Introduction
The family Haemulidae is one of the most conspicuous and diverse groups of marine fishes within the order Perciformes. Currently, this family contain 150 species included in 17 genera distributed worldwide (Froese & Pauly, 2019). In particular, the Neotropical genus Haemulon is one of the most species-rich, with 145 species (Tavera et al., 2012). In Mexico, species of the genus are distributed in the Pacific and Gulf of Mexico coasts, as well as in the Caribbean Sea. The genus is represented by 21 species of which 14 are exclusively found in the West Atlantic and Caribbean (Froese & Pauly, 2019). The genus Haemulon is one of the most well-represented in coral reefs of the New World. Few studies have documented the helminth parasite fauna of haemulids. Paschoal et al. (2015) reported 147 species of helminths (plus 37 undetermined taxa) infecting 48 species of grunts in a checklist of the metazoan parasites of Haemulidae in the Americas; trematodes were the most diverse, with 83 species reported. Centeno and Bashirullah (2003) compared the helminth parasite communities of 8 species of Haemulon in the Golfo de Cariaco, Venezuela. Later, Bashirullah and Díaz (2015) determined the infracommunity structure of the helminth parasite fauna of H. aurolineatum in the same locality. In Mexico, species of trematodes in grunts have been documented mostly as isolated reports (Pérez-Ponce de León et al., 2007); however, no studies have been conducted to evaluate the parasite community structure of haemulids.
The Puerto Morelos Reef National Park (PMRNP) is located in the Mexican state of Quintana Roo and is part of the second largest barrier coral reef in the world (Carabias-Lillo et al., 2000); the natural protected area is characterized by a large species diversity of fish and many invertebrate groups. Winfield et al. (2017) reported 19 species of tanaidaceans (Crustacea); Hernández and Álvarez (2019) reported 120 species of mollusks; interestingly, the large diversity of crustaceans and mollusks show the availability of intermediate hots for trematodes in the study area. Marine fish trematode life-cycles are usually complex, and involve 2 or 3 hosts. As definitive hosts, fishes are infected through trophic transmission when they fed upon vegetation, invertebrates or small fishes containing metacercariae. Mollusks are the first intermediate hosts (Galaktionov & Dobrovolskyj, 2013). In species of the family Opecoelidae, bivalvs act as first intermediate host, whereas echinoderms, crustaceans, annelids or even small fishes are the second intermediate hosts (Cribb, 2005). The Puerto Morelos reef also holds a diverse fish fauna, with 254 species, 13 of which belong to the family Haemulidae (Domínguez-Domínguez et al., 2015). However, only 4 studies have documented the presence of marine fish trematodes in the area (Caballero, 1990; Pérez-Ponce de León, 1992; Pérez-Ponce de León & Hernández-Mena, 2019; Rufino-González, 1989), accounting for 17 species of trematodes in 11 host species. This contrast with the trematode diversity that has been documented in other reef systems. For instance, Cribb et al. (2014) reported 240 species of trematodes described from marine fishes in the Great Barrier Reef, Australia. Considering the large fish species richness in the reef, it is clear that very few trematode species have been recorded in the study area. Moreover, no studies on the patterns and structuring processes of parasites operating at different scales in the PMRNP have been addressed. The aim of this paper was to report the trematode diversity in 6 species of grunts, and to describe their patterns of community structure, at the infracommunity and component community levels.
Material and methods
Between January and May 2015, specimens of grunts were collected using a fishing hawaiian sling in the Puerto Morelos Reef National Park (Fig. 1). The total sample consisted of: Haemulon aurolineatum Cuvier (n = 36), H. chrysargyreum Günther (n = 10), H. flavolineatum (Desmarest) (n = 80), H. plumierii (Lacepède) (n = 32), H. sciurus (Shaw) (n = 33), and H. carbonarium Poey (n = 19). Collected fish were placed on ice, transported to the laboratory, and examined a few hours after capture. Each individual fish was necropsied and all internal organs were separated and placed in Petri dishes with saline 8.5%, and observed under the stereomicroscope. Trematodes were separated from the organs and fixed in near-boiling saline. Trematodes were stained with Mayer's paracarmine and Gomori's thrichrome, cleared in methyl salicylate and mounted on permanent slides using Canada balsam (Lamothe-Argumedo, 1997). For the identification of trematodes, the keys to the Trematoda were used (Bray et al., 2008; Gibson et al., 2002; Jones et al., 2005), as well as specialized literature. All the trematode specimens were deposited in the Colección Nacional de Helmintos (CNHE), Instituto de Biología, UNAM, Mexico City (Table 1). Fishes were deposited in the Colección de Peces, Universidad Michoacana (CPUM) Morelia, Michoacán.
