Introduction
Poeciliids have been widely introduced in multiple aquatic ecosystems through their popularity as ornamental species in addition to their use in biological control programs against mosquitoes (García-Vásquez et al., 2017; Pyke, 2008). For these reasons, they are considered the most abundant and widely distributed exotic freshwater fish. Additionally, Poeciliids are successful invaders, an attribute mainly credited to their high fecundity, tolerance of habitat degradation, and diet flexibility (Magurran, 2009; Pyke, 2008).
The twospot livebearer Pseudoxiphophorus bimaculatus (Heckel, 1848) is a widespread poeciliid fish species with a natural distribution ranging from the Misantla River (State of Veracruz) in Mexico to the Prianzapolka River in Nicaragua, on the Atlantic slope (Miller et al., 2009). The trophic biology of the species in its native range indicates that it is a fish with herbivorous feeding behavior (Miller et al., 2009; Vega-Cendejas et al., 1997), but with a tendency towards carnivorous-insectivorous feeding behavior outside its natural distribution exhibiting a predominance of aquatic insect larvae in its gut contents (Mercado-Silva et al., 2002; Trujillo-Jiménez & Toledo-Beto, 2007). The twospot livebearer has been translocated into several basins of Central Mexico, such as those of the Balsas River and the Lerma-Chapala River, and has recently been reported in the Teuchitlán River, a hot spot of fish diversity in the headwaters of the Ameca River (Domínguez-Domínguez et al., 2008; Mejía-Mojica et al., 2012; Ramírez-Herrejón et al., 2012; Ramírez-García et al., 2017).
The Teuchitlán River is a highly anthropized lotic system that has undergone more than 60 years of human intervention (De la Mora-Orozco et al., 2014). The anthropogenic disturbances include modifications of the riverbanks, replacing them with concrete, uncontrolled domestic wastewater discharges, changes in the river bottom substrate, water-diversion for irrigation and livestock production, and the construction of a dam for the la Vega reservoir, disrupting the continuity to the Ameca River (Webb & Miller, 1998). In the Teuchitlán River, there is an historic fish richness of 15 native and 6 exotic species (López-López & Paulo-Maya, 2001). The butterfly goodeid Ameca splendens, and 3 microendemic fishes are species documented as present in the site and the only ones that currently survive in this area: the Ameca shiner Notropis amecae and golden skiffia Skiffia francesae, both of which are now extinct in the wild, and the tequila splitfin Zoogoneticus tequila, which was reintroduced to the area and brought back to the wild in 2018 (De la Vega-Salazar et al., 2006; Domínguez-Domínguez et al., 2018; IUCN, 2020; López-López & Paulo Maya, 2001; Webb & Miller, 1998). Nowadays, only 5 native and 6 exotic species are found in the Teuchiltán River (Herrerías-Diego et al., 2019).
A key factor proposed in the local extinction of native fish in the Teuchitlán River is the competition for food resources, particularly related to a high abundance of the introduced Poeciliids (Kingston, 1978; Webb & Miller, 1998). The introduction of Poeciliids is among the most harmful threats to native freshwater fishes in the Teuchitlán River and the recently introduced P. bimaculatus may be no exception, since it has been reported to be a successful competitor against native Goodeidae fishes in other geographic regions (Magurran, 2009; Ramírez-Carrillo & Macías-García, 2014). However, there are no data regarding the trophic biology of P. bimaculatus in the site and, consequently, the impact of the species on the Teuchitlán River food web and native species remains poorly understood.
The present study therefore characterizes the trophic biology of the invasive P. bimaculatus in the Teuchitlán River, in order to: 1) determine its trophic guild, diet breadth, omnivory, trophic level, and trophic strategy, and 2) analyze its temporal, spatial, and ontogenetic variation in a highly anthropized lotic system. We also discuss the possible role of P. bimaculatus in the transport of allochthonous energy into the Teuchitlán River system as well as its invasive potential.
Materials and methods
The Teuchitlán River is located in west-central Mexico, at the headwaters of the Ameca River basin (20°41’-20°40’ N, 103°51’-103°50’ W) (Fig. 1a). The lotic system has a length of 1.5 km from the origin to its termination in “La Vega” dam (López-López et al., 2004). To reflect different human impacts along the river, 5 different points were chosen as collection sites (Ramírez-García et al., 2017): “Rincón Spring” SpA, “Abrevadero Spring” SpB, “Upper part of river” RvC, “Middle part of river” RvD, and “End of the river” RvE (Fig. 1b, c).
