Introduction
Estimates of population size and life history are critical to effectively manage wildlife (Caughley, 1994). A basic requirement for many ecological and conservation studies is the identification of the individuals within a population (Bradshaw et al., 2007; Lusseau et al., 2006; McMahon et al., 2007; Schofield et al., 2008). Most studies on animal populations depend on different marking techniques to identify individuals. Highly utilized methods include permanent marks (tattoos and scissions), semi-permanent marks (tags, rings, necklaces, and belts), and temporary marks (fluorescent powders, paint marks, etc.) (McMahon et al., 2007; Sélem et al., 2004; Schofield et al., 2008).
Most of these marking techniques require the capture of organisms. However, capture involves risk of injury, and marking may influence the behavior, reproduction, or survival of the individuals in a population (Dugger et al., 2006; Nichols & Seminoff, 1998; Sélem et al., 2004). Furthermore, identification marks can fall off or disappear, which can interrupt the continuity of long-term studies (Limpus et al., 1992; Sibly et al., 2005; Schofield et al., 2008).
To minimize these disturbances, it is recommended to use automatic marking devices or natural markings for identification of individuals, especially when individuals are difficult to capture or mark (Beck & Osborn, 1995; Renton, 2004). Many individuals can be naturally identified by their markings or color patterns, scars, and lack or presence of a flight feather, among others (Best et al., 1993; Renton, 2004; Sélem et al., 2004). Together, these characteristics in each individual may form a unique pattern, known as a fingerprint, which allows individuals to be recognized on subsequent occasions. This identification technique represents one alternative to the capture-recapture method (Gamble et al., 2008).
The photo-identification by visual interpretation method consists in photographing individuals and identifying them according to individual natural markings. Individuals may be subsequently re-identified based on the visual correspondence of natural markings and patterns in the photographs. This method is capable of capturing the necessary details for distinguishing individuals (Gamble et al., 2008), and it has been already proven to be a useful tool in the long-term monitoring of several wildlife populations (Beck & Reid, 1995; Bradshaw et al., 2007; Thompson et al., 2000), such as in the study of marine mammals, in which identification is based on patterns of spots on their bodies (Acevedo et al., 2006; Calambokidis et al., 2009; Cañadas & Sagarminaga, 1995; Constantine et al., 2007; Glockner & Venus, 1983; Martínez-Aguilar, 2008; Valdes-Arellanes et al., 2011; Vigness-Raposa et al., 2010). In some terrestrial mammals, the identification is based on patterns of spots, lines, scars, and even behavior (Caro, 1994; Foster, 1996; Goodall, 1986; Kelly, 2001; Masclans, 2010; Reynolds et al., 2005).
Photo-identification based on specialized software has been little used for bird identification (Arroyo & Bretagnolle, 1999; Bretagnolle et al., 1994; Munn, 2012; Renton, 2004), therefore relying on visual interpretation for individual recognition. In these cases, identification has been based on distinguishing marks, such as plumage patterns, and specific individual coloration (e.g., eye rings, crown, eyebrows, tail feathers, primary and secondary feathers). In a couple of cases, Renton (2004) and Munn (2012) identified individuals of the Blue-Yellow Macaw (Ara ararauna) and Green-Winged Macaw (Ara chloroptera), respectively, in the field based on facial feather lines and the auricular area.
Although photo-identification by visual interpretation has been explored in a few bird species, the usefulness of this method using specialized software for the biomonitoring of bird species lacking sexual dimorphism has not yet been explored; moreover, these bird species generally have a lengthy longevity, and young individuals reach their adult size quickly therefore hindering the determination of different ecological and biological aspects, as is the case for macaws (genus Ara).
The Military Macaw (Ara militaris) is included in the Appendix I of the Convention on International Trade of Endangered Species (CITES, 1998), and is considered as globally vulnerable by International Union for Conservation of Nature (BirdLife International, 2020). In Mexico, the species is enlisted as endangered (Norma Oficial Mexicana, NOM-059-SEMARNAT-2010) (Semarnat, 2010), due to the destruction of its habitat and its capture for illegal trade (Íñigo-Elías, 2000). The Military Macaw has a disjunct distribution ranging from Mexico to northern Bolivia (Forshaw, 1989; Íñigo-Elías, 1999; Peterson & Chaliff, 1989). In Mexico, its historic distribution included the Pacific and Gulf slopes (Peterson & Chaliff, 1989; Ridgway, 1915; Rivera-Ortíz et al., 2013), inhabiting tropical deciduous, semi-deciduous, and pine-oak forests in lowland areas (Arizmendi & Márquez-Valdelamar, 2000; Carreón, 1997; Collar, 1997; Forshaw, 1989; Juniper & Parr, 1998).
