as predicted by the community assembly theory (Booth & Swanton, 2002; Colorado-Zuluaga, 2015; Götzenberger et al., 2011; Holzner, 1982).
Weeds have variable ecological requirements; some of them are specialists that may behave like the crops they grow with, and others are non-specialist that may have requirements similar to those of wild plants (Holzner, 1978). However, it is usually agreed that they are capable of high ecophysiological plasticity, fast-growth, and the capability of completing their life cycles within a short period of time (Baker, 1974; Holzner, 1982). Furthermore, they generally produce large amounts of seeds (the so-called r strategy) (Baker, 1974; Holzner, 1982; Storkey et al., 2010). For these reasons, weeds can compete with (and often, out-compete) crops for resources (light, space, water, nutrients), generating economic losses, which can be as high as 80% of the production (Espinosa-García & Sarukhán, 1997; Tanver et al., 2013). They can mechanically obstruct the harvest (Campiglia et al., 2018), or be hosts to pests or diseases (Datar, 2012; Nebreda et al., 2004; Santos-Martins et al., 2016).
However, despite the burden they may represent, weeds can also provide benefits. Weeds are usually pioneers in ecological succession and they can provide pollination services by maintaining populations of suitable pollinators or attracting them into the managed area; they can improve soil quality by preventing erosion. Also, they may contribute to maintaining a favorable microclimate for edaphic fauna; some species can even help to incorporate nitrogen into the soil (e.g., many Fabaceae) and aid in pest control, among other benefits (Blanco & Leyva, 2007; Campiglia et al., 2018; Holzner, 1982). From a socio-economic point of view, several weed species have potential as ornamental, medicinal, forage, and food plants (Blanckaert et al., 2007; Espinosa-García & Sarukhán, 1997; González-Amaro et al., 2009; Vieyra-Odilon & Vibrans, 2001).
About 12.3% of the Mexican flora is composed of species with potential weedy behavior (Espinosa-García et al., 2004a; 2009). If the vascular plants are represented in Mexico by 23,314 (Villaseñor, 2016) to 24,360 taxa (Sosa et al., 2018), then possibly as many as 2,800 species may display weedy behavior given the right circumstances (Espinosa-García et al., 2009). However, despite the importance that weeds may have for Mexico's agri-food industry, our knowledge of this group is, as a rule, relatively poor for many areas of the country (Espinosa-García & Villaseñor, 2017). The state of Colima is no exception to this assumption. In general, there is scanty information about its flora, although several authors have noted its great floristic diversity (Moreno-Gómez et al., 2016; Padilla-Velarde et al., 2006; Ruiz-Villarreal, 2016; Velarde et al., 2008; Villaseñor, 2016). Published knowledge of Colima's weeds is also poor and consists of only 3 publications (Orozco-Santos, 2001; Orozco-Santos & Farías-Larios, 2014; Villaseñor & Espinosa, 1998).
Here, we present the results of a survey of the agrestal weeds in agricultural areas of Colima devoted to cash-crops. We analyze the proportion of native and alien weed species. Then, we try to elucidate some of the biogeographical patterns of the weed communities with a non-metric multidimensional scaling analysis to identify weed communities and then, we identify the most influential bioclimatic variables determining the composition of the weed communities with the Maxent algorithm.
Materials and methods
The state of Colima is located in western Mexico, along the central Pacific coast (19°31'-18°41' N, 103°29'-104°41' W). It borders on the Pacific Ocean to the west and southwest, the states of Jalisco (north and northwest) and Michoacán (to the south); its elevation ranges from sea level to 3,820 m and it covers an area of approximately 5,543 km2 (Fig. 1). The main type of climate in the state is warm subhumid (Aw), followed by semi-dry warm (BSh), semi-warm subhumid (ACw), subhumid temperate (Cw), and a small area of subhumid semi-cold climate (Cew) (García, 2004). The average annual rainfall is 1,000 mm and it is strongly seasonal, with rainfall mostly concentrated from June through October. The average annual temperature is 26 °C (Conabio, 2016; Conafor, 2014). The main type of vegetation is dry forest (deciduous and sub-deciduous), although it is also possible to find small areas of other types of vegetation such as Quercus forests, coniferous forests, thorn scrub, gallery forests, mountain mesophilic forest, mangrove, and savanna-like vegetation (Martínez-Cruz & Ibarra-Manríquez, 2012; Padilla-Velarde et al., 2006).