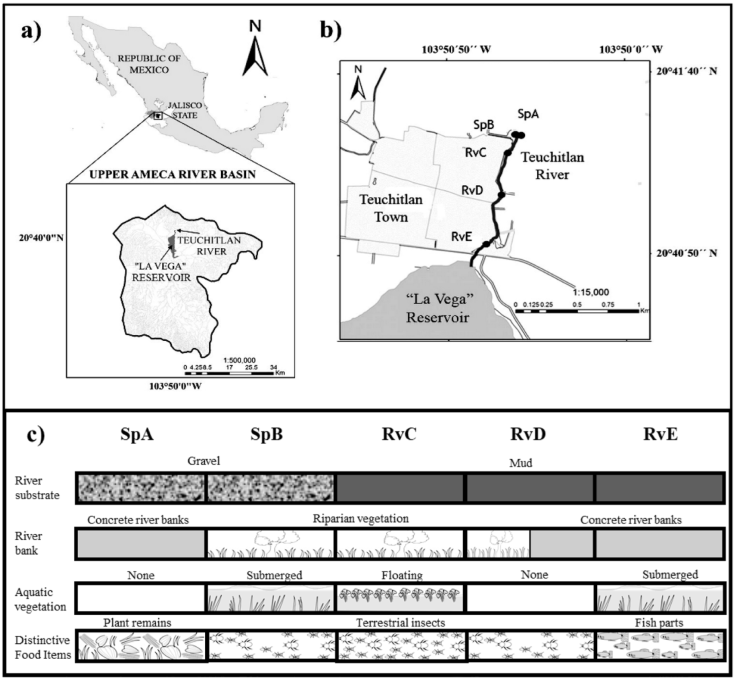
Figure 1 a) Geographic location of the Teuchitlán River; b) location of study sites in the lotic system; c) physical characterization and main prey in the study sites.
We collected samples during the day (10:00-16:00 hrs), twice per month over 1 annual cycle (January 2016 to January 2017), using a seine net (4.5 m in length, 2.3 m in height, and mesh size 1.35 mm) and electrofishing equipment (backpack DC electrofishing model ABP-3, ETS Electrofishing Systems LLC). We determined 2 seasons according to weather variations (Jiménez-Román, 1994): dry (January to June 2016) and wet (July to November 2016).
The captured fish were sacrificed by overdosing with the anesthetic tricaine mesylate (MS-222) according to the Official Mexican Norms NOM-051-ZOO-1995 and NOM-033-SAG/ZOO-2014, then labeled and fixed in 10% formaldehyde and transferred to 70% alcohol, following the criteria of Fournie et al. (2000).
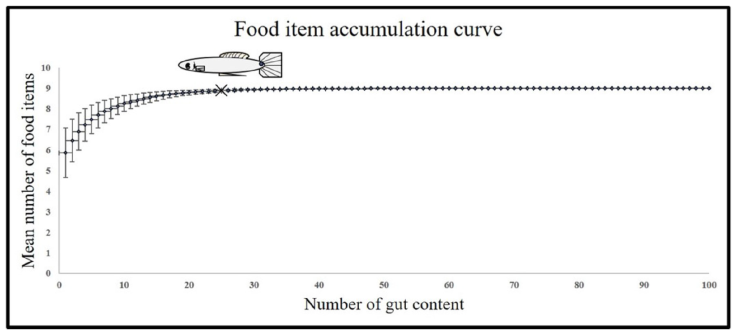
We used an exploratory analysis of prey accumulation using the Mao Tau index to determine the minimum number of individuals required to characterize the feeding habits of P. bimaculatus (Colwell et al., 2004; Appendix). We selected a minimum sample of 25 individuals and a maximum of 36 per size class, site, and season since the curve became asymptotic at 25 individual gut contents analyzed. We determined 2 size classes using a preliminary analysis of Sturges rule to obtain size class, and we used a principal component analysis on diet data and obtained the principal preys. The ANOVA test was performed on the principal preys to test differences among Sturges size classes, and we could only detect differences among 2 size classes, which correspond to size at first maturity for the species in the Teuchitlán River (Ramírez-García et al., 2017): juvenile fish (0-33.41 mm) and adult fish (> 33.42).
We obtained the weight (g) and standard length (mm) and removed the digestive tract of each fish. We measured the length of the intestine (mm) and the weight of the gut contents (mg). We performed the gut content analysis using the stomach content of P. bimaculatus because Trujillo-Jiménez and Toledo-Beto (2007) described the presence of a well-defined stomach in P. bimaculatus, as a saclike expansion of the digestive tube between the esophagus and the short intestine, which represented the first third portion of the intestine length. The gastric repletion was determined by following Borghetti et al. (1994). We used fish with a gastric repletion ≥ 50% in the trophic analysis. We evaluated the gut contents with a modification of the quadrant method (Hynes, 1950). We identified the components of the gut contents to the lowest taxonomic level possible, using the keys of Merrit and Cummins (1996) for insects, and those of Pennak (1978), and Thorp and Covich (2001) for zooplankton and other invertebrates. The insect parts for which identification was impossible due to high digestive degradation were catalogued as unidentified insect parts (UIP). We classified all unidentifiable gut content as detritus and this was excluded from the trophic analysis, along with gut samples that only had detritus in the gut content.
We evaluated the contribution of each food item to the diet of P. bimaculatus using a modified version of the relative importance index (RII) (Yáñez-Arancibia et al., 1976): RII = FO*PA/100, where FO is the frequency of occurrence and PA is the percentage of area occupied by a particular prey in the gut content. We calculated PA using a quadriculated microscope slide (1.9 mm × 1.9 mm) and the area was determined using a microscope camera (Amscope MD-35) with the software AmScope 3.7. This method has proved useful for fish with small components in their diet (e.g., microscopic algae, millimetric zooplankton, and small insect larvae) or when the gut content is difficult to separate and quantify (e.g., detritus or plant remains) (Canto-Maza & Vega-Cendejas, 2008; Ramírez-Herrejón et al., 2013; Vega-Cendejas, 1990). The RII was expressed as a percentage (Cortés, 1997).
