Introduction
Heteroxynematidae (Nematoda: Oxyuroidea) comprises a group of nematodes with a cosmopolitan distribution. The males in this family feature a tail with a regular shape, which is not bluntly truncated. Further, the males do not have long protruding papillae extending into cuticular alae and have genital papillae mainly in the perianal region (Petter & Quentin, 1976). Currently, this family contains 13 genera with more than 59 species parasites of birds and mammals (rodents and lagomorphs) (Hodda, 2011). In Mexico, Heteroxynematidae includes 3 genera: AspiculurisSchulz, 1927 (2 species), LamotheoxyurisFalcón-Ordaz, Fernández & García-Prieto, 2010 (1 species) and Dermatoxys Schneider, 1866 (2 species) (García-Prieto et al., 2012). Aspiculuris can be distinguished from other members of the family by having cuticular striations poorly marked and cervical alae well developed, extending from the cephalic vesicle (Petter & Quentin, 1976). The 19 nominal species of this genus known so far are parasites of rodents, including Cricetidae (Liu et al., 2012). This rodent family is cosmopolitan, with more than 130 genera and 680 species, from which 23 genera and 141 species are distributed in Mexico (Ceballos et al., 2005; Wilson & Reeder, 2005). As part of the authors commitment to inventory the helminth diversity associated with vertebrates, we found an undescribed species of the genus Aspiculuris which is herein described using light and scanning electron microscopy.
Materials and methods
In November 2010, 1 specimen of the tarabundi vole, Microtus oaxacensis Goodwin, 1966 and 2 of the Mexican harvest mouse, Reithrodontomys mexicanus Sassure, 1860 were collected at km 134 of the highway Oaxaca-Tuxtepec (17°25’10” N, 96°29’53” W), Oaxaca, Mexico. Hosts were collected under permission FAUT-0170 issued by Secretaría de Medio Ambiente y Recursos Naturales. Rodents were anesthetized by isoflurane inhalation, euthanized by cervical dislocation, and examined for endoparasites. Helminths were removed from the intestine and placed in 0.85% saline solution, fixed in hot 4% formaldehyde and stored in 80% ethanol. Nematodes were cleared with Amman's lactophenol and temporarily mounted for morphological study. Measurements, expressed in micrometers unless otherwise stated, are given as the mean, and followed by range and sample size in parentheses. Figures were drawn with the aid of a drawing tube. Two specimens for scanning electron microscopy (male and female) were dehydrated in a graded ethanol series, critical-point dried with CO2, and then coated with a gold-palladium mixture. Specimens were examined with a Hitachi SU1510 electron microscope. Type specimens were deposited at Colección Nacional de Helmintos (CNHE), Instituto de Biología, Universidad Nacional Autónoma de México, México City, Mexico.
Description
Aspiculuris mexicana n. sp.
http://zoobank.org/urn:lsid:zoobank.org:act:3115FA79-B9B3-4F10-82B8-2205D5F20C71
Diagnosis. Medium size nematodes. In both sexes, cervical alae begin at the prominent cephalic vesicle and end abruptly at the middle-length of esophageal bulb (Fig. 1) forming an acute angle (Fig. 2a, b). Triangular mouth with a small opening surrounded by 6 reduced lips. Four single, large cephalic papillae: 2 dorso and 2 ventro-laterals. Two prominent amphids between each pair of papillae (Fig. 3a).