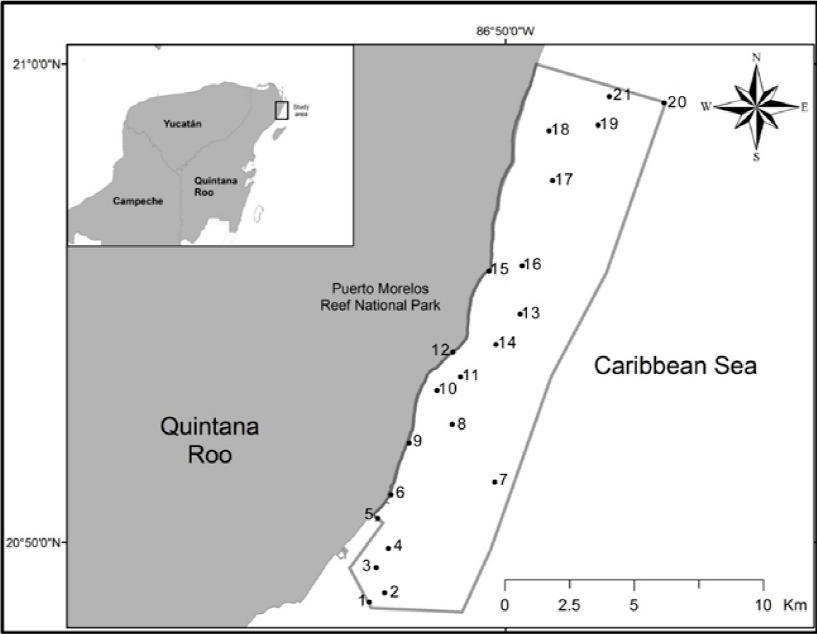
Figure 1 Map of the Puerto Morelos Reef National Park, Quintana Roo, Mexico, showing the collecting sites for this study. 1: Fish market, 2: La Luna, 3: La Pared, 4: Jardín frontal, 5: Muelle fiscal, 6: Ojo de agua, 7: Barco hundido, 8: Rordman, 9: Muelle UNAM, 10: Ojo norte, 11: Punta Caracol, 12: Mar Casa de playa, 13: Cuevones, 14: Cazones, 15: Bahía Petempich, 16: Herradura, 17: Bonanzas sur, 18: Punta Norte, 19: Boya zona norte, 20: Límite Punta norte del parque, 21: Limones.
Table 1 Trematodes of 6 species of Haemulon from the Puerto Morelos Reef National Park, Quintana Roo, México.
| Taxa | HAURO | HCARB | HCHRY | HFLAV | HPLUMI | HSCIU | CNHE |
|---|---|---|---|---|---|---|---|
| Megaperidae | |||||||
| Homalometron sp.1 | X | ||||||
| Homalometron sp. 2 | X | X | |||||
| Derogenidae | |||||||
| Leurodera decora | X | X | X | X | 1947,11221,11222, 11223, 11219, 11220 | ||
| Hemiuridae | |||||||
| Hemiuridae gen. sp. 1 | X | ||||||
| Hemiuridae gen. sp. 2 | X | 1951,1949 | |||||
| Monolecithotrema sp. | X | 11218 | |||||
| Lecithasteridae | |||||||
| Macradena sp. | X | ||||||
| Monorchiiidae | |||||||
| Lasiotocus haemuli | X | X | X | 1972, 11225 | |||
| Opecoelidae | |||||||
| Hamacreadium mutabile | X | X | X | 11224 | |||
| Pycnadenoides sp. | X | X | 11226 | ||||
| Helicometrina nimia | X |
HAURO: H. aurolineatum; HCARB: H. carborinarium; HCHRY: H. chrysargyreum; HFLAV: H. flavolineatum; HPLUMI: H. plumierii; HSCIU: H. sciurus; CNHE: Colección Nacional de Helmintos (UNAM, Mexico City).
Infection parameters describing the populations of trematodes in their fish hosts were calculated following Bush et al. (1997). Prevalence is the number of hosts infected with 1 or more individuals of a particular parasite species divided by the number of hosts examined for that parasite species. Mean Intensity is the total number of parasites of a particular species found in a sample divided by the number of hosts infected with that parasite. Mean Abundance is the average abundance of a parasite species among all members of a particular host population; these parameters were calculated using the statistics software Quantitative Parasitology v. 1.0.14 with 95% of confidence limits (Reiczigel et al., 2019).