We used the standardized Levins´ index (BI) to calculate a measure of niche breadth with the formula BA = B-1/n-1, where B = Levins´niche breadth (B = 1/Σpj2) and n = number of possible preys. The BI takes values between 0 and 1; fish are considered specialists when the BI value is lower than 0.60, and generalists when it is higher than 0.60 (Krebs, 1989). We used the omnivore index (OI) to estimate the variation in the trophic levels of the prey consumed by the species (Christensen & Pauly, 1992) with the formula OI = Σ (TLj-TL)2*DCij, where n is the number of groups in the system, TLj is the trophic level of prey j, TL is the average trophic level of the preys, and DCij is the fraction of prey (j) in the average diet of predator (i). Values equal to zero indicate that the species has only preys on 1 trophic level; large OI values indicate a variable trophic position of the species’ preys.
We estimated the trophic level (TL) with the TrophLab program (Pauly et al., 2000) using the equation TROPHi = 1+ΣDC×TROPHj, were DC represents the fraction of the prey j in the diet of species i, and TROPHj is the trophic position of species j. G is the number of species´i preys. We used Horn´s index (Krebs, 1989) to evaluate intraspecific diet overlap using the formula Ro = Σ (Pij+Pik) log (Pij+Pik) - Σpij logPij - Σpik logPik / 2 log2, where Ro represents the Horn´s overlap index among species j and species k; Pij, Pik = proportion of the resource i with respect to the total of resources shared by both species (i = 1, 2, 3..., n). The value of Horn´s index can vary from 0 when feeding resources are not shared, to 1.0 when maximum diet overlap occurs. Values higher than 0.6 are considered to represent a significant overlap due to limited resource availability (Wallace, 1981; Zaret & Rand, 1971).
We used the Costello trophic diagram to graphically determine the importance of components in the diet of the species and to identify the feeding strategy of the species (Costello, 1990). The abundance of the prey in the gut content (%PA) was placed on the axis of the ordinates and the frequency of appearance (%FO) on the axis of the abscissa. Four quadrants were determined, delimited by 50% of area and frequency of appearance. The prey items located in the upper right quadrant were considered as preferential (with a frequency of occurrence and percentage of area > 50%). Meanwhile, we considered accidental prey items those located in the lower left quadrant (with a frequency of occurrence and percentage of area < 50%)
To determine differences in the diet per site, season, and size, we conducted multivariate one-way PERMANOVAs using the prey consumption data (mm2) in PRIMER-E version 7 (Plymouth Marine Laboratory, UK) with the PERMANOVA+ package (Anderson et al., 2008). We used PERMANOVA design to detect overall differences in the diet among site, season, and size, and consequently, we performed an ANOVA analysis on the factors which presented statistically significant differences for PERMANOVA.
We conducted multi-factor analyses of variance using the R Stats Package to assess differences in prey consumption per season, site, and size class (R Core Team, 2013). We performed a posterior analysis to explore the distribution of the residuals and ensure no violation of normality and independence. A post hoc Tukey-Kramer honest significant difference test was used when the ANOVA showed significant differences (Zar, 1999).
Results
We analyzed the gut contents of 631 P. bimaculatus individuals with a standard length ranging between 11.74 and 66.31 mm. From these, 298 were sampled in the wet season and 333 in the dry season. Fifty-six percent (355) of the digestive tracts analyzed had gut repletion of between 75 and 100%. Detritus only in 18%, with a higher number (83) occurring in the wet season, compared to 29 in the dry season. Detritus-only gut content occurred at a high frequency in SpA (27), RvE (25), and RvD (16). The site with the lowest number of detritus-only gut contents was the RvC, with 1 in the dry season and 7 in the wet season.
We classified the diet composition into 10 prey categories (Table 1). Terrestrial insects showed the highest RII value (49.8-98.3) throughout the sites, size classes, and seasons. However, fish parts presented a high RII value (55.91) for adult fishes in the RvE site (Table 2). Diet differed among seasons (PERMANOVA: pseudo-F = 13.3, df = 1, p = 0.004) and sites (PERMANOVA: pseudo-F = 6.2, df = 4, p = 0.005), although not ontogenically (PERMANOVA: pseudo-F = 0.9, df = 1, p = 0.44). We found spatial and temporal differences in the consumption of plant remains (ANOVA: f = 8.5639, p < 0.001, df = 9), fish parts (ANOVA: f = 2.5409, p = 0.0388, df = 9), and terrestrial insects (ANOVA: f = 4.4135, p = 0.0016, df = 9; Fig. 2d-f), temporal differences for plant remains, fish parts, and unidentified insect parts (preferential food item by Costello: Fig. 3), and differences in the ingestion of plant remains and fish parts. In the wet season, the highest consumption of plant remains was found at site SpB, while the highest consumption of fish parts was at site RvE (ANOVA: f = 8.5, p < 0.0001, df = 1; Figs. 2a, b). Terrestrial insects were consumed to a greater extent at sites SpB, RvC, and RvD in both seasons, but the lowest ingestion of this component was found at sites SpA and RvE in the wet season (ANOVA: f = 4.4135, p = 0.0016, df = 9, Fig. 2c).