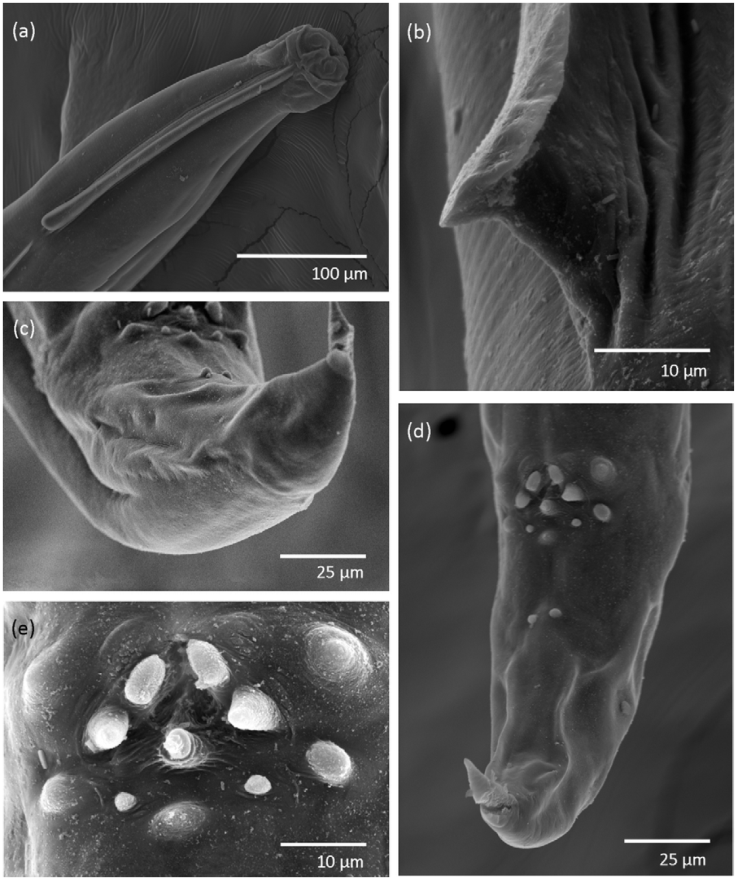
Figure 2 Scanning electron micrographs of Aspiculuris mexicana n. sp. (a) Male, anterior end, showing cervical ala extension; (b) cervical ala; (c) male, caudal end, lateral view; (d) male, caudal end, showing papillae arrangement: preanal (1PrA, 2PrA, 3PrA), adanal (1AA) and postanal (1PA, 2PA, 3PA, 4PA, 5PA); (e) male, papillae arrangement around anus.
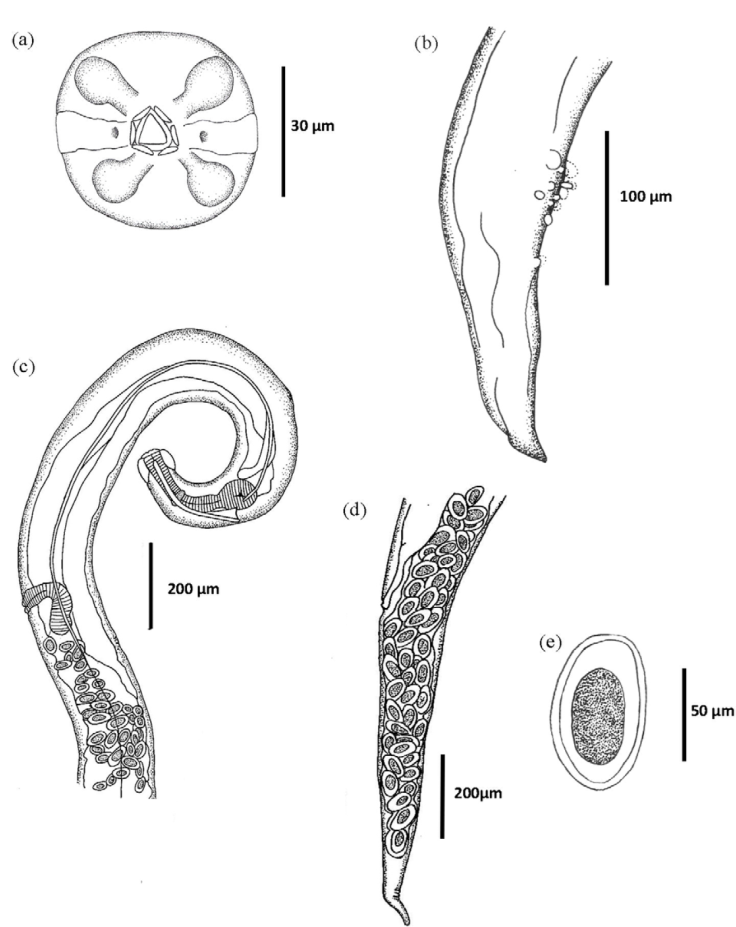
Figure 3 Aspiculuris mexicana n. sp. (a) Male, apical view; (b) male, caudal end; (c) female, anterior end; (d) female, caudal end; (e) egg.