Studies on Military Macaw populations in Mexico have mainly addressed natural history and aspects related to food preferences, reproduction, distribution, and population genetics (Carreón, 1997; Contreras-González et al., 2009; Gaucin et al., 1999; Loza, 1997; Rubio et al., 2007; Rivera-Ortiz et al., 2008, 2013, 2016). Although recent studies have provided valuable information, it is necessary to carry out more detailed studies on the behavior, reproductive biology, birth and mortality rates, and temporary spatial movements, among other aspects, which would require the identification of individuals. Therefore, we here tested the effectiveness of photo-identification based on specialized software as a technique for differentiating and recognizing captive Military Macaw individuals. We hope that our results provide a basis for the future recognition of Military Macaw individuals in wild populations.
Materials and methods
We consulted the database of Management Units for the Conservation of Wildlife (UMAs for its initials in Spanish) of the Mexican government to identify which UMA had a fair and manageable number of individuals of Military Macaws, and we selected the captive population at the Africam Safari Zoo in Puebla, Mexico. Military Macaws at this site are trained by specialized personnel, who helped in taking photographs. Also, the population at this site is likely heterogeneous, comprising more than 100 individuals of unknown geographical origin that have been recovered from illegal trafficking activities in the country (Núñez-López, 2014).
Twenty-four trained and highly manageable macaws were selected, which facilitated taking the photographs. Two photography sessions were held for each individual: one for each side of the face. Ten lateral pictures of each side were taken with a Nikon D60 camera using an 18-55 mm focal length lens, a F of 5.6, and an ISO of 200. The photographs were taken at a distance of 2 meters using a tripod (Fig. 1A). During the sessions, the following data were also recorded for each individual: i) number of rings (individual metal rings with a unique number for bird identification), ii) sex, iii) age, iv) number of photographs, and v) side of the face (Supplementary material, Appendix 1). The number of rings identified the individual during photoshoots.

Figure 1 Steps followed to process photographs and obtain the facial feather patterns: A) input of the original photograph, B) reframing of the face, and C) isolation of the final facial feather pattern.
To have a complete and defined view of the face feathers, we reframed all photographs to include only the face area of each of the macaws (Fig. 1B), in which the natural markings formed by the feathers in the cheeks form a unique pattern of black lines (hereafter feather pattern), which was isolated for further analysis (Fig. 1C). The size of the photographs was 750 × 750 pixels, which is required to input the photographs into SISREC 1.0 for the automatic recognition of images (Padilla-Ramírez, 2012).
Using ADOBE PHOTOSHOP CS6, we recovered these feather patterns as follows: i) for each individual, feather patterns on the right and left sides of the face were selected from the original photograph using the magic wand tool (Fig. 1B); ii) based on the orientation and size, feather patterns of all individuals were homogenized by cutting to show only feathers patterns; iii) then, the investment color tool was used to fill the background (as white), leaving only the black feather patterns of the face (Fig. 1C), and iv) final images were saved in .bmp format.
We used SISREC 1.0 to perform the automatic recognition of macaw individuals based on an automatic correlation procedure that minimizes the time required for manual recognition at a high reliability (> 90%). SISREC 1.0 manages a compact and simple interface, making identification easy and simple (Padilla-Ramírez, 2012). Figure 2 shows the main screen of the program, where the user loads the filter image (kernel), problem images (scene) and the type of filter to use. In particular, this software uses a filter image for the comparison created from a minimum of 5 different images of the same side of the face of the same individual (Padilla-Ramírez, 2012). The filter image is placed in the window on the left side; in the right window are placed scene images of all individuals to be compared, including those used as filter images (Fig. 2). Images are then processed by the program using a non-linear filter with a level of k = 0.1. A non-linear filter was used because several images were combined to generate the control image. Also, it can be optimized to achieve lower sensitivity to scale, rotation, and lighting of all images used (Guerrero-Moreno & Álvarez-Borrego, 2009). The resultant screen (Fig. 3), shows the correlation between images in the form of a three-dimensional graph where the location of the maximum peak of the correlation of each image is observed, with the option to import for calculations statistics (Padilla-Ramírez, 2012).

Figure 2 SISREC 1.0 main screen, where the user will load the kernel and scene images and select the type of filter to use.

Figure 3 Results screen, the user will be able to see the results of the correlation between their images and will observe the maximum peak of the correlation with the option of importing for statistical calculations.