Table 1 Food items of Pseudoxhiphophorus bimaculatus in the Teuchitlán River.
| Label | Food item | Identified biological groups |
|---|---|---|
| DET | Detritus | |
| PR | Plant remains | |
| ALG | Algae | Diatoms, genera: Achnanthes¸ Nitzschia, Terpsinoe |
| ARA | Araneae | Spiders |
| FP | Fish parts | Scales, flesh, and vertebrae |
| GA | Gastropoda | Exotic snails Melanoides tuberculata and Pomacea bridgesii |
| ZOO | Zooplankton | Calanoids copepods, cladocerans, ostracods |
| UIP | Unidentified insect parts | |
| AI | Aquatic insects | Orders: Coleoptera, Diptera, Ephemeroptera, Lepidoptera, Odonata, Trichoptera. |
| Families: Chironomidae, Dytiscidae, Isotomidae. Stratiomyidae, Tipulidae | ||
| TI | Terrestrial insects | Orders: Diptera, Coleoptera, Hymenoptera, Hemiptera, Thysanoptera. Families: |
| Vespidae, Staphylinidae. Genus: the exotic crazy ant Anoplolepis sp. |
Table 2 Index of relative importance (%RII) of each prey item of Pseudoxhiphophorus bimaculatus per size class and site in the Teuchitlán River. Values in bold show the highest RII.
| PR | ALG | ARA | FP | GA | ZOO | UIP | AI | TI | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| SpA | Wet | C-1 | 48.79 | 0.51 | 0.52 | 4.98 | 0 | 0.13 | 35.5 | 5.87 | 3.7 |
| C-2 | 18.51 | 0 | 0 | 17.31 | 0 | 0 | 51.97 | 0.72 | 11.49 | ||
| Dry | C-1 | 17.14 | 0.004 | 2.36 | 0.07 | 0 | 0.002 | 0.07 | 1.5 | 78.86 | |
| C-2 | 9.67 | 0 | 2.76 | 4.61 | 0 | 0 | 0 | 5.53 | 77.43 | ||
| SpB | Wet | C-1 | 18.34 | 0 | 0 | 3.79 | 0 | 0.006 | 27.21 | 0 | 50.66 |
| C-2 | 35.67 | 0 | 0 | 0.23 | 0 | 0.23 | 0 | 5.96 | 57.92 | ||
| Dry | C-1 | 0.79 | 0 | 0.42 | 0.87 | 0.37 | 0.25 | 0.22 | 0.91 | 96.15 | |
| C-2 | 0.16 | 0 | 0.14 | 2.98 | 0.46 | 0 | 0 | 1.18 | 95.08 | ||
| RvC | Wet | C-1 | 0 | 0 | 0.83 | 0 | 0.03 | 0 | 13.35 | 3.77 | 82.02 |
| C-2 | 0.01 | 0 | 0.56 | 3.86 | 0 | 0 | 7.99 | 8.46 | 79.11 | ||
| Dry | C-1 | 0.31 | 0 | 2.92 | 0 | 0.07 | 0 | 0 | 30.43 | 66.27 | |
| C-2 | 0 | 0 | 0.07 | 0 | 0 | 0 | 0 | 25.34 | 74.59 | ||
| RvD | Wet | C-1 | 0.4 | 0 | 0 | 19.95 | 0.47 | 0 | 29.38 | 0 | 49.8 |
| C-2 | 0 | 0 | 1.93 | 0.9 | 0 | 0 | 7.26 | 0 | 89.91 | ||
| Dry | C-1 | 3.62 | 0 | 3.71 | 0 | 1.89 | 0.02 | 5.79 | 1.98 | 82.98 | |
| C-2 | 6.87 | 0 | 0.15 | 1.06 | 5.87 | 0 | 16.88 | 0.009 | 69.15 | ||
| RvE | Wet | C-1 | 1.18 | 0 | 0.35 | 35.81 | 0 | 0 | 61.75 | 0 | 0.9 |
| C-2 | 12.12 | 0 | 0 | 55.91 | 0 | 0 | 31.98 | 0 | 0 | ||
| Dry | C-1 | 1.7 | 0 | 34.48 | 0.22 | 0.1 | 0.32 | 28.47 | 9.7 | 25 | |
| C-2 | 0.02 | 0 | 0.79 | 0.1 | 0 | 0 | 0.07 | 0.72 | 98.3 |
PR = Plant remains, ALG = algae, ARA = Araneae, FP = fish parts, GA = Gastropoda, ZOO = zooplankton, UIP = unidentified insect parts, AI = aquatic insects, TI = terrestrial insects.