Male (based on holotype, 2 paratypes, one of them studied under SEM). Body length 2.81 mm (2.21-3.42; n = 2), width at middle body 110 (80-130; n = 2); cephalic vesicle length 65 (60-70; n = 2) by 70 (50-80; n = 2) width. Esophagus length 340 (310-370; n = 2). Esophageal bulb 95 (90-100; n = 2) length by 60 (50-70; n = 2) width. Nerve ring 100 (90-110; n = 2) from anterior end; excretory pore not observed. Cervical alae 270 (210-330; n = 2) length, begins at 40 (30-50; n = 2) from the anterior end and 20 (n = 1) from the lateral alae; length of lateral alae 3.09 mm (n = 1). Tail 190 (180-210; n = 2) curved ventrally, with pointed tip, provided with 3 pairs of alae (Fig. 3b): 1 preanal and 2 postanal (lateral and sublateral), not enclosing the caudal apex (Figs. 2c, d). The 16 caudal papillae are arranged as follows: 3 preanal pairs (1 subventral and 2 medio-ventral pairs near to the anus); 1 adanal pair; a single papilla immediately postanal; 2 postanal pairs (1 subventral pair, 1 smaller ventral pair); 1 single ventral papilla, and 1 ventral pair far away from the anus (Figs. 2d, e; 3b). Two postero-lateral fasmids situated posterior to the last pair of papillae.
Female (based on alotype and 1 paratype). Body length 5.85 mm (5.69-6.02; n = 2), width at middle body 250 (220-270; n = 2). Cephalic vesicle 85 (80-90; n = 2) length by 125 (120-130; n = 2) width. Esophagus 310 (290-320; n = 2) length. Esophageal bulb 110 by 90 (n = 1). Nerve ring and excretory pore 145 (140-150; n = 2), and 950 (890-1010; n = 2) from anterior end, respectively. Cervical alae begin 25 (20-30; n = 2) from the anterior end (Fig. 3C); length 395 (390-400; n = 2). Pre-equatorial vulva 2.15 mm (2.04-2.26; n = 2) from anterior end (Fig. 3d). Tail 940 (n = 1). Eggs 74 (60-90; n = 10) by 39 (30-40; n = 10) (Fig. 3e).
Taxonomic summary
Type host: Tarabundi vole, Microtus oaxacensis Goodwin, 1966 (Cricetidae: Arvicolinae).
Additional host: Mexican harvest mouse, Reithrodontomys mexicanus Sassure, 1860 (Cricetidae: Neotominae).
Type locality: Oaxaca: km 134 of the highway Oaxaca-Tuxtepec (17°25’10” N, 96°29’53” W), Mexico.
Site of infection: colon.
Prevalence (%) and mean intensity (MA) of infection: M. oaxacensis: 100%; MA: 6. Reithrodontomys mexicanus: 50%; MA: 1.
Material deposited: CNHE 8658 (holotype male); CNHE 8659 (1 paratype, male; 1 paratype female); 8660 (alotype, female); CNHE 8661 (1 voucher, female).
Etymology: the parasite is named after Mexico, the country where the specimens were collected.
Remarks
Aspiculuris is currently composed by 19 species parasites of rodents worldwide (Falcón-Ordaz et al., 2010; Liu et al., 2012). Quentin (1975) divided this genus in 2 groups based on the shape of the cervical alae. The first group includes 14 species with cervical alae that are interrupted at the level of the esophageal bulb and have an arrow shape-like end (Table 1). The second group encompasses 5 species, in which the width of the alae decreases progressively and connects with the lateral alae (Table 2). The specimens described herein belong to the first group, which includes Aspiculuris tetraptera (Nitszch, 1821), A. dinnickiSchulz, 1927, A. schulzi Popov and Nasarova, 1930, A. kazakstanica Nasarova and Sweschnikova, 1930, A. americanaErickson, 1938, A. lahorica Akhtar, 1955, A. pakistanica Akhtar, 1955, A. tschertkoviTarzhimanova, 1969, A. aserbaidjanicaTarzhimanova, 1969, A. arianicaKotrla and Daniel, 1970, A. rysavyiKotrla and Daniel, 1971, A. versteraeHugot, 1980, A. huascaensisFalcón-Ordaz, Pulido-Flores and Monks, 2010 and A. tianjinensisLiu, Bu and Zhang, 2012 (Falcón-Ordaz et al., 2010; Liu et al., 2012). Based on the number of caudal papillae, Aspiculuris mexicana n. sp. can be differentiated from A. americana, A. arianica, A. dinnicki, A. huascaensis, A. kazakstanica, A. lahorica, A. tetraptera, A. rysavyi, A. schulzi, A. tianjinensis and A. versterae, because they have a smaller number (Table 1). In addition, cervical alae in the new species are abruptly interrupted at mid-length of esophageal bulb while in A. schulzi, A. arianica and A. versterae alae end in the pre-bulb region and in A. americana, A. dinnicki, A. kazakstanica, A. rysavyi and A. tianjinensis cervical alae surpass the esophageal bulb. The smaller body size of females in A. huascaensis and A. tetraptera allow us to separate them from A. mexicana, which measures 5.85 mm (Table 1).