To determine if the sampling effort was sufficient to recover the majority of existent species in the trematode community of each Haemulon species in the study area, accumulation curves of species were carried out using Estimates v. 8.20 (Colwell, 2016), with the data of the accumulated trematode richness (Y axis), and the sampled individuals (X axis), randomized 100 times. A comparison was made between the accumulated wealth observed and the estimator Chao 2 to decide the accuracy of sampling effort; only hosts with a representative sampling effort greater than 50%, representing the majority of the trematodes in the community were considered in further community analyses; these were made at the infracommunity (all trematodes in each individual host, representing a subset of the parasite fauna of the host species) and component community (all trematodes in all hosts collected, i.e., the parasites found in a host population) (Holmes & Price, 1986, Poulin, 1997). Component community parameters included the total number of parasite species, total number of individual parasites, the Shannon-Wiener index (H) as a measure of diversity (Krebs, 1999), and the Berger-Parker index (BPI) as a measure of numerical dominance (Magurran, 2004). To test the significance of differences among trematode infracommunities, we conducted a Permutational Multivariate Analysis of Variance (PERMANOVA), a semiparametric method that simultaneously retains robust statistical properties of rank-based nonparametric multivariate methods (Anderson, 2005), and a Multidimensional Scale Analysis (MDS) to visualize differences among infracommunities (Kruskal & Wish, 1978).
Considering the difficulties that arise when trying to compare the different diversity indices, due to the significant effect of the units (Simpson index, Shannon-Wiener index), each species was weighted by its abundance, obtaining the Hill numbers, where N0 represents the 'total number of species' in the sample; N1 is the 'number of the abundant species', and N2 is the 'number of the very abundant species' in the sample (Magurran, 2004). Additionally, to show the relative abundance of species and dominance in the communities, a curve range-abundance was performed. The b-diversity of the infracommunities and component community were calculated with the Jaccard index to analyze qualitative similarity, and with the Bray Curtis index to analyze quantitative similarity (Magurran, 2004). All indices were calculated using PAST 2.01 (Hammer et al., 2001).
Results
Trematode species composition in Haemulon spp.
A total of 210 specimens of grunts were collected in 21 sampling sites of the Puerto Morelos Reef National Park. Eleven species of trematodes allocated in 6 families were collected as parasites of Haemulon spp. in the Puerto Morelos Reef National Park, including 3 species of Opecoelidae (Hamacreadium mutabileLinton, 1910, Helicometrina nimiaLinton, 1910, and Pycnadenoides sp.), 2 species of Megaperidae (Homalometron sp. 1 and Homalometron sp. 2), 3 species of Hemiuridae (Monolecithotrema sp. and 2 of Hemiuridae gen. sp.), 1 of Derogenidae (Leurodera decoraLinton, 1910), 1 of Monorchiidae (Lasciotocus haemuliOverstreet, 1969), and 1 of Lecithasteridae (Macradena sp.) (Fig. 2). Some species were not determined up to species level because of the low number of sampled specimens required for the taxonomic identification, or due to the poor quality of the fixed specimens. However, we are certain that 2 species of Homalometron were collected. They can be easily differentiated by the anterior distribution of the vitelline follicles, and the position of testes along the body. In Homalometron sp. 2, vitelline follicles reach anteriorly the ventral sucker, testes are postequatorial, and both testes and ovary are lobulated. The 2 hemiurids were not identified due to the low number of sampled specimens (3 and 1, respectively), although they can be differentiated as separate species. Hemiuridae gen. sp. 1 possesses a uterus occupying most of hindbody, from the caecal bifurcation to the posterior end of body and rounded and relatively small eggs (150μ long); instead, in Hemiuridae gen. sp. 2 the uterus is postequatorial and eggs are ovoid and large (300 μ long). The most frequent trematode species in grunts was Leudorera decora since it was found in 4 of the 6 fish species, followed by Lasiotocus haemuli and Hamacreadium mutabile, found in 3 species. Each host species harbored between 1 and 8 trematode species. The French grunt (H. flavolineatum) reached the highest trematode species richness with 8 species, followed by the White grunt (H. plumierii) and the Bluestriped grunt (H. sciurus) with 4 species each. The other 3 haemulids (H. aurolineatum, H. carbonarium, and H. chrysargyreum) were infected by 2 or only 1 species of trematode (Table 1).