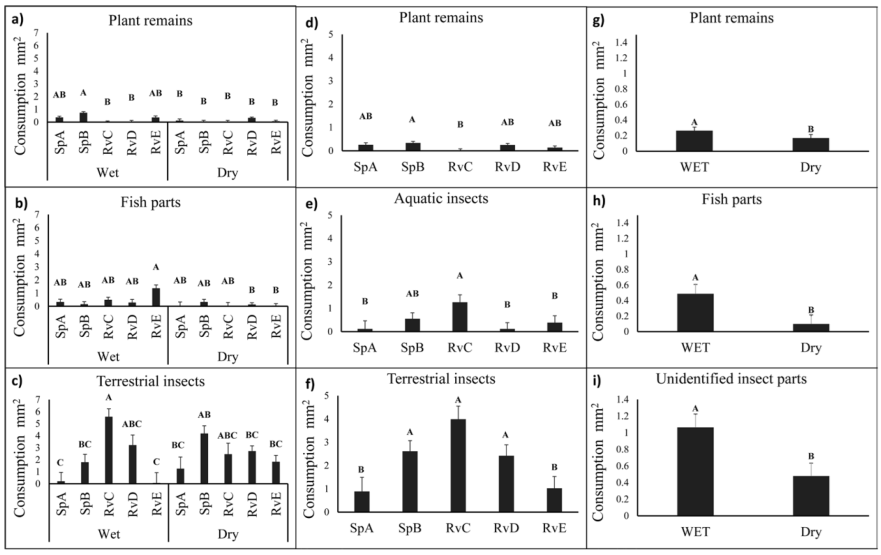
Figure 2 Spatio-temporal (a-c), spatial (d-f) and temporal (g-i) variation of prey consumption (mm2) by Pseudoxiphophorus bimaculatus in the Teuchitlán River. Bold upper-case letters A, B, C in the bars refer to among site-season, among sites and among season differences by ANOVA results, respectively (Tukey-Kramer honest significant difference [HSD] post hoc test, p < 0.05)
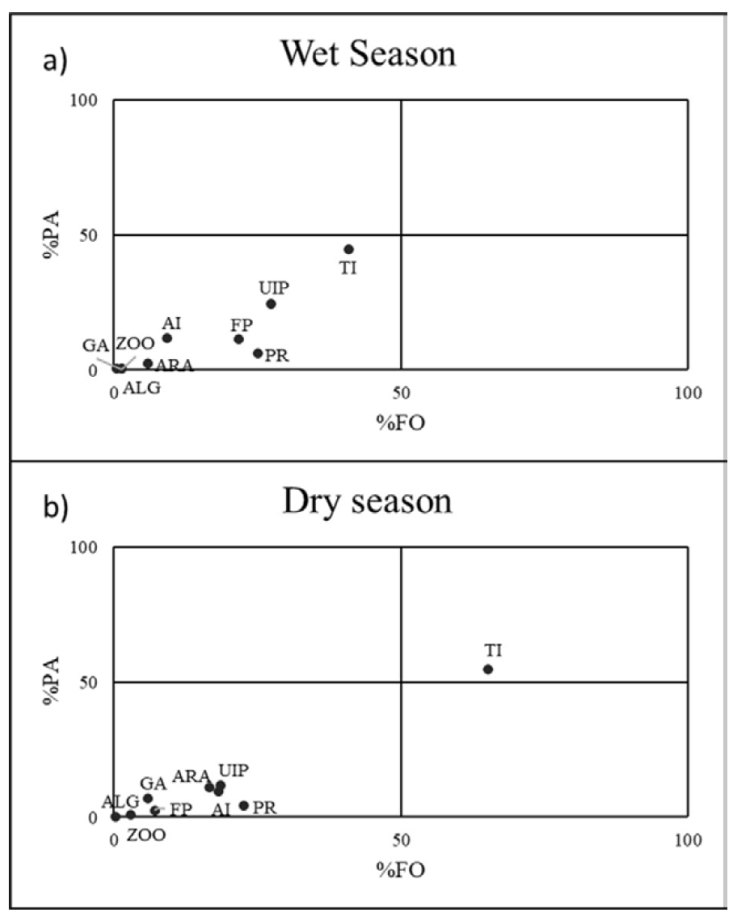
Figure 3 Costello graphics representing trophic strategy based on plotting the relationship between the abundance of the prey in the gut content (% PA) and the frequency of appearance (% FO). PR = Plant remains, ALG = algae, ARA = Araneae, FP = fish parts, GA = Gastropoda, ZOO = zooplankton, UIP = unidentified insect parts, AI = aquatic insects, TI = terrestrial insects.
Niche breadth per size class, season, and site were variable (Bi mean = 0.22±0.18, Bi minimum = 0.007-Bi maximum = 0.66; Table 3). This indicates that the twospot livebearer has a specialist trophic niche breadth, with the lowest value presented in the dry season (Bi mean = 0.14±0.13) and a tendency towards a generalist trophic niche breadth in the wet season with the highest value (Bi mean = 0.31±0.19). The adults of the RvE site in the wet season presented the highest niche breadth value (Bi = 0.66) (Table 3).
Table 3 Niche breadth, omnivore index, and trophic level of Pseudoxhiphophorus bimaculatus by size class and sites in the Teuchitlán River. Values in bold are the highest values.
