Introduction
South American tropical forests concentrate highly significant reserves of carbon, biodiversity (Meister et al., 2012; Asner et al., 2014) and occupy approximately 6,300,000 km2 throughout the Amazon, of which 50,000 km2 are in Peru (Ruokolainen et al., 2001). Part of it is represented by marshes, peat bogs, palm swamps, and other forested wetlands, commonly located at low altitude (< 300 m asl) but these environments also occur in the foothills of the Andes. The palm swamps and forested wetlands are connected with other biomes, which allows environmental heterogeneity and different soil types (chemical composition, type of drainage and peat formation), favoring the various vegetation types in the landscape (Klink & Machado, 2005).
Studies on forested wetlands in the foothills are scarce in Peru. The few studies on forested wetlands are insufficient due to the extension of these ecosystems in the country (more than 5 million hectares). In addition, they have contributed little to the knowledge about floristic composition and structure. In Peruvian Amazonian, most of these studies have been carried out in floodplain lowland territories in the department of Loreto and recently in Madre de Dios; highlighting its importance as a fragile ecosystem (Bhomia et al., 2018; Lähteenoja & Page, 2011). This has generated a large information gap on the flora of the highest-elevation floodplain forests in Peru, especially in the piedmont.
Melack and Hess (2010) identified at least 12 wetland types in the Peruvian Amazon, including “aguajales” (M. flexuosa palm swamp forests), renacales (Ficus sp. and Coussapoa sp.), and pacales (Guadua sp.). This spectrum of ecosystems is generated by a high variability of climate, geomorphology and soils in the region (Fujiyoshi et al., 2009; Gentry & Ortiz, 1993). The “aguajales” are a wetland type with soil made up of accumulated remnants of partially decomposed vegetation (peat) and dominated by individuals of Mauritia flexuosa palm that is maintained by the water balance of the ecosystem (Janovec et al., 2013). The “aguajales” stand out for the quality of the ecosystem services they provide: they are the main carbon sinks, since they store more than twice as much carbon as all the world’s forests combined (Barthelmes et al., 2015). Furthermore, they are important in the reserve, purification and regulation of water cycles and food supply, both for human populations and for native fauna; among others. In “aguajales” exist species that are not adapted to seasonal hydrological influences during their lifespan, and are commonly associated with anoxic environments that retard soil respiration rates and allow the buildup of thick peat (Householder et al., 2012; Luize et al., 2018). The border zone is an area with anthropogenic activities (agriculture and cattle ranching) that occurs surrounding the palm swamps. The central portion is a saturated area near the river. The moisture gradient thus permits the establishment of species with different ecological requirements, ranging from flooded experts to generalist species (Kurtz et al., 2013).
Palm trees, especially M. flexuosa, is the main species of palm swamps in Peruvian Amazon due to their high abundance in all growth categories and their presence in the emerging canopy (Balslev et al., 2010; Urrego, 2018). Other important canopy forming species are: Virola elongata, Symphonia globulifera, Euterpe precatoria, and other Leguminosae, Moraceae and Lauraceae species (Quinteros et al., 2016). Herbs from the Maranthaceae, Costaceae, Araceae (Anthurium, Philodendron, Syngonium) families and ferns (Dryopteridaceae, Polypodiaceae, Selaginellaceae) are commonly found in the understory of palm swamps (Quinteros et al., 2016); as well as shrubs of the families Rubiaceae, Melastomataceae and Leguminosae that develop in organic soils with low nutrient content and slightly acidic pH (Fajardo et al., 1999).
The presence and high abundance of hyperdominant species lead to a relatively low diversity of tree species compared to other Amazon forests, especially “várzea” which are the most diverse wetland forest worldwide, containing more than 1,000 tree species (Wittmann et al., 2006). Few studies classify the growth categories from palm trees and woody species, leaving a gap of information in the structure of the understory, so the present study is an important contribution (Table 1).
Table 1 Growth categories, according morphological characteristics, making a differentiation between (a) woody species and (b) palm species.
| Woody species | Palm species | |
| Saplings Poles Small tree/ palm Large tree/ adult palm |
1 - 2.5 cm DBH ≥ 2.5 cm < 7.5 cm DBH ≥ 7.5 cm < 17.5 cm DBH ≥ 17.5 cm DBH |
≥ 150 cm and < 300 cm in height ≥ 300 cm and < 600 cm in height ≥ 600 cm tall with stem but without reproductive structures ≥ 600 cm tall with stipe and reproductive structures |
The forests of the Alto Mayo Valley have large-scale destruction due to the increasing land use change from palm swamps to rice crops by settlers since the Marginal highway construction in 1975, which is the major road connecting the northern Amazon region with the highlands in Peru (Dietz et al., 2003). Most facies of the Alto Mayo biome present subtropical vegetation, ranging from open areas such a grassland formations and rice crops to closed formations as the premontane forest and forested wetlands which shows a floristic and structural complexity in contrast to the usual open formations (Rull & Montoya, 2014).