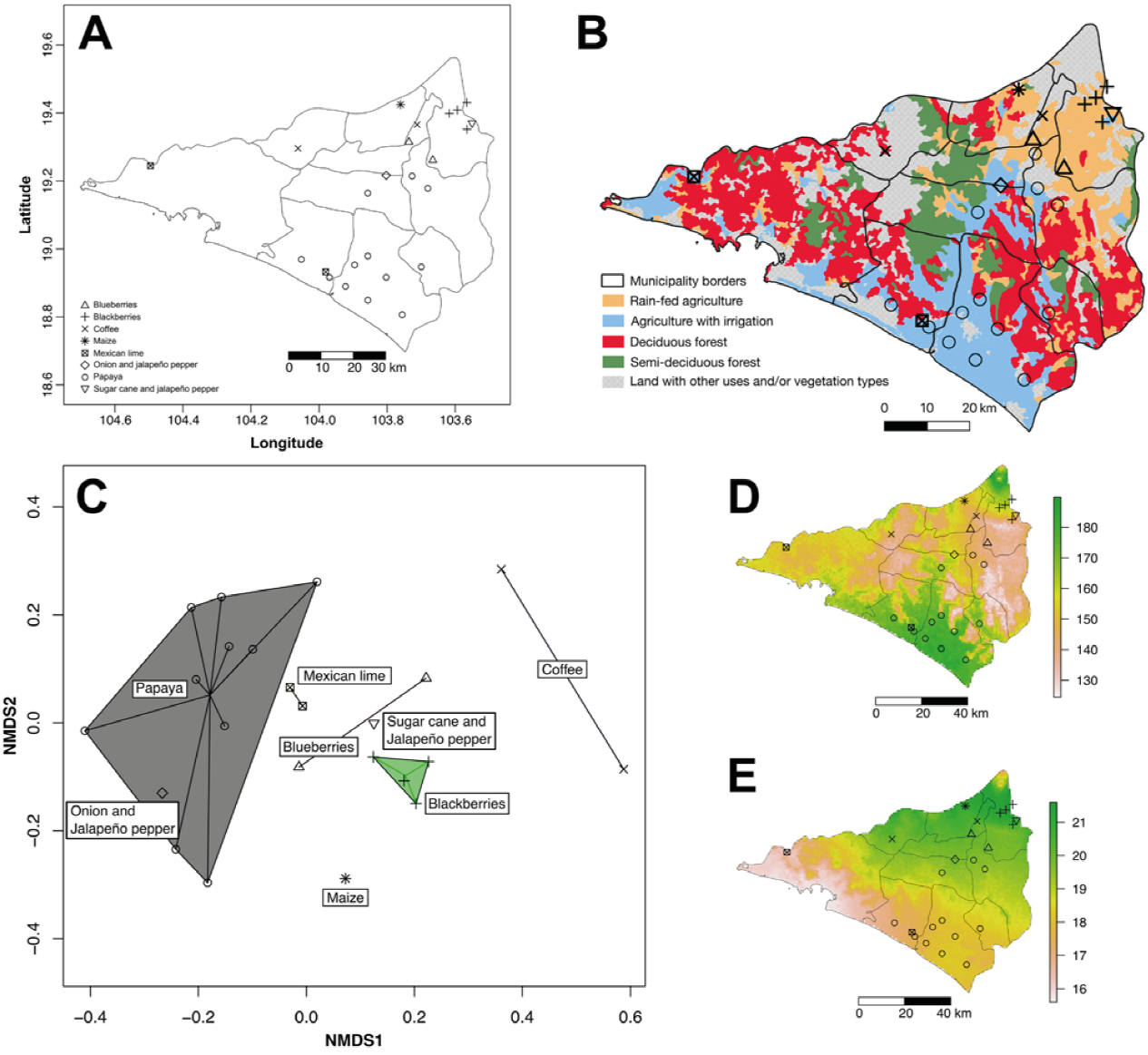
Figure 1 Distribution of surveyed fields and plantations across Colima, some bioclimatic variables, and general information about irrigation and vegetation types. A) Distribution of surveyed fields and plantations; B) map of Colima indicating the borders of each municipality, the distribution of the main vegetation types in the state (deciduous and semi-deciduous forest), and the agricultural areas with irrigation and without irrigation (rain-fed agriculture); C) non-metric multidimensional scaling (NMDS) analysis of weed communities using the sampled crops as proxy; D) temperature seasonality in Colima (Bioclim variable 4), the side scale indicates the standard deviation × 100; E) temperature annual range (Bioclim variable 7), the sidebar represents the variation in Celsius degrees (°C) between the max temperature in the warmest month and the minimum temperature in the coldest month. In all panels the symbology to represent the fields sampled by crop is the same as is indicated in A.
According to SIAP (2018), the state has approximately 760,000 inhabitants, 90% of which live in urban areas, and 10% in rural areas. The primary sector employs 11.5% of the population. Almost 90% of the people in the primary sector work in agriculture, which in 2017 contributed ca. 4.6% of the Colima gross domestic product (GDP). In 2017, the state yielded about 3.7 million tons of food, of which 97% corresponded to agricultural products. Due to the diversity of climates in Colima, it is possible to find a wide range of crops, from bananas in the tropical lowlands to others that require more temperate conditions, such as blackberry. The most important crops (in terms of surface area and contribution to GDP) are papaya and Mexican lime. In Colima, 45% of the planted area is irrigated, and 55% is rain-fed. Perennial crops grow on 85% of the planted area and contribute 91% of the harvested volume. The municipality with the highest production value is Tecomán, followed by Manzanillo and Armería.