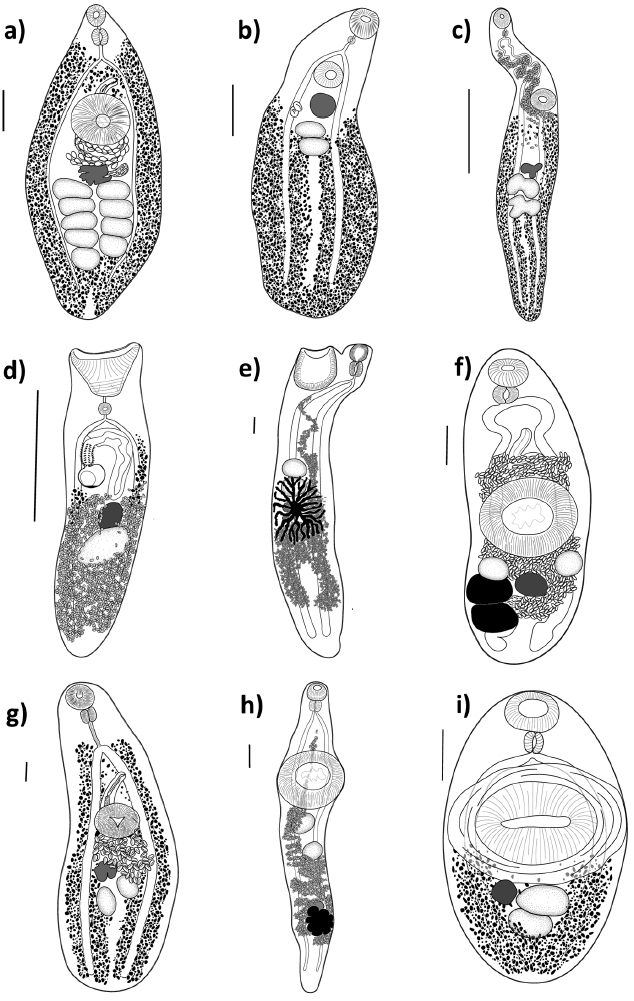
Figure 2 Line drawings of the trematodes sampled in this study. a) Helicometrina nimia, b) Homalometron sp. 1, c) Homalometron sp. 2, d) Lasiotocus haemuli, e) Macradena sp., f) Leurodera decora, g) Hamacreadium mutabile, h) Monolecithotrema sp., i) Pycnadenoides sp. All figures are ventral view; scale bar = 0.2 mm.
Characterization of infrapopulations
In total, 101 trematode specimens were collected in 42 of the 210 analyzed fish, corresponding to 6 species of Haemulon. The values of prevalence for each trematode species did not yield either dominant species (prevalence > 40%) nor common species (prevalence 20-40%); in consequence, all trematodes are considered rare species (prevalence < 20%). The trematodes that reached the highest prevalence of infection were Lasiotocus haemuli in H. plumierii (15.6%), and Leurodera decora in 2 hosts, H. sciurus (15.2%), and H. flavolineatum (11.2%) (Table 2). All other trematodes reached even lower prevalence values in all the fish species analyzed. The mean abundance was relatively low, with the highest value recorded for L. decora with 0.48 individuals per analyzed host (H. sciurus). Mean intensity values varied between 1 and 14 trematodes per infected host.
Table 2 Ecological parameters of the infection of trematodes in 6 species of Haemulon spp. of the Puerto Morelos Reef National Park, Quintana Roo, Mexico.
| Host species | Digenean species | NR | NI | TP | P (%) | MA | MI |
|---|---|---|---|---|---|---|---|
| H. aurolineatum | Monolecithotrema sp. | 1 | 1 | 2.8 | 0.03 | 1.0 | |
| Leurodera decora | 1 | 2 | 2.8 | 0.06 | 2.0 | ||
| H. carbonarium | Pycnadenoides sp. | 19 | 2 | 3 | 10.5 | 0.16 | 1.5 |
| H. chrysargyreum | Lasiotocus haemuli | 10 | 1 | 1 | 10.0 | 0.10 | 1.0 |
| H. flavolineatum | Homalometron sp. 1 | 80 | 5 | 6 | 6.2 | 0.75 | 1.2 |
| Homalometron sp. 2 | 1 | 2 | 1.2 | 0.03 | 2.0 | ||
| Leurodera decora | 9 | 14 | 11.2 | 0.18 | 1.5 | ||
| Hamacreadium mutabile | 1 | 3 | 1.2 | 0.04 | 3.0 | ||
| Hemiuridae gen. sp. 2 | 2 | 3 | 2.5 | 0.04 | 1.5 | ||
| Helicometrina nimia | 1 | 1 | 1.2 | 0.01 | 1.0 | ||
| Lasiotocus haemuli | 1 | 14 | 1.2 | 0.18 | 14 | ||
| H. plumierii | Leurodera decora | 32 | 2 | 4 | 6.6 | 0.13 | 2.0 |
| Lasiotocus haemuli | 5 | 15 | 16.6 | 0.47 | 3.0 | ||
| Hamacreadium mutabile | 2 | 3 | 6.6 | 0.1 | 1.5 | ||
| Hemiuridae gen. sp. 1 | 1 | 1 | 3.3 | 0.03 | 1.0 | ||
| H. sciurus | Leurodera decora | 33 | 5 | 16 | 15.2 | 0.49 | 3.2 |
| Hamacreadium mutabile | 1 | 9 | 3.0 | 0.27 | 9.0 | ||
| Homalometron sp. 2 | 1 | 2 | 3.0 | 0.06 | 2.0 | ||
| Macradena sp. | 1 | 1 | 3.0 | 0.03 | 1.0 |
NR = Number of individual fish examined, NI = number of individual fish infected, TP = total number of parasites per individual fish sample, P (%) = prevalence, MA = mean abundance, MI = mean intensity.