We analyzed the floristic composition, forest stand structure, and the conservation status of the tree flora of piedmont Alto Mayo Valley (AMV) in the department of San Martín, Peru. The results of this study were expected to confirm the hypothesis that the family Arecaceae concentrates the highest density and basal area in forested wetlands.
Materials and methods
The study was carried out in 4 sites in the localities of Tingana and Posic in the Department of San Martín, north-central Peru. The Tingana forest is protected by the Area de Conservación Aguajal Renacal del Alto Mayo (ADECARAM/Tingana) since 2002, and the area is rarely flooded by the rivers Huascavacu, Avisado and Mayo (Borner & Zimmermann, 2003; Dietz et al., 2003).
Tingana is located at 870 m asl in the municipality of Pueblo Libre, between 5°53’52” - 5°56’36” S, 77°05’55” - 77°11’47” W longitude. The Posic forest is located in the municipality of Posic (6°01’08” - 6°02’23” S, 77°09’22” - 77°10’13” W; Fig. 1).
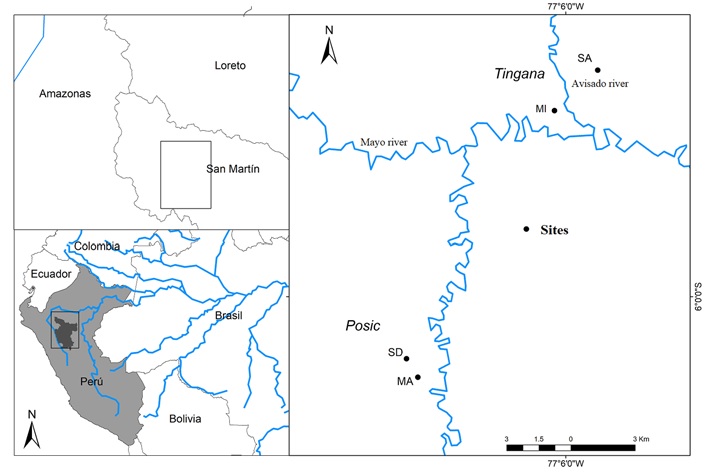
Figure 1 Forested wetlands located in Tingana and Posic, with sampled plots. Legend: SA and MI plots in Tingana; SD and MA plots in Posic.
Both forests are in the Andean-Amazonian piedmont (20-30% slope) in the western Alto Mayo Valley, which is an ecotone between seasonally dry forests of Central Huallaga and the yungas (mountain forests of the eastern Andes of Peru). The climate is humid subtropical, with a mean annual temperature of 22.8 oC and average annual rainfall of 1,265 mm (PEAM, 2004). Rainfall is concentrated in the wet season between October and April (Toivonen et al., 2017).
The stratigraphic composition and the geochronology of the exposed rocks of the study area were formed by a sequence of pure marine grey limestone from the Triassic-Jurassic eras with great structural deformations and Quaternary fluvial deposits in the floodplains, which provide nutrient-poor substrate for soil development (Alva et al., 1992; Fujiyoshi et al., 2009). The soils are inceptisols with imperfect to poor drainage and a fluctuating water table (40-80 cm depth; ONERN, 1983). The AMV is characterized by its variety of natural forest physiognomies and its particular location in hollows with depths of more than 20 m composed of different layers of Quaternary peat deposits and 10-30 m canopy with dense understories (Alva et al., 1992; Young & León, 2000). The study area has an altitude between 800 and 900 m asl and includes the floodplains at the lower end of the watersheds (Dietz et al., 2003).
One hundred plots of 20 × 20 m were established, 25 plots in each of the 2 sites in Tingana and 25 plots in each of the 2 sites in Posic; the plots were separated at least 40 m from each other. Plots in Tingana included semi-dense “aguajales” (SA site) and mixed “aguajales” (MI site) without extractive activity, and plots in Posic included relict forests in semi-dense to mixed “aguajales” (SD site) and mixed “aguajales” (MA site) that have been strongly affected by the change of land use for rice crops and undergoes sporadic floods of the Tonchima river due to deforestation of its banks.
All free-standing plants including trees, shrubs and palm individuals (herbs, grasses and sedges were not included) with diameter at breast height (DBH) ≥ 1 cm were tallied, tagged and the species name recorded. The diameter at breast height (DBH) was measured with a diameter tape, and tree height using a Suunto clinometer. Unidentified species were collected in the field, the vouchers were prepared and deposited in the San Marcos University Herbarium (USM).