Between February 2015 and May 2019, we surveyed 25 cultivated sites devoted to cash-crops in the state of Colima (municipality of Armería [3 plots], Colima [2 plots], Comala [1 plot], Coquimatlán [2 plots], Cuauhtémoc [6 plots], Ixtlahuacán [1 plot], Manzanillo [1 plot], Minatitlán [1 plot], Tecomán [7 plots], and Villa de Álvarez [2 plots]; Fig. 1, Appendix 1). Each site was surveyed once, but at a different time of the year or in different years; thus, we sampled across all seasons, although some surveyed crops (coffee, maize) were sampled only in one season. Safety and ethical considerations limited the survey to companies or individuals willing to participate. Due to the importance of papayas to the Colima agri-food industry, our sample was biased toward this crop; but we also sampled other relevant crops, such as Mexican lime and some currently expanding crops like blackberry and blueberry (see Appendix 1 for a complete list).
Most of the surveyed crops grew on units of several hectares, and they were divided into blocks. We selected one block (usually of ca. 1 ha) and searched the interior and the edges (within the managed area) of the field until we could find no additional weed species. Exceptions to this procedure were coffee and sugarcane plantations. In the first case, because there is no division, we worked within the plantation without a previously determined direction, continuously documenting the species until we were unable to find additional ones. In sugarcane fields, we only searched at the edge because the plant density of the crop made it difficult to work inside. Our sample was heterogeneous because it included perennial and annual crops. Some crops were grown inside greenhouses or used occasional protection such as covers, like plastic tunnels; also, most of the sampled agricultural fields and plantations had irrigation systems and intensive or semi-intensive management programs (Appendix 1).