| Wet | Dry | |||
|---|---|---|---|---|
| SpA | ||||
| P. bi 1 | P. bi 2 | P. bi 1 | P. bi 2 | |
| Diet breadth | 0.24 | 0.47 | 0.08 | 0.16 |
| Omnivory index | 1.31 | 1.20 | 0.24 | 0.14 |
| Trophic position | 2.68±0.33 | 3.2±0.46 | 2.98±0.36 | 3.15±0.41 |
| SpB | ||||
| P. bi 1 | P. bi 2 | P. bi 1 | P. bi 2 | |
| Diet breadth | 0.43 | 0.29 | 0.01 | 0.02 |
| Omnivory index | 0.74 | 0.49 | 0.02 | 0.007 |
| Trophic position | 3.03±0.39 | 2.83±0.35 | 3.19±0.4 | 3.24±0.42 |
| RvC | ||||
| P. bi 1 | P. bi 2 | P. bi 1 | P. bi 2 | |
| Diet breadth | 0.11 | 0.11 | 0.22 | 0.31 |
| Omnivory index | 0.24 | 0.15 | 0.02 | 0.01 |
| Trophic position | 3.2±0.4 | 3.25±0.42 | 3.2±0.4 | 3.2±0.4 |
| RvD | ||||
| P. bi 1 | P. bi 2 | P. bi 1 | P. bi 2 | |
| Diet breadth | 0.42 | 0.08 | 0.07 | 0.16 |
| Omnivory index | 0.55 | 0.13 | 0.16 | 0.40 |
| Trophic position | 3.45±0.5 | 3.2±0.4 | 3.17±0.4 | 3.15±0.41 |
| RvE | ||||
| P. bi 1 | P. bi 2 | P. bi 1 | P. bi 2 | |
| Diet breadth | 0.24 | 0.66 | 0.38 | 0.007 |
| Omnivory index | 1.16 | 0.80 | 0.58 | 0.004 |
| Trophic position | 3.64±0.57 | 3.78±0.64 | 3.18±0.4 | 3.2±0.4 |
P. bi 1 = P. bimaculatus juvenile fish (0-33.41 mm), P. bi 2 = P. bimaculatus adult fish (> 33.42).
Trophic level was variable across class size, site, and season (trophic level minimum = 2.68±0.33-trophic level maximum = 3.78±0.64). The lowest value was found for juveniles of site SpA, while the highest value was found for adults at site RvE, both in the wet season (Table 3).
The omnivorous index was also variable (OImean = 0.42 ± 0.43, OI minimum = 0.004-OI maximum = 1.31, see Table 3) and mostly indicated low levels of omnivorous behavior, with the lowest value in the dry season (OImean = 0.16 ± 0.2) compared to the wet season (OImean = 0.68 ± 0.44). The highest values of the omnivorous index were for juveniles (OI = 1.31) and adults (OI = 1.2) of site SpA and juveniles (OI = 1.16) of site RvE in the wet season (Table 3).
Diet overlap between size classes was high (0.97), but the overlap presents spatial and temporal differences. The size classes of site RvE did not overlap in terms of trophic resources, with SpA, SpB, RvC, and RvD across seasons (values from 0 to 0.58). In the wet season, classes I and II of the SpA site did not present diet overlap (0.19 to 0.55). The main trophic resource in the diet overlap was unidentified insect parts (UIP).
The Costello diagrams showed a temporal difference in the feeding strategy of the twospot livebearer (Fig. 3). In the dry season, the preferred prey are terrestrial insects, while the rest of the prey items are accidental (Fig. 3b). In the wet season, no such preferential item was found (Fig. 3a).
Discussion
According to the information presented here, the invasive species P. bimaculatus presents a dynamic trophic strategy and flexible feeding behavior in the Teuchitlán River. The species is mainly a carnivorous-insectivorous fish, mostly consuming terrestrial insects (Table 2); however, we found behavior that was generalist in the wet season and specialist in the dry season, as well as an herbivorous trend in some sites, occupying different trophic levels and variable trophic width (Table 3, Figs. 2, 3). This variable feeding strategy enables P. bimaculatus to exploit resources from different trophic levels, such as plants and algae in producers and other fishes in secondary consumers.
These findings are congruent with the trophic biology data of the species throughout its native and non-native distribution; although Vega-Cendejas et al. (1997) and Miller et al. (2009) state that P. bimaculatus is herbivorous in its native range, Trujillo-Jiménez and Toledo-Beto (2007) found that the twospot livebearer is a carnivorous-insectivorous fish that feeds mainly on terrestrial insects within its non-native distribution. The morphology of structures related to trophic acquisition, such as the teeth, mouth, a well-defined stomach, and short digestive tract, indicates that the twospot livebearer is a carnivorous fish, as has been reported in invaded areas (Trujillo-Jiménez & Toledo-Beto, 2007). These data, far from being contrasting, provide evidence that the twospot livebearer can present flexible feeding habits, as we found for the Teuchitlán River according to trophic width, trophic level, and omnivory, which are similar to the flexible feeding behavior presented by other related species (Ramírez-Herrejon et al., 2013; Schaefer et al., 1994).
We did not find differences in diet among size classes (PERMANOVA pseudo-F = 0.9, df = 1, p = 0.44). Dietary shifts are expected as a function of increased body size and are common in many fish species (Davis et al., 2012; Feyrer et al., 2003; King, 2005). However, the invasive P. bimaculatus in the Teuchitlán River exhibits an ontogenetic overlap in diet, which is indicative of shared food items (Horn´s index = 0.97) (Copp, 1992; McCormick, 1998). This could be explained by the niche overlap hypothesis, which states that a high availability of prey reduces competition and allows coexistence between different species (Pianka, 1974). This theory could explain the ontogenetic diet overlap and coexistences at the same site on the river, since the ontogenetic stages of a species have been proposed as different ecological units (Davis et al., 2012; Stoner & Livingstone, 1984).