For each plot, we calculated richness, abundance and number of families. The density, basal area and biomass were calculated for sites. One-way analysis of variance (ANOVA) and Fisher’s least square difference (LSD) post-hoc test was used to test the significant effects in the abundance, richness and basal area at 5% probability significance threshold among the sites (SA, MI, SD and MA).
Density, frequency and dominance (basal area) values were calculated as well as the importance value index (IVI; DBH ≥ 1 cm) per site (SA, MI, SD and MA) according to Curtis and McIntosh (1950) and Nebel et al. (2001). For each site, species richness was estimated and compared with ACE (Abundance-based Coverage estimator) and Chao1 polynomial curve adjustment in EstimateS 9.1.0 software (Colwell, 2013; Colwell & Coddington, 1994).
All individuals per site were classified based on their height as understory (< 10 m in height), lower canopy (≥ 10 and < 15 m in height), mid canopy (≥ 15 and < 20 m in height), and upper canopy (≥ 20 m in height) according to Endress et al. (2013). Forest structure was also shown for each site using diameter classes.
A Mantel test was used to assess whether there was a relationship between geographic distance and floristic similarity (Sokal & Rohlf, 1995). We elaborated 2 data distance matrices with Chao-Sorensen and Chao-Jaccard estimators for all 100 plots (Chao et al., 2005). Mantel test by a Monte Carlo permutation test using 999 permutations was calculated with XLSTAT (2017) version 2020.1.
Non-metric multidimensional scaling (NMDS) was used to show the floristic relationship among the 100 plots using the Bray-Curtis distance for species abundance. NMDS collects similarity between pairs of samples (plots) preserving any distance between them. Moreover, NMDS looks for the space that best represents the original distances in terms of order ranges (< stress) with the aim of summarizing the dissimilarities between samples and finding homogeneous groups of samples. Significance of the floristic similarity among sites was tested using Analysis of Similarity (ANOSIM; Quaresma et al., 2017) and was performed using PAST software version 3.0 (Hammer et al., 2001).
The conservation status of the recorded species was assessed according to the Red List criteria, using the categories from CITES (I, II, III) and the Peruvian categorization of threatened flora species (“Decreto Supremo N° 043-2006-AG”).
Results
A total of 5,795 individuals (canopy: 932; understory: 4,863) were assessed corresponding to 112 species, 71 genera, and 35 families (Appendix). Leguminosae (13 species), Rubiaceae (12), Moraceae (10), Melastomataceae (9), Primulaceae (7) and Arecaceae (6) were the richer families, and together they accounted for 51% of total richness. The most diverse genera were Ficus and Inga, with 8 species each. Most individuals belong to the Arecaceae (28.5%), followed by Malvaceae, Leguminosae and Rubiaceae with more than 500 individuals each. Only 6 of 35 families were common to the 4 sites: Arecaceae, Clusiaceae, Myristicaceae, Annonaceae, Urticaceae and Araliaceae, while Combretaceae and Hernandiaceae were exclusive to MI plots.
Arecaceae and Myristicaceae were more abundant in the canopy, whereas Arecaceae, Leguminosae, Malvaceae, Rubiaceae, Clusiaceae, Myristicaceae and Annonaceae shrubs or treelets were the most abundant flora in the understory.
Mauritia flexuosa, Virola elongata, Matisia bracteolosa and Mauritiella armata were the most abundant canopy species, while M. bracteolosa, M. flexuosa, Ferdinandusa chlorantha, Machaerium floribundum, V. elongata and Symphonia globulifera were the most abundant species in the understory (each one with more than 300 individuals).
Matisia bracteolosa was the most abundant woody species (812 individuals, canopy: 82, understory: 730). Only 3 palm trees reported adult individuals (M. flexuosa, Euterpe pracatoria and Mauritiella armata). Mauritia flexuosa was the most abundant palm (1,083 individuals, canopy: 442, understory: 641) and was present in 94% of the plots.
Richness, abundance and number of families were higher in Sa plots, while canopy and understory density were lower in Mi site. Moreover, the basal area and biomass were higher in Tingana sites (Table 2).
Table 2 Structural characteristics of vegetation ≥ 1 cm DBH in 4 sites (20 × 20 plots) of the Alto Mayo Valley (AMV) swamp forest, Peru. SA: Tingana semi-dense “aguajales”, MI: Tingana mixed “aguajales”, SD: posic semi-dense to mixed “aguajales”, MA: posic mixed “aguajales”.
| SA | MI | SD | MA | |
| No. of individuals | 72.8 ± 17.66*a | 33.28 ± 9.60*b | 65.92 ± 48.48*a | 59.8 ± 45.48*a |
| Richness | 11.32 ± 2.72 | 10.92 ± 2.72 | 9.92 ± 4.31 | 9.8 ± 3.78 |
| No of families | 7.72 ± 1.99 | 7.64 ± 1.66 | 7.44 ± 3.34 | 7.4 ± 2.65 |
| Canopy density (N ha-1) | 275 | 171 | 207 | 279 |
| Understory density (N ha-1) | 1545 | 661 | 1441 | 1216 |
| Basal area (m2 ha-1) | 40.93 | 34.52 | 33.19 | 32.78 |
| Biomass (Mg ha-1) | 217.31 | 194 | 138.54 | 139.86 |
a, b Different literals for each row, indicate significant differences (p < 0.05) in Fisher’s Least Square Difference (LSD) post-hoc.