Each weed morphospecies was photographed. Then, vouchers were collected and processed according to standard herbarium techniques. For specimens with few, small, or delicate flowers, all or part of the material was preserved in a solution of 96% ethanol and glycerol (3:1) for further study; this material was used for identification and later integrated into a formal spirits collection of Colima weeds. The specimens were identified using general and regional floristic treatments (Cullen, 2006; Espinosa-García & Sarukhán, 1997; Levin & Gillespie, 2016; McVaugh & Anderson, 1984; Pruski & Robinson, 2018; Vibrans, 2012) or sent to specialists in particular plant groups for determinations (see acknowledgments for a list of specialists). The first set of the collections (including the spirit collection) was housed at the herbarium of the Universidad de Colima (UCOL), and duplicates were sent to the herbarium of the Centro de Investigación Científica de Yucatán (CICY), and the herbarium of the Universidad de Guadalajara, Centro Universitario de la Costa Sur (ZEA); acronyms according to Thiers (2020, continuously updated). The nomenclature was updated using the Taxonomic Resolution Service (Boyle et al., 2013), but we cross-checked to verify the status of the names and synonymy following Villaseñor (2016) and international databases such as IPNI (2020), The Plant List (2013), and Tropicos (2020). In cases of nomenclatural conflicts, we preferred the names used by the most recent or authoritative source (Levin & Gillespie, 2016; Pruski & Robinson, 2018).
The status of the species as native or alien was assessed based on Villaseñor and Espinosa-García (2004), Villaseñor (2016), Espinosa-García and Villaseñor (2017), and Sosa et al. (2018). We assigned native species to one of 3 levels of geographic restriction, according to the distribution data available in Villaseñor (2016): i) native species that grow naturally in Mexico, but their distribution is not restricted to the country, ii) the endemics of Mexico (their distribution is naturally restricted to the country, but are widely distributed across it), and iii) the endemics to the Mexican Pacific Coast. The third level is comprised of 2 biogeographical provinces: the Pacific Coast province, which is in the Neotropical Domain and the Sierra Madre del Sur province, which is in the Mexican Transition Zone. Both provinces are included because according to Morrone (2019) regionalization system, Colima is encompassed within both; the lowlands correspond to the Pacific Coast province and the highlands are in the Sierra Madre del Sur province.
To analyze the distinctiveness of the weed floras of the surveyed crops and fields or plantations, a non-metric multidimensional scaling (NMDS) was carried out in R with the vegan package (Oksanen et al., 2019; R Core Team, 2019). We used data from 25 surveyed fields and plantations in the analysis; because we only had presence/ absence data, we used the Jaccard index as a distance measure. The NMDS is a non-parametric multivariate ordering technique that is based on the rankings of distances between points (Chahouki, 2012). In addition, we calculated the proportion of the samples that each weed species represented and examined whether it is exclusive to any crop or bioclimatic zone of the state. The NMDS clustered the crops according to the similarities of their weed flora. Then, we selected the locations to assess the bioclimatic variables most relevant in determining the composition of the weed floras. These bioclimatic variables were used at the finest grain size available (ca. 1 km2) from the WorldClim database (Fick & Hijmans, 2017). Data were analyzed with Maxent within R using the dismo package (Hijmans et al., 2017; Phillips et al., 2017). The R script used is available in the data resources section (see below). For illustrative purposes, we include a map with information on the main vegetation types and irrigation regimes, using land-use data layers series V (INEGI, 2013) and assembled in QGIS 3.12.
The underlying data of the analysis reported in this paper (including Matrices and R scripts used in NMDS and bioclimatic analyses) are available in https://doi.org/10.7910/DVN/JIU7LP.