The obtained data were analyzed using non-parametric tests, since these were not normal distributed. First, for all 24 individuals, we compared the images of the feather patterns of the right side of the face with those of the left side of the face of the same individual. We used a Wilcoxon-T test adjusted with a Bonferroni correction to identify whether the right and left feather patterns of the face of the same individual were identical or different. This test evaluates the probability that the differences found between 2 related samples are due solely to the sampling error from the comparison of pairs and has the advantage that it gives more weight to the larger differences (Zar, 1999). Then, the images of both sides of the face were compared to those of the remaining 23 individuals. This same procedure was performed for all individuals. We then used a Friedman Q test adjusted to a Bonferroni correction to determine whether there were differences in the facial feather patterns (on the right and left sides of the face) among the 24 individuals. This test compares 3 or more related samples and determines that the differences found are not due to randomness (Zar, 1999). All statistical tests were carried out in Statistica 6 (http://www.statsoftiberica.com; StatSoft Inc., 1984).
Results
A total of 240 photographs (10 photographs per individual, 5 photographs of each side of the face) of the facial feather patterns of 24 Military Macaw individuals were taken. We found that the patterns of the feathers on the right side of the face (32 ± 1.5 maximum correlation) are significantly different from these on the left side of the face (0.5 ± 0.03 maximum correlation), in each individual (Fig. 4). These differences were consistent in all 24 analyzed individuals (Table 1).
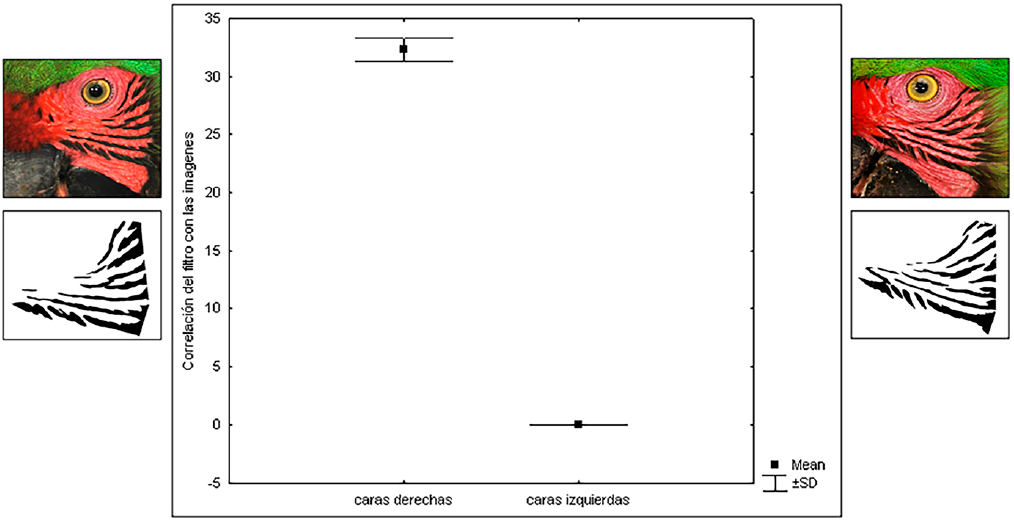
Figure 4 Mean and standard error of the maximum correlation between the filter and the images of the feather patterns on the right and left side of the face in Military Macaw individual AFRI-B-025. The comparisons of the other 23 individuals are shown in Table 1.
Table 1 Significant differences in the feather's patterns of the right side versus the left side of the face in each Military Macaw individual based on the Wilcoxon T test with the Bonferroni correction. T = Wilcoxon T test; * Bonferroni correction (PBC) p < 0.05.