Abundance showed significative differences (F = 5.85, p < 0.01; MI site showed significant differences) among sites, contrary to richness (F = 1.15, p = 0.33) and basal area (F = 0.51, p = 0.67). Besides, canopy and understory showed significative differences in abundance (t = 11.1, p < 0.001) and richness (t = 16.78, p < 0.001).
From the total species, only 10 species had IVI greater than 15%. The most ecologically important species of site SA were M. flexuosa (68.2%), Machaerium floribundum (58%), Virola elongata (40.2%) and Symphonia globullifera (34.4%). Whereas for MI site, M. flexuosa (66%), Virola elongata (58.4%) and Inga stenoptera (20.3%) showed the highest values of the importance value index (Table 3). In SD site (Posic), the most ecologically important species were M. flexuosa (80.2%) and Matisia bracteolosa (72.4%) followed by Virola elongata (40.2%), Ferdinandusa chlorantha (13.5%) and Mauritiella armata (12.6%). M. flexuosa (74.4%), F. chlorantha (46.7%) and M. bracteolosa (36.7%) were the top 3 species with the highest IVI values in the Ma site.
Table 3 Five most ecologically important species per site (1 ha) in the swamp forest of Alto Mayo Valley (AMV). Sites: Tingana (SA: Semidense aguajal, MI: Mixed-aguajal), posic (SD: semi-dense aguajal, MA: mixed aguajal). D = density, F = frequency, DO = dominance, RD = relative density (%), RF = relative frequency (%), RDO = relative dominance (%), Importance value index (IVI%).
| Site | Species | D | RD | F | RF | DO | RDO | IVI% |
| SA | Mauritia flexuosa L.f. | 184 | 11.87 | 24 | 8.54 | 19.58 | 47.83 | 68.24 |
| Machaerium floribundum Benth. | 539 | 34.77 | 23 | 8.19 | 6.17 | 15.07 | 58.03 | |
| Virola elongata (Benth.) Warb. | 227 | 14.65 | 25 | 8.90 | 6.82 | 16.66 | 40.20 | |
| Symphonia globulifera L.f. | 230 | 14.84 | 24 | 8.54 | 4.53 | 11.06 | 34.44 | |
| Euterpe precatoria Mart. | 85 | 5.48 | 23 | 8.19 | 1.29 | 3.15 | 16.82 | |
| Other 57 species | 285 | 18.39 | 162 | 57.65 | 2.55 | 6.23 | 82.27 | |
| MI | Mauritia flexuosa L.f. | 121 | 16.40 | 25 | 9.36 | 13.87 | 40.19 | 65.95 |
| Virola elongata (Benth.) Warb. | 104 | 14.09 | 23 | 8.61 | 12.31 | 35.66 | 58.37 | |
| Inga stenoptera Benth. | 92 | 12.47 | 18 | 6.74 | 0.37 | 1.07 | 20.28 | |
| Inga cayennensis Sagot ex Benth. | 64 | 8.67 | 20 | 7.49 | 0.45 | 1.30 | 17.46 | |
| Guatteria blepharophylla Mart. | 62 | 8.40 | 17 | 6.37 | 0.32 | 0.93 | 15.70 | |
| Other 42 species | 295 | 39.97 | 164 | 61.42 | 7.19 | 20.84 | 122.23 | |
| SD | Mauritia flexuosa L.f. | 156 | 10.20 | 20 | 8.77 | 20.35 | 61.30 | 80.27 |
| Matisia bracteolosa Ducke | 634 | 41.44 | 21 | 9.21 | 7.22 | 21.77 | 72.41 | |
| Virola elongata (Benth.) Warb. | 65 | 4.25 | 16 | 7.02 | 0.90 | 2.72 | 13.99 | |
| Ferdinandusa chlorantha (Wedd.) Standl. | 113 | 7.39 | 9 | 3.95 | 0.72 | 2.18 | 13.51 | |
| Mauritiella armata (Mart.) Burret | 93 | 6.08 | 8 | 3.51 | 1.01 | 3.05 | 12.64 | |
| Other 34 species | 469 | 30.65 | 154 | 67.54 | 2.98 | 8.98 | 107.18 | |
| MA | Mauritia flexuosa L.f. | 124 | 9.17 | 20 | 8.47 | 18.60 | 56.73 | 74.38 |
| Ferdinandusa chlorantha (Wedd.) Standl. | 479 | 35.43 | 11 | 4.66 | 2.17 | 6.61 | 46.70 | |
| Matisia bracteolosa Ducke | 177 | 13.09 | 21 | 8.90 | 4.82 | 14.70 | 36.69 | |
| Mauritiella armata (Mart.) Burret | 85 | 6.29 | 8 | 3.39 | 0.95 | 2.88 | 12.56 | |
| Virola elongata (Benth.) Warb. | 48 | 3.55 | 12 | 5.08 | 0.96 | 2.93 | 11.57 | |
| Other 34 species | 439 | 32.47 | 164 | 69.49 | 5.29 | 16.14 | 118.1 |
ACE showed a better response curve (R2 > 0.92) as a function of abundance for Tingana plots (Fig. 2A). In the MI plots the values of the estimators exceeded the observed species by less than 10%, so we believe that ACE and Chao1 are good estimators for the aguajales, reporting 92% and 95% of the observed cases, respectively. In the MA plots, ACE and Chao1 adjustment curves showed overlap with similar responses and very close adjustment values (ACE R2 = 0.847, Chao1 R2 = 0.816; Fig. 2B).