Table 1 Selected features of the Aspiculuris species with cervical alae interrupted at level of the esophageal bulb (measurements in millimeters).
| Aspiculuris spp. | Female length | Male length | CA/EB | CP number | CA/T | Eggs size | Host family | Distribution | Reference |
|---|---|---|---|---|---|---|---|---|---|
| A. americana | 3.17-3.88 | 2.25 - 2.46 | Posterior | 8 | No |
|
Cricetidae | Nearctic | Erickson (1938) |
| A. arianica | 4.0 | 3.3 | Anterior | 11 | No | - | Sciuridae | Palearctic | Kotrla & Daniel (1970) |
| A. aserbaidjanica | 4.3 - 4.62 | - | Posterior | - | - |
|
Cricetidae | Palearctic | Tarzhimanova (1959) |
| A. dinnicki | 8.0 - 8.25 | 3.86 | Middle | 10 | No |
|
Cricetidae | Palearctic | Schulz (1927) |
| A. huascaensis | 3.22 | 2.48 | Middle | 12 | No |
|
Muridae | Nearctic | Falcón-Ordaz et al. (2010) |
| A. kazakstanica | < 6 | - | Posterior | 9 | - |
|
- | Palearctic | Miller &Schmidt (1982) |
| A. lahorica | - | - | Middle | 10 | - |
|
- | Palearctic | Miller & Schmidt (1982) |
| A. mexicana | 5.85 | 2.81 | Middle | 16 | No |
|
Cricetidae | Neotropic | Present study |
| A. pakistanica | < 6 | - | Posterior | - | No | - | - | Palearctic | Miller & Schmidt (1982) |
| A. rysavyi | 6-8 | 5-5.8 | Posterior | 11 | Yes |
|
Cricetidae | Palearctic | Kotrla & Daniel (1971) |
| A. schulzi | 2.4 - 3.6 | 2.2 | Anterior | 10 | - |
|
Muridae | Palearctic | Cambieri (1957) |
| A. tetraptera | 3.8 (3.5-4.5)* | 0.7 | Middle | 10+2 double | Yes |
|
Muridae | Neotropic | Hugot (1980) |
| A. tianjinensis | 6.46 | 4.69 | Posterior | 12 | No |
|
Cricetidae | Palearctic | Liu et al. (2012) |
| A. tschertkovi | 3.82 - 5.31 | 2.51 - 2.55 | Middle | 16 | Yes |
|
Cricetidae | Palearctic | Tarzhimanova (1959) |
| A. versterae | 1.91 | 1.59 | Anterior | 10+1 double | Yes |
|
Muridae | Ethiopian | Hugot (1980) |
CP = Caudal papillae number; CA/EB = cervical alae ending level respect to esophagus bulb; CA/T = caudal alae enclosing or not the tail;
* measurements after Behnke et al. (2015).
Table 2 Selected features of Aspiculuris species with non-interrupted cervical alae (measurements in millimeters).
| Aspiculuris spp. | Female length | Male length | CP number | CA/T | Eggs size | Host family | Distribution | Reference |
|---|---|---|---|---|---|---|---|---|
| A. asiatica | 8.61-9.71 | 5.40 | 10 + 1 double | Yes | 0.08 × 0.04 | Muridae | Palearctic | Schulz (1927) |
| A. africana | 3.2-4.2 | 2.3-3.6 | 10 + 1 double | No | 0.09 × 0.05 | Muridae | Ethiopian | Quentin (1966) |
| A. ratti | 3.1-3.25 | - | Unknown | Yes | 0.09 × 0.04 | Muridae | Oriental | Johnson (1969) |
| A. witenbergi | 8.1 | 4.4 | 11 (1 pair is raspberry) + 1 double | No | 0.07 × 0.04 | Muridae | Palearctic | Quentin (1975) |
| A. shikoloueta | 5.33 | 3.30-3.50 | 12 (1 pair is raspberry) + 1 double (possibly) | No | 0.09 × 0.06 | Muridae | Ethiopian | Inglis et al. (1990) |
CP = Caudal papillae; CA/T = caudal alae enclosing or not the tail.