Species accumulation curves
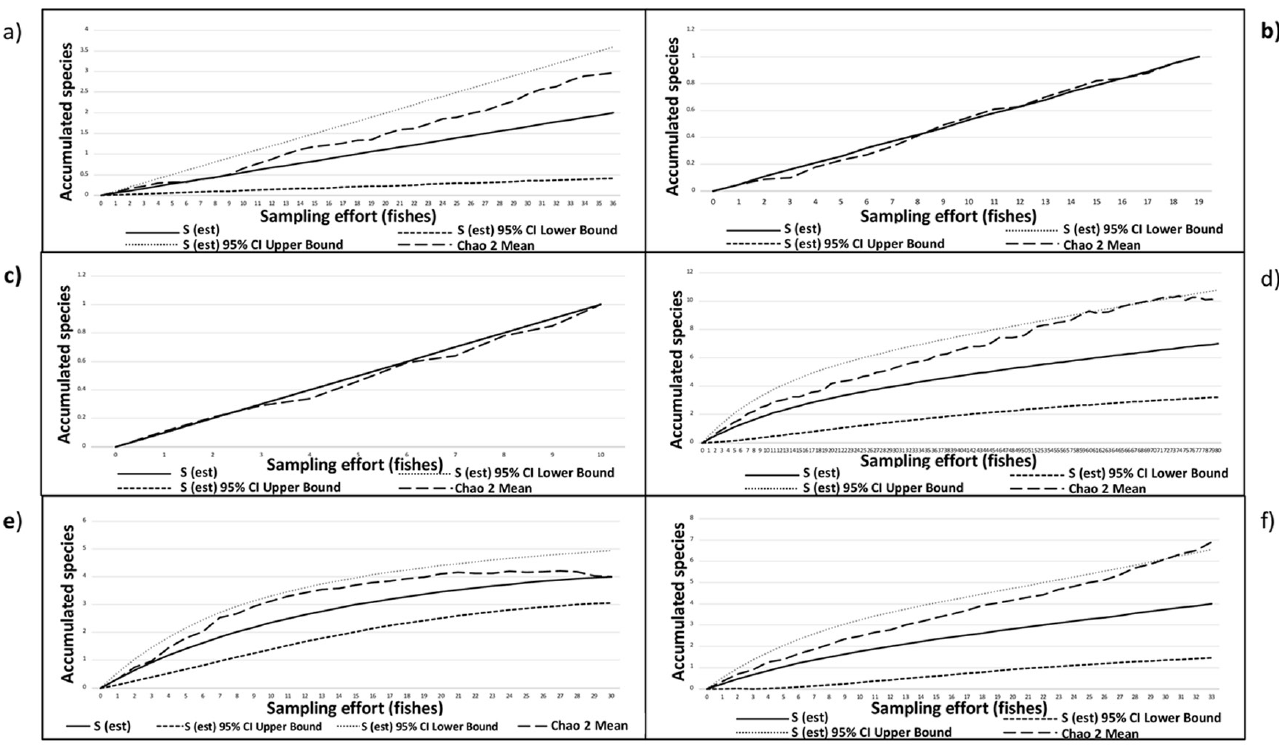
Species accumulation curves showed that only 3 of the 6 host species, i.e., H. flavolineatum, H. plumierii, and H. sciurus, presented more than half of the expected trematode species. The values for the non-parametric species richness estimator (Chao2) for these 3 species showed that 3 missing species remain to be found, except for the curve of H. plumierii where Chao2 does not predict that more species will be found (Fig. 3). Based on the latter result, the analysis of the trematode community structure we present next only includes these 3 host species.
Infracommunity
The trematode infracommunities of H. flavolineatum reached the highest values of mean species richness, whereas those of H. sciurus exhibited the highest values of mean abundance with 4.67 trematodes per infected host. However, the PERMANOVA analyses showed that differences of trematode infracommunities among hosts are not statistically significant (PERMANOVA-J: F = 1.01, p = 0.346; PERMANOVA-Bray Curtis: F = 0.845, p = 0.447). This result was further confirmed through multiscale dimensional analysis showing that trematode infracommunities in haemulids are overlapped (Fig. 4). The Shannon-Wiener diversity index was very low and indicated that H. flavolineatum had the highest trematode diversity (0.21 ± 0.38), although the evenness was very similar among infracommunities. Using Hill's numbers, it was possible to determine the representative number of species for the diversity indices, which showed a richness represented by the mean number of species (N0 = 1.33-1.42), diversity by the abundant species (N1 = 1.11-1.42) as well as its dominance (N2 = 1.10-1.15) (Table 3). Overall, the infracommunities of H. plumierii and H. sciurus were dominated by 1 species, whereas that of H. flavolineatum was dominated by 2 species of trematodes, all with a Berger Parker index higher than 0.9; in the abundance range curve, L. haemuli and L. decora stand out in H. flavolineatum, while L. haemuli was the dominant species in H. plumierii, and L. decora in H. sciurus (Fig. 5).