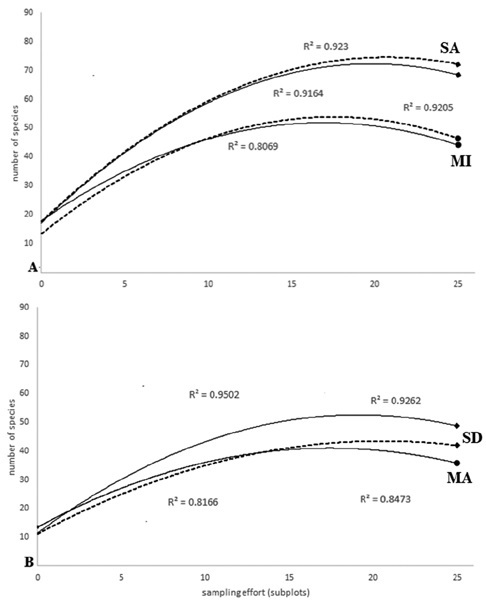
Figure 2 Species-area curve (species accumulation; p < 0.05) of 25 plots of sampling effort, with continuous and broken lines corresponding to Chao1 and ACE index, respectively. A) The diamond at the end of the line refers to SA site while the circle does to MI site. B) Circles at the end of the line represent a MA site and diamonds refer to SD site.
In Tingana most of the stems (61.7%) had the highest number of individuals in the lowest DBH class and the number of individuals progressively decreased with increasing diameter class with the exception of the category (30 - 39.9 cm). Moreover, we found higher density in SA, but lower abundance in the last 2 diameter class (Fig. 3A). In Posic, it was also observed that among 30 and 49.9 cm there is an increase in abundance, both in SD and in MA (Fig. 3B). The highest DBH values (> 73 cm) corresponded to individuals of V. elongata, M. bracteolosa and Pachira. aquatica. For V. elongata and M. bracteolosa, their individuals were arranged in all the diametric classes, unlike P. aquatica, which was found in the categories > 40 cm in DBH.
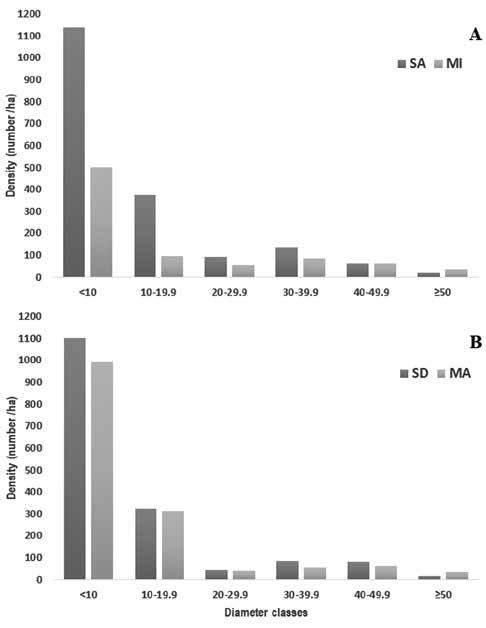
Figure 3 Palm and woody stem among diametric classes. Diametric classes using total number of individuals for Tingana sites (A) and Posic sites (B).
Height distribution was heterogeneous and presented higher number individuals in the second height class (5 - 9.9 m; Fig. 4). In fact, palms contributed with 34% of individuals in the understory (< 10 m) and 87% in the emerging canopy (> 20 m). Indeed, the emerging canopy consisted of 2 palm species (Euterpe precatoria: 21% and M. flexuosa: 78%) in SA site, while 2 species were also recorded in Mi site (M. flexuosa and V. elongata). In Posic, the emerging canopy registered only 1 specie (Ficus krukovii) at both sites.