| Number of individuals |
Band number | Right side vs. left side | |
|---|---|---|---|
| T | PBC | ||
| 1 | AFRI-B-025 | 9.5061 | 0.0002* |
| 2 | AFRI-B-O29 | 2.0041 | 0.0001* |
| 3 | AFRI-B-036 | 7.5268 | 0.0004* |
| 4 | AFRI-B-210 | 9.5301 | 0.0002* |
| 5 | AFRI-B-214 | 5.2035 | 0.0003* |
| 6 | AFRI-B-215 | 8.5960 | 0.0001* |
| 7 | AFRI-B-216 | 4.0069 | 0.0002* |
| 8 | AFRI-B-220 | 7.2640 | 0.0002* |
| 9 | AFRI-B-224 | 4.5061 | 0.0006* |
| 10 | AFRI-B-231 | 5.4480 | 0.0004* |
| 11 | AFRI-B-232 | 4.5920 | 0.0001* |
| 12 | AFRI-B-234 | 9.0023 | 0.0009* |
| 13 | AFRI-B-235 | 9.5061 | 0.0007* |
| 14 | AFRI-B-236 | 6.5040 | 0.0002* |
| 15 | AFRI-B-237 | 8.0580 | 0.0001* |
| 16 | AFRI-B-238 | 9.4640 | 0.0005* |
| 17 | AFRI-B-239 | 5.2416 | 0.0002* |
| 18 | AFRI-B-241 | 6.8032 | 0.0008* |
| 19 | AFRI-B-242 | 7.6069 | 0.0010* |
| 20 | AFRI-B-243 | 5.4750 | 0.0009* |
| 21 | AFRI-B-244 | 6.7261 | 0.0003* |
| 22 | AFRI-B-408 | 6.1556 | 0.0002* |
| 23 | AFRI-B-411 | 8.0368 | 0.0001* |
| 24 | AFRI-B-415 | 9.0865 | 0.0002* |
Significant differences were also found in the feather pattern of the right side of the face of each individual with respect to that of all other macaws, as well as in the feather pattern of the left side of the face of each individual with respect to that of all other macaws. These findings indicate that the facial feather patterns of Military Macaws are not identical among individuals (Table 2). Differences in the right and left side of the face are shown for all individual Military Macaws (Supplementary material, Appendix 2).
Table 2 Significant differences in the feather patterns of the right side and left side of the face in 24 Military Macaws based on the Friedman Q test with the Bonferroni correction. *Bonferroni correction (PBC) p < 0.05. Q = Friedman Q test.
| Number of Individuals | Band number | Right faces | Left faces | ||
|---|---|---|---|---|---|
| Q 5,23 | PCB | Q 5,23 | PCB | ||
| 1 | AFRI-B-025 | 52.9680 | 1.6E-05* | 50.1680 | 0.0000* |
| 2 | AFRI-B-O29 | 35.0400 | 0.0023* | 63.2800 | 4.5E-07* |
| 3 | AFRI-B-036 | 44.2658 | 0.0002* | 52.7600 | 1.8E-05* |
| 4 | AFRI-B-210 | 53.3520 | 0.0001* | 52.4025 | 0.0002* |
| 5 | AFRI-B-214 | 47.2480 | 0.0009* | 50.3120 | 3.8E-05* |
| 6 | AFRI-B-215 | 34.9600 | 0.0023* | 44.6000 | 0.0002* |
| 7 | AFRI-B-216 | 48.5920 | 6.4E-05* | 54.9360 | 9.0E-06* |
| 8 | AFRI-B-220 | 41.4480 | 0.0004* | 50.4640 | 3.6E-05* |
| 9 | AFRI-B-224 | 55.2640 | 8.1E-06* | 57.5200 | 4.0E-06* |
| 10 | AFRI-B-231 | 40.7520 | 0.0005* | 59.8400 | 1.8E-06* |
| 11 | AFRI-B-232 | 42.9920 | 0.0003* | 53.5920 | 1.3E-05* |
| 12 | AFRI-B-234 | 49.8863 | 4.3E-05* | 47.0000 | 0.0001* |
| 13 | AFRI-B-235 | 41.9040 | 0.0004* | 60.4880 | 1.3E-06* |
| 14 | AFRI-B-236 | 56.8080 | 0.0005* | 54.1120 | 1.1E-05* |
| 15 | AFRI-B-237 | 48.8880 | 5.8E-05* | 54.0640 | 1.1E-05* |
| 16 | AFRI-B-238 | 59.3920 | 2.2E-06* | 62.4640 | 9.0E-07* |
| 17 | AFRI-B-239 | 45.1600 | 0.0001* | 51.2480 | 2.8E-05* |
| 18 | AFRI-B-241 | 62.2400 | 9.0E-07* | 51.3280 | 2.8E-05* |
| 19 | AFRI-B-242 | 70.5920 | 0.0000* | 62.8960 | 4.5E-07* |
| 20 | AFRI-B-243 | 53.4720 | 1.4E-05* | 50.2320 | 3.9E-05* |
| 21 | AFRI-B-244 | 56.7360 | 0.0005* | 62.4954 | 9.0E-07* |
| 22 | AFRI-B-408 | 56.4720 | 5.4E-06* | 45.1440 | 0.0001* |
| 23 | AFRI-B-411 | 47.3680 | 9.1E-05* | 58.1200 | 3.1E-06* |
| 24 | AFRI-B-415 | 31.6560 | 0.0048* | 61.0800 | 1.3E-06* |
Discussion
Several studies have shown that photo-identification based on natural markings is useful for identifying individuals of species with similar phenotypic characteristics, which allows generating more accurate demographic or migratory information (Bretagnolle et al., 1994; Connolly et al., 2002; De Oliveira & Rosso, 2008; Forcada & Aguilar, 2000; Foster, 1966; Foster et al., 2006; Gamble et al., 2008; Mizroch et al., 2004; Peterson, 1972; Reynolds et al., 2005; Valdes-Arellanes et al., 2011). In addition, this technique represents an alternative to traditional marking methods, because it does not require the physical capture of animals, thereby preventing stress. It is non-invasive, inexpensive, and as our study shows, it may be reliable, and might potentially be used in long-term studies or monitoring. Photo-identification could be particularly suitable for species of conservation concern, both in captivity and wildlife (Buonantony, 2008). In the case of species in wildlife, to generate the photographic catalogue and use this technique, the first thing to take into account is to have information on the ecology of the species, locate feeding, rest or sleeping areas, know the daily activity, etc. Second, identify areas where more individuals congregate, and that the photographic sample can be taken without disturbances. Third, have the professional help of a photographer and photographic equipment that allows taking the details of the natural marks (Dixon, 2003; Masclans, 2010; Trujillo-González, 1994).