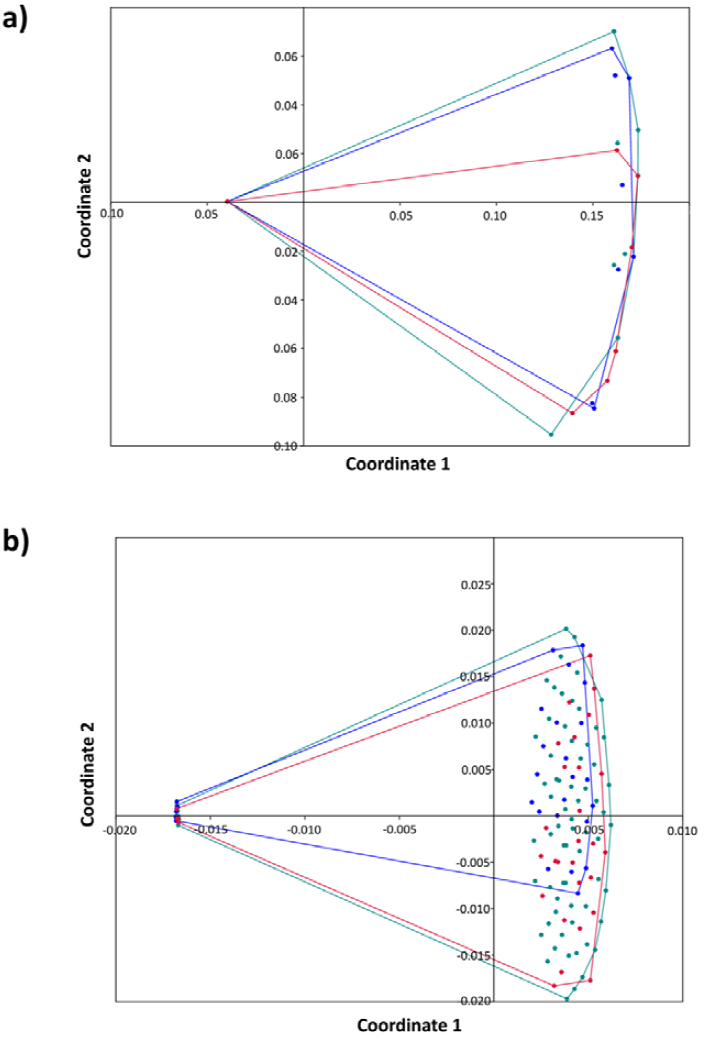
Figure 4 Multidimensional scaling analysis (MSD) among 3 species of haemulids of the Puerto Morelos Reef National Park, Quintana Roo, based on a) Jaccard similarity index and b) Bray-Curtis similarity index. Red dots = Haemulon flavolineatum, blue dots = Haemulon plumierii, green dots = Haemulon sciurus.
Table 3 Trematode infracommunities of 3 species of Haemulon in the Puerto Morelos Reef National Park, Quintana Roo, México. Values are presented as the mean ± standard desviation.
| Host | Overall prevalence |
MSR | MA | H´ | N0 | N1 | N2 | d | Dominant species |
|---|---|---|---|---|---|---|---|---|---|
| H. flavolineatum | 17.5 | 1.42 ± 0.76 | 3 ± 3.68 | 0.21 ± 0.38 | 1.42 | 1.23 | 1.15 | 0.90 ± 0.20 |
Lasiotocus haemuli and Leurodera decora |
| H. plumierii | 26.66 | 1.25 ± 0.47 | 2.88 ± 2.1 | 0.14 ± 0.26 | 1.25 | 1.14 | 1.10 | 0.93 ± 0.12 |
Lasiotocus haemuli |
| H. sciurus | 18.18 | 1.33 ± 0.51 | 4.67± 3.07 | 0.16 ± 0.27 | 1.33 | 1.11 | 1.12 | 0.93 ± 0.13 |
Leurodera decora |
MSR = mean trematode species richness, MA = mean abundance of trematodes, H'= Shannon-Wiener diversity index, N0 = total number of species, N1 = number of abundant species, and N2 = number of most abundant species, d = Berger-Parker dominance index.

Figure 5 Range-abundance curves showing the trematode infracommunity of 3 species of Haemulon of the Puerto Morelos Reef National Park, Quintana Roo. HMUTAB: Hamacreadium mutabile, HNIMIA: Helicometrina nimia, HEMIUR2: Hemiuridae sp. 2, HOMALO1: Homalometron sp. 1., HOMALO2: Homalometron sp. 2. LHAEMU: Lasiotocus haemuli, LDECOR: Leurodera decora, MACRAD: Macradena perfecta. The most abundant species in each species of Haemulon are indicated in bold.