The basal area in 4 sites was 141.4 m2 ha-1. About 74% of basal area was contributed by individuals with a diameter above 30 cm. The basal area in SA site was found to be higher (40.93 m2 ha-1) than Mi site (34.52 m2 ha-1). Mi site registered the second largest basal in spite of low density (individuals ha-1) attributed to the fact that the dominant palms and woody species found here were higher in sizes.
The Mantel test indicated significant correlations between geographical distances and floristic similarity for plots (Chao-Jaccard index: R = -0.38, p < 0.001; ChaoSorensen index: R = -0.357, p < 0.001). Axes 1 and 2 of the ordination analyses showed SD and MA plots more separated from each other than the SA and MI plots (Fig. 5; ANOSIM: R = 0.58, p = 0.001). Moreover, it was possible to identify 3 floristic groups with some similar features and M. flexuosa dominance (Fig. 5): (a) Mauritia-MachaeriumVirola (SA plots): vegetal association that represents a palm swamp in Tingana with clear waters due to the accumulation of rainwater and poor drainage of the soil (no runoff was observed). The terrain is slightly undulating (30-60 cm) with some unevenness of up to 1 m and the species reported in this association prefer permanently flooded soils (> 50 cm). The community was dominated by M. flexuosa (22.8%), M. floribundum (19.3%), V. elongata (13.4%), and S. globulifera (11.5%). Palm and woody species richness in the canopy accounted for 26.7% of understory richness. Only 2 species present in the canopy were absent in the undergrowth: Triplaris poeppigiana and V. pavonis. The upper canopy consisted exclusively of M. flexuosa and E. precatoria (22.1 ± 1.9 m; heliophyte species). Mid canopy (16.8 ± 1.3 m) included some individuals from S. exorrhiza, O. mapora and V. elongata, while in the lower canopy (11.9 ± 1.2 m) we found subadults of palm trees and V. elongata, M. floribundum and S. globulifera individuals. The most representative species in the understory were Hura crepitans, O. sphaerocarpa, H. alchorneoides, N. pulverulenta and Psychotria alba. In canopy gaps, M. flexuosa saplings and poles were observed. Thirty-one taxa were found only once or twice and were considered as rare species, most of them were scyophytes. (b) Mauritia-Virola-Inga (MI plots): association that represents a palm swamp in Tingana with muddy soils and characterized by permanent flood episodes triggered by the rise in water level of the Avisado and Mayo rivers (> 3 m), especially during the heavy rainfall period (January-April). MI community was represented by the codominance of M. flexuosa (22%) and V. elongata (19.5%). All the species present in the canopy were also present in the understory but not vice versa. The upper canopy was formed only by M. flexuosa (22 ± 0.9 m), while mid canopy (16.8 ± 1.4 m) includes some individuals of the P. aquatica, E. precatoria, V. elongata and V. pavonis. Within the lower canopy (12.4 ± 1.5 m), Virola was the largest basal area. The most representative understory species were H. crepitans, Picramnia latifolia, P. alba and Inga genus. In most plots many ponds (spaces without vegetation with of up to 200 cm depth and poor regeneration) were found producing an incomplete canopy. On the branches of Chullachaqui renaco (C. trinervia), Huasca renaco (F. trigona) and the Inga genus were observed, as well as numerous orchids (Elleanthus, Epidendrum, Maxillaria) and bromeliads (Racinaea and Tillandsia). (c) MauritiaMatisia-Ferdinandusa (SD & MA plots): association that represents 2 palm swamps in Posic with dark but shallow waters (20-40 cm), adjacent to Tonchima river. In this territory there has been a rapid change in the land use from evergreen forest and “aguajales”to rice crops. The accumulation of organic matter was considerable, especially under adult palms where the remains of leaves and fruit racemes were concentrated. Since canopy forests are characterized by high levels of competition for light, palm trees tend to favor growth in height over diameter (common in several genera of palms). The community was dominated by M. flexuosa (25.8%), M. bracteolosa (18.2%) and F. clorantha (10.1%). All canopy species were found in the understory. Exclusively, Ficus krukovii and 1 individual of M. flexuosa formed the upper canopy (24.9 ± 3.2 m). The mid canopy (15.9 ± 0.9 m) was formed by M. flexuosa and the lower canopy (11.9 ± 1.4 m) was represented by Mauritia flexuosa, Matysia bracteolosa and Mauritiella armata with incidence of some individuals of F. clorantha and V. elongata. In the understory, we found individuals of C. longifolium, H. alchorneoides, Leonia glycycarpa, Clusia loretensis, Clitoria javitensis and Vismia pozuzoensis. Seedlings and small saplings have a higher probability of survival due to the low-level flooding (up to 60 cm) from Tonchima River; especially palm species which are adapted to these conditions (M. flexuosa, O. mapora and S. exorrhiza). Three species of Miconia and 1 Cyatheaceae (Cyathea pilosissima) were rare species.