Results
We recorded 222 weed species for Colima, from 53 families and 163 genera (Table 1, Appendices 2, 3). Ferns and liverworts were represented by a single family and genus each; 2 families and 3 genera were Magnoliids, 6 families and 28 genera were Monocotyledons, and 43 families and 129 genera were Eudicotyledons. The most speciose plant families were Poaceae (29 species), Asteraceae (25), and Fabaceae (18). The genera with the most species were Euphorbia L. (7 species), Solanum L. (6), Physalis L., and Ipomoea L. (5 species each; Appendix 2). Also, 84.2% of the species were native and 15.8% were alien; 21 (9.4%) of 222 species included on our list were endemic to Mexico, 5 restricted to the Mexican Pacific, the remaining 16 with broader distributions (Table 1, Appendix 2).
Table 1 Families and genera by sampled crop, as well as their number and percentage of native, endemic, and exotic species.
| Crop | Families | Genera | Species | Native (%) | Endemics (%) | Aliens (%) |
|---|---|---|---|---|---|---|
| Blueberries | 24 | 39 | 43 | 33 (76.7) | 4 (9.3) | 6 (14) |
| Blackberries | 26 | 58 | 73 | 56 (76.7) | 3 (4.1) | 14 (19.2) |
| Coffee | 18 | 32 | 36 | 28 (77.8) | 6 (16.7) | 2 (5.6) |
| Maize | 10 | 17 | 21 | 18 (85.7) | 1 (4.8) | 2 (9.5) |
| Mexican lime | 25 | 47 | 54 | 43 (79.6) | 0 | 11 (20.4) |
| Onion and jalapeño pepper | 9 | 9 | 12 | 11 (91.7) | 1 (8.3) | 0 |
| Papaya | 26 | 84 | 106 | 83 (78.3) | 8 (7.5) | 15 (14.2) |
| Sugarcane and jalapeño pepper | 19 | 32 | 38 | 29 (76.3) | 3 (7.9) | 6 (15.8) |
| Total | 53 | 163 | 222 | 166 (74.8) | 21 (9.4) | 35 (15.8) |
Forty-six percent (46.85%, 104/222) of the species were found in 4% (1/25) of the surveyed sites and 20.72% (46/222) of the species were in 8% (2/25) of the sites; together this represents 67.57% of the species. In contrast, only 4 species occurred in more than 40% of the sites and were thus found in most of the sampled crops: Euphorbia hirta (60%, 15/25 sites), Heliotropium procumbens (48%, 12/25 sites), Richardia scabra (44%, 11/25 sites), and Euphorbia heterophylla (40%, 10/25 sites). We also found a few, much more crop-specific species, such as Piper hispidum, which grows only in coffee plantations, whereas Marchantia polymorpha or Lemna aequinoctialis were found only in blueberry plantations with intensive management. Papaya plantations were commonly invaded by Amaranthus palmeri, along with Anoda cristata, Acalypha aristata, and Boerhavia erecta. In Mexican lime plantations, a distinctive weed species was Struthanthus interruptus, a hemiparasite, whereas in maize Castilleja arvensis was commonly found (Appendix 2).
In a more detailed comparison of the surveyed crops, we found that the crop with the most associated weed species was papaya (106 species), followed by blackberry (73), and Mexican lime (54) (Table 1). The crop associated with the largest number of endemic species was coffee (6 species, 16.7%), whereas not a single endemic species behaving as a weed was found in Mexican lime plantations, although these plantations, along with those of blackberries, sugarcane, and papaya, had the highest proportion of alien taxa: 20.4%, 19.2%, 15.8%, and 14.2%, respectively (Table 1, Appendix 2).
In comparing crops and all species using NMDS, we found that coffee and maize had very characteristic weed floras. Papaya, onions, and Mexican lime fields were more similar, whereas blueberry, blackberry, and sugarcane also had weed components in common (Fig. 1C). This pattern reflects the 2 biogeographic areas in the state: the onion field and all our papaya and Mexican lime plantations were within the Pacific Coast province. In contrast, the sugarcane, blackberry, maize fields, and one of the coffee plantations were located within the Sierra Madre del Sur province. One coffee orchard was in a highly conserved area near the Sierra de Manantlán protected area, whereas the blueberry fields were found in areas that can be considered as transitional between both biogeographic areas.