Our results from the relative importance index and the PERMANOVA of diet variation indicate the presence of spatial variation in resource consumption (Table 2). The ANOVA result indicates that consumption of terrestrial insects is statistically high in sites with the presence of riparian vegetation. This vegetation has a major role in the transfer of allochthonous energy to the river system, since it provides habitat for insects that may eventually fall into the water, increasing the availability of this food supply for the fishes (Tabacchi et al., 1998; Wipfli & Baxter, 2010). This trend of P. bimaculatus was observed in the RvC site, where we found the highest ingestion of terrestrial insects (Fig. 2c), which is congruent with the presence of well-established riparian vegetation (Fig. 1).
According to the ANOVA results, the twospot livebearer presented the highest ingestion of plant remains in the SpA. In spring-fed stream systems, the periphyton is fundamental to the web trophic dynamic (Battin et al., 2003), and the deposition of vegetal detritus from the adjacent vegetation, as well as detritus of algal origin, could represent food resources (Garman, 1992; Pound et al., 2011). The sites SpA and SpB are dominated by rocky bottom substrate, and both present concrete banks and high periphyton productivity (pers. obs), which could explain the high rate of algae consumption. In the case of the high rate of plant remains, we suggest that these 2 sites are characterized by a low abundance of macroinvertebrates and zooplankton (Escalante-Jimenez, unpublished data), and SpB is used for watering cattle, which increases the input of vegetal matter via animal defecation. The low abundance of animal prey items and increased input of vegetal material could be causes for the high consumption of primary producers in these sites (Figs. 1c, 2d). In terms of exploiting the available resources, this represents flexibility in P. bimaculatus feeding.
The results of the trophic indices (Table 3), trophic strategy (Fig. 3), and diet overlap (Horn´s index) suggest temporal differences in the diet of P. bimaculatus. The Teuchitlán micro-basin is one of the driest sites in the Ameca basin, and a severe difference in rainfall occurs between the dry and wet season (Jiménez-Román, 1994). We found that a change occurs in the feeding strategy between the wet and dry seasons. During the dry season, terrestrial insects are preferred, while in the wet season the consumption tends to be of primary producers and unidentified insects. The preference for terrestrial insects during the dry season could be the result of the temporal availability of this food. In other streams systems, a high productivity coming from the nearby riparian-terrestrial zone are reported during the summer with a consequent high availability of terrestrial insects (Nakano & Murakami, 2001). However, the consumption of plant remains in the wet season could be a result of the temporal transport of this material through the drainage system into the river via flood events (Wantzen et al., 2008). This is also supported by the increased quantity of unidentified terrestrial insects in the diet, which could also be the result of the transport of dead and partially degraded insects by rainwater (Wantzen & Junk, 2000). Rivers are known to suffer temporal changes due their variations in flood and drought (Hill & Boston, 1991). As a result, their associated biotic communities also vary seasonally and the availability of food for fishes in river systems show high spatial and temporal variation (Angermeier, 1982, 1985; Angradi. 1997; Bae et al., 2016).
Temporal shifts in diet have been reported for other introduced species in different freshwater systems as a response to environmental differences in the availability of food resources (Maitipe & De Silva, 1985). Our results regarding the temporal switch in feeding strategy are consistent with other fish species and for other vertebrates (Fig. 3), where the temporal shift is reported as a function of an increased abundance of food items in the wet season (Rayner et al., 2009; Wilson, 1971; Zaret & Rand, 1971). Thus, the temporal change of P. bimaculatus diet may reflect the seasonal variation of food resources; however, the abundance of food resources was not measured in the present study. Further studies should focus on the seasonal change of food resources in order to explore the effect of resource variability on the diet of P. bimaculatus.
We used a multivariate analysis (PERMANOVA) to test overall diet differences among the sites, seasons, and ontogenetic factors, but to evaluate specific differences in the use of particular resources we used multi-factor analysis (ANOVA). The PERMANOVA analysis enables us to demonstrate a spatial and temporal variation in the diet of P. bimaculatus in Teuchitlán River, but multi-factor ANOVA permits the detection of specific variation in plants remains, fish parts, and terrestrial insects among sites/ seasons. The use of both ANOVA and PERMANOVA is useful to better elucidate the effects of biological invasions (Gioria & Osborne, 2009), and therefore our study shows the utility of both PERMANOVA and ANOVA in trophic studies with a community approach to understand complex biological information.
The results of the relative importance index indicate a high consumption (%RII > 50) of terrestrial insects, which was the main food item in the diet of P. bimaculatus. This could indicate that P. bimaculatus may function as a vehicle of allochthonous energy into the Teuchitlán River system. Terrestrial insects are abundant items in freshwater stream habitats with different levels of human perturbation and, as explained above, riparian vegetation seems to be key in the transfer of terrestrial insects to water, making them available for fish ingestion (Carbajal-Becerra et al., 2020; Nakano et al., 1999; Wipfli & Baxter, 2010). In other water bodies, human perturbation can affect the terrestrial-aquatic energy flux, increasing the allochthonous input by terrestrial insects (Vital-Rodríguez et al., 2017). However, study of the energetic flux is necessary to corroborate this terrestrial-aquatic trophic linkage and to evaluate the role of P. bimaculatus in the transfer of energy into the aquatic system.