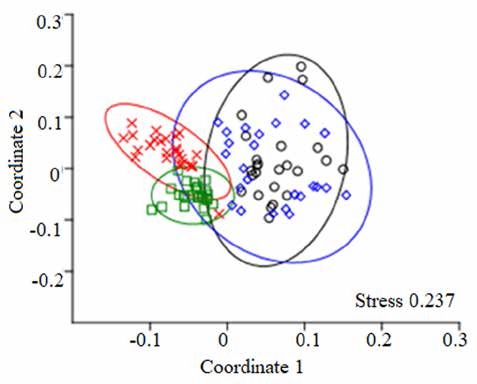
Figure 5 Nonmetric multidimensional scaling (NMDS) ordination of the 100 inventory plots based on abundance data. Green = SA plots, red = MI plots, black = SD plots and blue = MA plots. Ellipses show 95% confidence interval around the group centroids, based on the standard deviations of point scores. The p-value is based on 999 permutations, where the ordination scores were randomly assigned to the groups.
Eight endemic species to Peru were reported in the plots, 13 species were included in the IUCN Red List Categories and Cedrela odorata and Cyathea pilosissima in Appendix III and II from CITES, respectively (Appendix).
Discussion
The Amazon “várzea” forest is the most species-rich floodplain forest worldwide (Wittmann et al., 2006). However, in those same floodplain territories we can find the “aguajales” that are considered the main Amazon tropical peatlands that covers poorly drained territories with relatively stable vegetation types (Junk & Piedade, 2010) and characterized by low richness plant communities (Honorio et al., 2015; Householder et al., 2012) due to strong environmental filters (Draper et al., 2018), which indicates a high habitat specialization which favors the presence of M. flexuosa and other hyperdominant species adapted to flood conditions (Ter Steege et al., 2013). Emilio et al. (2014) argue that palms and woody species in “aguajales” have developed adaptations such as aerial roots (Ficus and Coussapoa), stilts (S. exorrhiza), buttress roots (Virola sp.) and pneumatophores (M. flexuosa) that allow oxygen transport to the roots in anaerobic conditions (Aguilar & Jiménez, 2009; Melack & Hess, 2010; Parolin et al., 2010).
Tree adaptations to different levels and periods of flooding determining the distribution of flood-tolerant forests and long-term inundated type forest (Parolin et al., 2004, 2010). Indeed, waterlogging may be the cause of most of the variation in the composition, distribution, dynamic and abundance of species (Costa et al., 2008; Eiserhardt et al., 2011; Junk, 1989; Junk et al., 2010).
The study area is partially covered by different types of flood-tolerant forests and long-term inundated forest along Mayo, Avisado Huascayacu and Tonchima river courses; where the species did not occupy the zone homogeneously, they changed continuously in response to environmental mosaics associated with the distance to the basins, seasonal effects and anthropic activity (Drucker et al., 2008; Siqueira et al., 2012; Tilman & Lehman, 2001). After flooding and sedimentation, herbaceous plants (Costus, Scleria, Eleocharis, Calathea), small shrubs (Miconia, Tococa, Neea, Piper), vines (Tassadia, Cissus, Heteropsis) and successional trees as Cecropia become visible, especially near the concave river banks contributing to considerable increase in swamps extension and occasionally cause the destruction of the woody species regeneration and favoring the recruitment of palm trees in all growth categories suggesting a shift to palm swamp, in an advanced succession process (Kalliola et al., 1991; Hergoualc’h et al., 2017; Urrego, 2018).
The richness, diversity values and floristic composition of the studied “aguajales” were similar to other floodplain “aguajales” in the Peruvian Amazon and Amazon basin (Galeano et al., 2015; Urrego, 1997), registering the same representative genera (Mauritia, Euterpe, Socratea, Ficus, Xylopia, Ceiba, Inga, Mauritiella) and families (Arecaceae, Moraceae, Annonaceae and Leguminosae; Endress et al., 2013; Pitman et al., 1999; Zárate et al., 2013).
Of the 112 species found, 42% can be considered rare because they presented an absolute density of less than 1 individual per hectare. Because they present a low density of individuals by area, they are considered rare species and can be used as benchmarks for monitoring genetic reserves (Kageyama et al., 1998). Conservation of populations of rare species ensures that other, less rare and more common species are also conserved (Van Dyke, 2008).