The results of the similarity indices determined a low beta diversity among species of Haemulon herein studied; these diversity values implied a low overlap of parasite species among individuals. The infracommunities of H. flavolineatum shared several trematode species, but the values of qualitative (0.31) and quantitative similarity were very low (0.25). Within H. plumierii, infracommunity similarity is even lower, and in H. sciurus exchange occurs in all individuals, but in low quantities (Table 4).
Table 4 Beta diversity in Haemulon infracommunity with Jaccard and Bray-Curtis coefficients. Values are presented as the mean and range.
| N | Jaccard coefficient (qualitative) | Bray-Curtis coefficient (quantitative) | |
|---|---|---|---|
| H. flavolineatum | 13 | 0.31 (0.25-0.67) | 0.25 (0.11-0.67) |
| H. plumierii | 8 | 0.43 (0.5) | 0.26 (0.22-0.83) |
| H. sciurus | 5 | 0.70 (0.33-0.5) | 0.48 (0.13-0.91) |
N = number of pairs of individuals compared.
Component community
At the component community level, H. flavolineatum was the species that reached the highest values for the descriptors of the community structure. This haemulid had the highest abundance with 43 individual trematodes, the highest diversity index (1.66), the highest species richness, with 7 species; the Shannon-Wiener Diversity Index of the trematode component community in H. flavolineatum was 1.66 which was the highest value compared to the other component communities. The Berger Parker index did not show a marked dominance for the hosts (Table 5).
Table 5 Trematode in component community of 3 species of Haemulon in the Puerto Morelos Reef National Park, Quintana Roo, Mexico.
| Component community | ||||||
|---|---|---|---|---|---|---|
| A | H´ | N0 | N1 | N2 | d | |
| H. flavolineatum | 43 | 1.66 | 7 | 4.99 | 4.1 | 0.32 |
| H. plumierii | 23 | 0.99 | 4 | 2.68 | 2.1 | 0.65 |
| H. sciurus | 28 | 1 | 4 | 2.69 | 2.29 | 0.57 |
A= Abundance of trematodes, H = Shannon-Wiener diversity index. N0 = Total number of species, N1 = number of abundant species, and N2 = number of most abundant species, d = Berger-Parker dominance index.
Similarity indices showed that Haemulon species show low beta diversity, both in composition (Jaccard index) and in structure (Bray-Curtis). The highest similarity was found between H. flavolineatum and H. plumierii, with values of 0.57 and 0.67, respectively. Instead, the lowest similarity was found between H. plumierii and H. sciurus, with 0.33 and 0.27, respectively.
Discussion
In this study, 11 trematode species were identified as parasites of 6 species of Haemulon in the PMRNP area. Previous studies indicate that the trematode fauna of these species of Haemulon, distributed along the Western Atlantic coast, may harbor up to 173 taxa, 150 of them taxonomically determined to species level (Paschoal et al., 2015). Our results contrast with the records of the trematode fauna of these haemulids because they possess a higher trematode species richness, i.e., the number of species of trematodes reported for H. aurolineatum across its distributional range is 25, that of H. carbonarium is 17, 12 in H. chrysargyreum, 29 in H. flavolineatum, 36 in H. plumierii, and 54 in H. sciurus (Paschoal et al., 2015). Instead, in the PMRNP, these same species are only infected by 2, 1, 1, 8, 4 and 4 trematode species, respectively. The most striking contrast was found for the trematode fauna of H. sciurus that include 54 species, while in our study, only 4 species were reported in the same host species. Interestingly, only 1 of the trematode species (L. decora) had been previously reported in H. sciurus in Florida (Linton, 1910). The other 3 represent new host records (Hamacreadium mutabile, Macradena sp., and an unidentified and probably new species of Homalometron).
Overall, 16 new host records are reported in this study. Eight of the 11 trematode species are reported in the PMRNP for the first time. Only the opecoeliids Helicometrina nimia, and Hamacreadium mutabile, and the derogenid Leurodera decora were previously reported in the area, although in different host groups the first 2 species (Caballero, 1990; Pérez-Ponce de León, 1992); L. decora was found in H. sciurus (Rufino-González, 1989). The species Pycnadenoides sp.,Monolecithotrema sp., and Macradena sp., as well as the 2 species of Homalometron, represent new taxa for the family Haemulidae (Paschoal et al., 2015). Furthermore, the 2 unidentified species of Homalometron reported in our study are new host records. According with Paschoal et al. (2015), 3 species of Homalometron have been reported in haemulids along the Western Atlantic coast, namely H. cryptum (Overstreet, 1969), H. dowgialloiDyer, William and Bunckley-Williams, 1992, and H. foliatumSiddiqi and Cable, 1960 (Dyer et al., 1992; Overstreet, 1969; Sidiqi & Cable, 1960). However, our specimens (representing 2 separate species) are morphologically very different to the species previously reported, and they can represent new species. Unfortunately, the specimens collected are in poor condition to accomplish the proper species description.