A key factor proposed in the local extinction of native fish in the Teuchitlán River is the competition for food resources (Kingston, 1978; Webb & Miller, 1998). However, the main problem for evaluating the potential trophic impact of P. bimaculatus is the lack of specific information about the trophic role of the native species and how the Teuchitlán trophic web is structured. The endemic Ameca splendens is possibly an herbivorous fish based on its possession of a long and convoluted intestine and lack of stomach (Miller & Fitzimon, 1971). Because of that, trophic overlap of P. bimaculatus and A. splendens is expected to be low. However, in laboratory conditions, the interactions of poeciliids and native species have shown a disadvantage of the natives when there is low food availability (Escalera-Vázquez et al., 2016). Meanwhile, the natives Zoogoneticus tequila and Zoogoneticus purhepechus have short intestines, similar to their relative Zoogoneticus quitzoensis, which is a carnivorous fish with preference for aquatic insect preys (Acuña-Lara et al., 2006). Our results showed that P. bimaculatus are carnivorous and thus trophic competition between the native and the invasive carnivores are possible. More detailed study must be done to test this interaction. However, our study provides a baseline for further understanding of the Teuchitlán River trophic web, with the addition of the Teuchitlán River fish species trophic data to our results we could determine the trophic impact of P. bimaculatus in the site.
The poeciliid P. bimaculatus is a species with a native distribution in rivers of the Atlantic slope of Central America but has also been widely introduced by human action into several other drainages (Mejía-Mojica et al., 2012; Miller et al., 2009; Ramírez-García et al., 2017; Ramírez-Herrejón et al., 2012). The introduction of the twospot livebearer in the Teuchitlán River is relatively recent (< 15 years). It was not reported until 1996 (Dzul-Caamal et al., 2012; López-López & Paulo-Maya, 2001). And not recorded during fieldwork conducted in the area in 2008 (ODD per. obs). However, this fish species has become successfully established all along the river and is the dominant species, representing more than 50% of the fish assemblage (Herrerías-Diego et al., 2019). Studies of reproductive biology have shown that the invasive twospot livebearer in Teuchitlán is iteroparous, the dominating sex ratio being female (1.9:1, female:male), and presents early reproduction and high fecundity. This indicates the high effectiveness of the fish in terms of resource exploitation (Gómez-Márquez et al., 2016; Ramírez-García et al., 2017).
The flexible behavior in the trophic strategies of P bimaculatus presented here is indicative of a successful invasive species. Variation in the trophic biology throughout sites has been described as characteristic of an adaptive response of non-native fish species to environmental prey availability (Davis et al., 2012; Jepsen & Winemiller, 2002). Seasonally trophic flexible behavior seems to be a key factor in the abundance-dominance of the species in the Teuchitlán River (Herrerías-Diego et al., 2019), helping the twospot livebearer to tolerate anthropogenic perturbation of the water body, such as processes of habitat modification or eutrophication (Ruehl & DeWitt, 2005). The twospot livebearer is a euryphagous species, which confers an advantage in terms of avoiding seasonal food limitation and has been related to increased abundance of invasive species (Weliange & Amarasinghe, 2003). This life trait of some species of the Poeciliid fish family enables individuals to tolerate fluctuations in prey availability in their environment and has been proposed as a key factor in their invasive success (Arthington, 1991; De Carvalho et al., 2019; Pollux & Reznick, 2011). In summary, the twospot livebearer shows flexibility in its trophic biology; it can occupy different trophic levels, modify its trophic width, change its trophic guild, modify its omnivorous behavior, and utilize allochthonous and autochthonous trophic sources.
These results, as well as other biological traits such as continuous reproduction, a high proportion of female individuals (Ramírez-García et al., 2017), parental care associated with viviparity (Gross & Shine, 1981), and high tolerance to environmental degradation (Mercado-Silva et al., 2002) are consistent with successful invasive species (Sakai et al., 2001), since this success is related to their establishment, spread, and abundance (Hayes & Barry, 2008; Marchetti et al., 2004; Ricciardi, 2013; Ricciardi et al., 2013). Moreover, in the present study we found ontogenetic trophic overlap of P. bimaculatus and, considering the iteroparous reproductive biology of the species in the site (Ramírez-García et al., 2017), a clear generational overlap over time, facilitating the potential for spread and colonization and giving rise to the apparently rapid and successful establishment of P. bimaculatus in the Teuchitlán River (Bateman et al., 2015; Herrerías-Diego et al., 2019).
It is clear that P. bimaculatus should be considered a species with high invasive potential and a serious risk for the freshwater ecosystems of central Mexico, a region that has been recognized as a very important hotspot for freshwater fish conservation (Carbajal-Becerra et al., 2020; Domínguez-Domínguez et al., 2006; Miller, 1986), with endemicity of up to 70% and a dramatic decrease in native fish populations (De la Vega-Salazar, 2006; Domínguez-Domínguez et al., 2008; Lyons et al., 1998). The introduction of this species into water bodies of the area is therefore to be avoided, and more attention must be paid to the stocking process of fish species of commercial value and the release of exotic fishes for mosquito control or ornamental purposes. Educational programs to prevent the introduction of this and other species to areas of importance for the conservation of freshwater diversity must be conducted, with management plans developed and control of established populations carried out.











 text new page (beta)
text new page (beta)