To further explore the pattern of weed composition across the surveyed sites, we reviewed our data to identify unique species for each group of crops or area and found that taxa such as Acalypha setosa, Cissus verticillata, Boerhavia erecta, Corynandra viscosa, Momordica charantia, and Priva lappulacea, among others (Appendix 2), were common or unique in papaya and Mexican lime plantations. Also, species like Bidens alba, Glandularia bipinnatifida, Lepidium virginicum, Pseudognaphalium viscosum, and Verbena carolina, among others, were common or restricted to blueberry, blackberry, and sugarcane fields (Appendix 2).
To identify the potentially most influential bioclimatic variables in each area, we pooled localities of papaya, onion, and Mexican lime plantations and found that the main bioclimatic variables identified by Maxent (potentially with more than 40% of weight each) were "temperature seasonality" (bio4, Fig. 1D), and "mean temperature of wettest quarter" (bio8). This implies that the weed species that grow in the Pacific Coast province of Colima are adapted to a high monthly average variability in temperature across the year (ca. 17 °C). At the same time, they appear to prefer warmer temperatures in the most humid period (ca. 28 °C). In contrast, the species occurring within the Sierra Madre del Sur province or near its limits, were favored by the combination of temperature with a lower monthly variation (ca. 15 °C), and lower mean temperature (ca. 23 °C) in the most humid quartile.
The same analyses for localities with blueberry, blackberry, and sugarcane plantations found that the main bioclimatic variables (potentially with more than 40% of weight each one) were "temperature annual range" (bio7, Fig. 1E) and "precipitation of driest quarter" (bio17). This means that weed species that grow in or near the Sierra Madre del Sur province tend to withstand more extreme temperatures, with an annual variation of up to 20 °C, when compared with the species that grow in the Pacific Coast province zone of Colima (up to 18 °C). Similarly, there was variation in the amount of precipitation during the driest quartile of the year, although the surveyed areas tended to behave similarly, on average 8.92 vs. 8.37 mm of rainfall in the crops sampled in the Pacific Coast and Sierra Madre del Sur provinces, respectively.
Discussion
The first compilation of weeds of Colima was published by Villaseñor and Espinosa (1998), who conceptualized weeds as plants that were reported as unwanted in human-controlled habitats. Their list included agrestal, ruderal, grassland, aquatic, forest, and environmental weeds, and comprised 510 species. The large difference between lists (510 vs. 222 species) can be explained by the differences in scope, but also by different methods based on either bibliography or fieldwork. Both lists share 121 species (54% of the 222 species listed in Appendix 2), including such common taxa as Anoda cristata, Euphorbia hirta, Amaranthus palmeri, and Argemone mexicana, among others. However, we also report 101 additional species for the state; Evolvulus alsinoides, Chloris barbata, and Urochloa meziana are some of the more common in surveyed sites.
The other published weed lists for Colima were restricted to tamarind plantations, and Mexican lime plantations (Orozco-Santos, 2001; Orozco-Santos & Farías-Larios, 2014). In the first study, 21 species of weeds (8 monocots and 13 eudicots) were reported, but only 3 species were not shared with our list, namely Digitaria sanguinalis, Euphorbia hypericifolia, and Commelina erecta (Appendix 2).
Orozco-Santos and Farias Larios (2014) reported 44 species (15 monocots and 29 eudicots), but only 10 (22.7%) of their species were not found on our list (Digitaria sanguinalis, Chloris virgata, Brachiaria fasciculata, Rottboellia cochinchinensis, Acalypha alopecuroides, Euphorbia hypercifolia, Commelina erecta, Cuscuta americana, Senna occidentalis, and Crotalaria incana. Of the remaining 34 species, 18 were shared by both lists whereas 16 species were found by us associated with other crops, but not with Mexican lime; our list also includes another 34 species associated with Mexican lime that were not reported by Orozco-Santos and Farias Larios (2014) (Appendix 2).