The diametric distribution of the communities in the study area was characterized as a false reverse J-shaped curve in palm swamp forests. The M. flexuosa presence explains an increase in the diametric classes (30, 40) and (40, 50), concentrating 11% of all individuals. These categories were similar to those reported in Loreto (Zárate et al. 2013). The highest concentration of individuals in the first diametric class (0, 10) characterizes a community with reserves of individuals, a typical pattern of stable tropical forests of age and varied composition of species that make up the bank permanent seedling (Scolforo 1998).
The study area has a large contingent of shrub and arboreal individuals in the understory (64%), while in the emerging canopy Moraceae (13%) and Arecaceae (87%) species were dominant. The diametric and altimetric distribution revealed abundance in the natural regeneration component due to a large number of juvenile plants that are part of the lower stratum of the community. Indeed, the species ensure their representativeness in the community structure when present in all the forest strata (Scolforo 1998). Species that do not follow this rule, in the future, may not be present in the plant community, due to not reproducing or because they did not regenerate locally, with the exception of those that are characteristic of the lower strata and forest intermediate (Scolforo 1998). Lack of recruitment can create a setback in the forest by impairing the replacement of forest cover, exerting pressure on the understory (Assis & Wittmann, 2011). The canopy remains mostly closed -except MI plot -, generating a high level of competition for space and light, which tends to favor the development of height above diameter (Endara et al., 2012).
The abundance of woody species was 3 times greater than palm species, palms registered higher DBH and height and, therefore, represented 55% of the basal area in the studied aguajales, corroborating the hypothesis proposed (Endress et al., 2013). Indeed, the profile vegetation representation allowed characterizing the “aguajales” in strata and the analysis of the vertical phytosociological structure revealed that there are dominant species for each stratum of the community.
According to the Mantel test, geographical distance and similarity between plots were related. We observed that the closest plots were the most floristically similar, suggesting that the proximity between plots influences the proportion of shared species. Within each site, the floristic similarity tended to produce high Mantel correlation with almost all geographical plots distance (Ruokolainen et al., 2007).
The NMDS analysis showed that in the floristic group of Posic, the plots were more dispersed than the plots in the other 2 floristic groups in Tingana. This suggests that the plots in Posic greatly differ from each other; and that the floristic groups in Tingana are more homogeneous, sharing more species. That is, a greater geographical proximity between plots could determine a higher similarity value (Colwell, 2000; Diniz-Filho et al., 2013). These patterns could also be influenced by the number of geographical barriers, as happened in Tingana unlike the Posic plots that were shown as a single set (Montufar & Pintaud, 2006: Palminteri et al., 2011; Soininen et al., 2007). Another factor to explain the floristic composition among plots is the vagility of palm species and some woody species, which can have very active dispersers that transport fruits over long distances (Acevedo-Quintero & Zamora-Abrego, 2016; González-Ramírez et al., 2017). These factors could directly influence the abundance of palm and hyperdominant species found in this study. On the contrary, distribution and abundance of rare species can be affected by environmental barriers (Fujiyoshi et al., 2009; Jiménez-Valverde & Hortal, 2003).
We consider the suggestions of Kalliolla et al. (1991 that studies in wetlands and palm swamps should contain descriptions of plant communities (floristic composition, key species, endemic and protected). However, the description of this type of vegetal formation is complicated and, in general, provisional due to the physiographic and environmental conditions of the study area where in certain periods of time some species may be absent from the sampling area or not to be detectable (Gentry y Ortiz, 1993). Moreover, the inconsistent terminology applied for its description and the different criteria used to classify the vegetation (Kalliola et al., 1991).
As a result of their composition species, diversity, structural complexity and utility, the “aguajales” (fragile and threatened ecosystems) are exposed to different anthropogenic activities such as selective extraction of timber and non-timber forest products, hunting of native fauna, change of land use and other activities generating loss of diversity with negative ecological implications (Börner & Zimmermann, 2003; Slade et al., 2007; Tilman & Lehman, 2001). An example of this is Virola surinamensis, a timber species, was represented by only 24 individuals among 3 -14 m, which is an indicator that the population trend is decreasing and confirmed their conservation status (endangered). Furthermore, Centronia laurifolia (endemic) is also in a vulnerable situation therefore the “aguajales” requires a better understanding of its composition, ecological interactions and should have high priority in future management and conservation management plans (Endress et al., 2013).
It is imperative to continue the study of forest diversity in the AMV in order to propose conservation strategies that link research and conservation projects in these territories and to help decision-makers understand the complex, realistic and inclusive arguments that pertain to most conservation decisions (Ludwig, 1999). A successful case of management conservation is Tingana that provides conservation rules, includes meaningful community participation, and can be self-sustain. Indeed, detailed knowledge of species composition, communities and trophic unions is the key to the success of conservation projects and, in this specific case of aguajales, they can be used to monitor the impacts of global environmental change on piedmont ecosystems (Begón et al., 2006).











 nova página do texto(beta)
nova página do texto(beta)