Although photo-identification of natural markings is a promising technique, it still relies on visual interpretation, and processing of large amounts of data can be labor intensive and highly subject to human error. Also, the accuracy and reliability of photographic comparisons can be hampered by low image quality (light intensity and clarity), and the size of the database (Schofield et al., 2008). On the other hand, a higher number of photographs per individual would also increase the reliability in photo-identification results by visual interpretation. However, this technique is useful for the identification of Military Macaws only if the photographs are of good quality and clearly show the feathers patterns of the face. One alternative to reduce human error in photo-identification by visual interpretation is the use of specialized software for pattern recognition, as in our study. An additional benefit of software use is that it provides different tools for more effective, automatic, or fast identification (Kelly, 2001; Padilla-Ramírez, 2012).
In the case of birds, natural markings have previously been used to visually differentiate individuals (Munn, 2012; Renton, 2004). In this study with Military Macaw, we considered the feather patterns on its face. Our findings showed that facial lines form unique feather patterns allowing for individual recognition, moreover, these differences can be observed visually and statistically characterized. These findings underscore photo-identification as a useful technique for identifying gregarious species such as the Military Macaw, where it may be nearly impossible to identify individuals, especially considering that there are no noticeable visible differences between males and females, or between young and adult individuals (Íñigo-Elías, 1999). Demographic studies of such species would be more accurate and reliable if their life histories could be closely tracked, including individual seasonal movements and habitat use (Foote et al., 2009; Mackey et al., 2007).
In conclusion, the utility of the photo-identification technique for identifying Military Macaws opens the door to more specific and reliable studies in wild populations that require individuals to be distinguished. This information will help to implement this technique in wild populations. It must also be taken into account that when implementing this technique in wild populations, determining the time of the molt is essential, since the results may vary. It has been documented that Psittaciformes have complete annual (small species) or biannual (larger species) molts and usually before the reproductive season (Durán, 2003), so the photo shoot must be made in the reproductive season, when the macaws have completed their molting cycle. Other limitations of photo-identification should also be taken into consideration. It cannot be used for detailed biological descriptions such as the characterization of populations, since differentiation between juveniles and adults is not possible. Also, if the feather pattern of the face of individuals in the field is not clearly seen after photographed, the technique could not be reliably applied (Williams & Thomson, 2015). Another further potential limitation is that not all individuals have equal probability for face feather patterns obtention. Unequal capture probability is a potential problem in all photo-identification techniques (Williams & Thomson, 2015).
Photo-identification is a potentially useful tool for understanding the demographic patterns, as well as movements of individuals of the species to other populations, with this information one could identify the natural corridors, conserve the habitat and the species itself (Langtimm et al., 2004; Patiño-Valencia et al., 2008; Pompa-Mansilla, 2007; Reyes et al., 2002; Reynolds et al., 2005). The Military Macaw is an umbrella species, meaning that the protection of this species could indirectly result in the protection of other species (Íñigo-Elías, 1999, 2000; Jiménez-Arcos et al., 2012). It is important to build a catalog of photographs of wild populations of Military Macaws for both sides of the face, because these are good predictors of individuality that can facilitate the continued evaluation of individuals over time in complex studies.











 nueva página del texto (beta)
nueva página del texto (beta)


































































