The most speciose families on our list, Poaceae and Asteraceae (Appendix 2), are consistent with most weed studies in Mexico (León-de la Luz et al., 2009; Martínez-De La Cruz et al., 2015; Orozco-Santos, 2001; Orozco-Santos & Farías-Larios, 2014; Vibrans, 1998; Villaseñor & Espinosa, 1998). This reflects the high number of taxa that these families have in Mexico and elsewhere (Baker, 1974; Simpson, 2019; Villaseñor, 2016).
Other important families were Fabaceae and Euphorbiaceae, which are usually well-represented in the dry forests that are prevalent in Colima (Ceballos et al., 2010; Conafor, 2014; Martínez-Cruz & Ibarra-Manríquez, 2012; Padilla-Velarde et al., 2006). Some of the most widely distributed weedy plants in the state are taxa such as Euphorbia heterophylla (Aarestrup et al., 2008). One interesting pattern was that the most frequently collected weeds were several taxa of Euphorbia subgenus Chamaesyce, particularly Euphorbia hirta. This trend most likely reflects their wide climatic and edaphic tolerance, the fact that some of them feature C4 photosynthesis, the numerous seeds produced by each individual, toxic latex that deters herbivores, and their resistance to pesticides (Ferreira et al., 2017; Tanver et al., 2013).
Our results show that 15.8% of our list is composed of alien species; but the proportion of alien weeds ranges from 0-20.4% (Table 1), depending on the crop. These general numbers are consistent with the national estimates of 12.3% of the total flora being weedy (Espinosa-García et al., 2004a, 2009) and is below the range of 25.2-39.2% estimated for the alien weed flora of Colima (Espinosa-García et al., 2004a; Villaseñor & Espinosa-García, 2004).
The relatively small proportion of alien species in some of the surveyed crops, particularly in maize, coffee, and onions, may be related to their resistance to the establishment of alien species because of the history of agriculture in the region (Espinosa-García & Villaseñor, 2017). The somewhat higher levels in other types of crops (such as papaya, blueberry, blackberry, and Mexican lime) may be related to the movement of plantlets, seedlings, or other propagules for crop establishment, along with soil or other substrates. Good examples of introduced species that are currently weeds in crops are several grass species used for forage, including Echinochloa colona, Eleusine indica, Melinis repens, and Megathyrsus maximus, among others. These species can disperse with contaminated equipment or material, by adherence to vehicles or animals (Sánchez-Ken et al., 2012; Vibrans, 2012), or even by local birds that use them as food (Petit et al., 2013; Viana et al., 2016).
Most crops had their own weed assemblage, except for a field of onions and jalapeño pepper, which was embedded in the papaya group in the analysis. This pattern has been found in other crop systems such as wheat, watermelon, banana, and others (Gomaa, 2012; Holzner, 1982; Quintero-Pertúz et al., 2018; Suárez et al., 2001). In our data, the most distinctive communities were maize and coffee. Probably the key to understand this are the crop management practices: coffee is managed with traditional techniques, and also has a permanent tree cover; these characteristics tend to be friendly to native, shade-loving flora, and also explain the high weed endemicity, compared to other crops, although we report only 36 species, a far smaller figure than the 58 species found by Sanginés et al. (2014) in coffee plantations at Comapa (Veracruz). The differences may be associated with the study region -the flora of Veracruz is different from that of Colima; it may also be related to our survey techniques. We only sampled in the dry season and only once in each site.
Maize also had a distinctive community, with traditional milpa-like management (we found squash, but not beans plants inside the maize field), which is also friendly to native, sun-loving species. We found only 21 species associated with this crop, but we do not have much comparative data.