Parasite infrapopulation parameters exhibited variable values; only 2 parasite species reached a prevalence of 15% (Lasiotocus haemuli in H. plumierii, and Leurodera decora in H. sciurus); Bashirullah and Díaz (2015) recorded prevalence values higher than 30% in H. aurolineatum studied off the coast of Venezuela; in our study, in the same host species the highest prevalence value reached only 2.8%. This difference can be attributed to the environmental and biological conditions of the sampling site, even if it is the same host species (Holmes, 1990; Scott, 1982; Violante-González et al., 2010); the variation between habitats affects the composition of parasite species; therefore, the variation in spatial distribution of different taxa of local benthic invertebrates can be expected to reflect the distribution of parasites that use them as intermediate hosts (Marcogliese, 2002).
At the component community and infracommunity levels, trematodes of Haemulon spp. exhibited a similar pattern, with low species richness and abundance, as well as low diversity values, and having communities dominated by at least 1 species of trematode. This overall pattern has been found in other studies, where the feeding habits and the differential availability of the intermediate hosts harboring metacercarial stages of the parasites have been discussed as the main factors structuring parasite communities. (e.g., Violante-González, Aguirre-Macedo, & Rojas-Herrera, 2008). The low diversity of the trematode communities analyzed in this study at both levels indicate that Haemulon species are not top predators in the food web in the study area; high values of parasite diversity indicate that their hosts occupy such position in the food web (Bush et al., 2001; Poulin, 2007). The low species richness values of the trematode grunt infracommunity at the PMRNP in comparison to the parameter at the component community shows that each infracommunity simply represents a subset of the species present in the component community; instead, the component community reflects a subset of a larger collection of species that represent the entire trematode fauna of these host species along its distributional range (Poulin, 1997).
Moreover, patterns of similarity were very similar at both scale levels of our study; this indicates a low beta diversity which can be the result of high dispersion rates (vagility), which acts to homogenize communities (Moos et al., 2020). At the infracommunity level, a low exchange of trematode species was shown, indicating that not all the species occur in all individual hosts; meanwhile, the b-diversity of the component community, despite being low, presented some species that are shared among host species, e.g., L. decora and H. mutabile, as well as other species that exhibited some level of host specificity for some of species of Haemulon; that is the case for Homalometron. sp. 1, Hemiuridae gen. sp. 1, Hemiuridae sp. 2, and Macradena sp., indicating a particular parasite structure for each Haemulon species.
The food preference of each host species explains the parasitic structure of each Haemulon species; for example, H. flavolineatum mainly feeds on amphipods and scaphopods, H. plumierii on brachyura and caprellidae, while H. sciurus on ascidians; in addition, the 3 hosts share other food items represented by taxonomic groups such as Anthozoa, Decapoda, Foramminifera, Gastropoda, Asteroidea, Holoturioidea, and Ophiuroidea (Cipresso-Pereira et al., 2015, Froese & Pauly, 2019). The hosts diet is known to be one of the most influential factors structuring the parasite fauna (Carvallho et al., 2015; Prasanna et al., 2011); changes in prey availability and host-parasite compatibility have been also described as important factors (Ojaveer et al., 2020). Further, Esch et al. (1990) and Violante-González, Aguirre-Macedo and Vidal-Martínez (2008) argued that infracommunities are separated by guild, through feeding and microhabitat mechanisms of the host; the variation in diet and the amount of food items eaten by hosts is related to the dynamics of the parasite population, and their community structure; differences in the assemblages of parasites among marine fish species are usually explained by these ecological characteristics of the host (Timi & Lanfranchi, 2009).
In conclusion, the trematode communities of the species of Haemulon analyzed in this study at both, infracommunity and component community levels, were characterized by presenting low diversity indices, placing them as predators at a low trophic level. Furthermore, trematode communities differed among species of grunts, indicating that ecological factors such as feeding habits, geographic distribution, and vagility of the hosts play a major role structuring these communities. In general, our results are consistent with several studies that indicate that ecological selection can strongly structure parasite communities (Moos et. al., 2020). Clearly, more studies are required to fully understand the parasite community structure of marine fishes in the PMRNP, considering the inclusion of other fish species since the species richness of this vertebrate group is very high in the area (Domínguez-Domínguez et al., 2015). This information will contribute to generate the necessary data to address aspects of conservation in an important area of the Mexican Caribbean Sea.











 nueva página del texto (beta)
nueva página del texto (beta)