The other crops, which clustered in 2 larger groups (Fig. 1C), can probably be explained by several filtering factors (apart from management). They were related to the biogeographic provinces (Pacific Coast and Sierra Madre del Sur), and their different climates and species pools (Appendix 2), as was found elsewhere (Booth & Swanton, 2002; Nagy et al., 2017; Poggio, 2012).
Several temperature variables (bio4, bio7 and bio8; Fig. 1D, E) influenced the composition of the local weed flora. Group one (lowland, Pacific Coast province) was associated with the hottest temperatures (average at wettest quarter 28 °C) with less extreme values (range up to +/-17 °C), and group 2 (highland, Sierra Madre del Sur province) with the coldest temperatures (average at wettest quarter 23 °C) and more extreme values (range up to +/-20 °C). According to Espinosa-García et al. (2004b), in Mexico, low temperature is one of the limiting factors for the distribution of vegetation. The same was only partially true for Colima perhaps because no survey sites were located in areas with winter frosts, also the Maxent analysis did not identify that the minimum temperature of the coldest month (bio6) as a relevant variable, as suggested by Espinosa-García et al. (2004b).
Noteworthy is the high number of species collected in one or a few sites (ca. 70%) and the proportion of endemics (ca. 9%; Table 1; Appendix 2). Both observations are probably related to the heterogeneity of the landscape. The 9.4% endemic species found is within the expected value according to the information available for maize (5.7-13.7%) (Vibrans, 1998) and for ruderal floras such as Malinalco in the state of Mexico (8.7%) (Martínez-De La Cruz et al., 2015) and Coronango in Puebla (6.3%) (Flores-Huitzil et al., 2020).
The crops do not have the same proportion of endemics. There were no endemics in the Mexican lime plantations, whereas in coffee plantations they reached 16.7% of the total flora. Perhaps these variations are related to the fact that the first are irrigated and located in a highly modified landscape matrix. In mature plantations weeds are controlled by brush cutters and herbicides. On the other hand, coffee plantations are in a landscape matrix that tends to be more conserved and managed less intensively.
From our species list, only Manihot chlorosticta (which is widely distributed in Mexico) is included in the IUCN red list (as near threatened, NT) (Vera-Sánchez & Nassar, 2019). But we found another 15 endemic species of wide distribution in the country and 5 restricted only to the Pacific Coast: Tridax dubia and Melampodium tepicense were found in blackberries, Phyllanthus hexadactylus and Hilaria annua in papaya, and Piper abalienatum in coffee. They are currently under no legal protections (Appendix 2).
Finally, 222 species were found, of these 166 (74.8%) are native. Furthermore, 21 of the listed species are endemic to Mexico, whether they are widely distributed (16 species) or restricted to the Pacific Coast (5 species). In general, each crop has its own distinctive community of weeds; however, they are grouped into 2 large clusters: those that correspond to lowland crops in the Pacific Coast province and that tend to have warmer and more stable temperatures throughout the year. The other group is made up of highland crops found within the Sierra Madre del Sur province, which tends to be subject to lower temperatures and greater annual variation. Of course, some crops are in transitional areas and have a flora that reflects this, whereas others such as maize and coffee feature very distinctive floras, due to different management requirements and particular characteristics.
The predictive power of our results is constrained because they are focused on commercial crops, most of them perennial and with a relatively high degree of technification; they also have a significant bias towards papaya. Although the sampling is multi-year and covers all seasons of the year, each site was visited just once. Therefore, it cannot be ruled out that by including more annual crops as well as other crops managed in a more traditional manner (low technification), it would be possible to add more species to this list, in accordance with the high richness of native species in the state (ca. 4,300 species sensu Villaseñor, 2016). Also, ruderal vegetation and aquatic weeds were not included. We expect to document the weed flora of urban areas of Colima and increase the sampling in crops such as maize as well as include other crops such as banana, coconut, melon, and cucumber, and with different management requirements (i.e., traditional, technified or organic).











 nueva página del texto (beta)
nueva página del texto (beta)