The morphological features of 2 of the 3 remaining species (A. aserbaidjanica and A. pakistanica) are poorly known, even males of the first species are unknown. Nonetheless, in A. aserbaidjanica, the cervical alae extend beyond the posterior region of esophageal bulb (Tarzhimanova, 1969) and in A. pakistanica cervical alae end in the posterior region of this structure (Miller & Schmidt, 1982), whereas in the species herein described, the cervical alae end at mid-length of esophageal bulb. In addition, A. pakistanica and A. aserbaidjanica are distributed in the Palearctic region meanwhile A. mexicana is a species distributed in the transition zone between the Nearctic and Neotropical realms. Considering the number of caudal papillae, A. tschertkovi most closely resembles A. mexicana because both species have 16 papillae. Nevertheless, arrangement of caudal papillae of both species enables their differentiation: A. tschertkovi has 2 preanal papillae pairs, 2 adanal pairs and 4 postanal pairs (Tarzhimanova, 1969); on the other hand, in A. mexicana caudal papillae are disposed as follows: 3 preanal pairs; 1 adanal pair; 1 single papilla immediately postanal; 2 postanal pairs; 1 single ventral papilla, and 1 ventral pair. In addition, cervical alae end at different level in both species (Tarzhimanova, 1969) and their geographic distribution is distinct (Palearctic and Neotropical zone, respectively).
We considered that the specimens described herein correspond to a new species based on the morphological and morphometric features described in this study, particularly the extension of the cervical alae, and the number of caudal papillae and their particular distribution. Aspiculuris mexicana n. sp. is the twentieth species described for the genus and the fourth recorded in Mexico (Pulido-Flores et al., 2019). To the best of our knowledge, this study constitutes the first record of a helminth parasitizing both species of mammals: M. oaxacensis and R. mexicanus.
Behnke et al. (2015) analyzed the molecular phylogeny and morphology of 5 species of Aspiculuris parasites of voles and house mice. They concluded that the use of DNA is the most informative way to distinguish between species which are very similar to one another since number and configuration of caudal papillae are not reliable. We partially agree with this statement since only 5 of the 20 species of the genus have been studied from a molecular perspective (Behnke et al., 2015; Goswami et al., 2015). In addition, the identification of Aspiculuris is not based exclusively on the number and arrangement of the caudal papillae, but on a combination of morphological characters additional to these, such as the terminal region of the cervical alae with respect to the bulb of the esophagus, size of the eggs, length of the male and female body, tail enclosed or not by caudal alae, among others (Falcón-Ordaz et al., 2010; Liu et al., 2012). Based on these morphological data, in this work is presented an updated taxonomic key for 17 of the 20 species of Aspiculuris described to date, which can be well differentiated using a combination of morphological features.
Identification key for species of Aspiculuris.
(A. aserbaidjanica, A. kazakstanica and A. pakistanica, whose morphological descriptions are incomplete, are not included):
| 1a. | Cervical alae interrupted at level of esophageal bulb with an arrow shape-like end. . . . . . . . . . . . . . . . . . . . . . . | . . . . . . . . . . . . . . . . . . . . . . 2 |
| 1b. | Width of the cervical alae progressively decreasing, connected with lateral alae. . . . . . . . . . . . . . . . . . . . . . . | . . . . . . . . . . . . . . . . . . . . . . 16 |
| 2a. | Cervical alae end anterior or at middle of esophageal bulb . . . . . . . . . . . . . | . . . . . . . . . . . . . . . . . . . . . . 3 |
| 2b. | Cervical alae end posterior to esophageal bulb . . . . . . . . . . . . . . . . . | . . . . . . . . . . . . . . . . . . . . . . 11 |
| 3a. | Caudal region with 10-12 papillae . . . . . . . . . . . . . . . . . . . . . . | . . . . . . . . . . . . . . . . . . . . . . 4 |
| 3b. | Caudal region with 14-16 papillae . . . . . . . . . . . . . . . . . . . . . . | . . . . . . . . . . . . . . . . . . . . . . 10 |
| 4a. | Caudal region with double papillae . . . . . . . . . . . . . . . . . . . . . . | . . . . . . . . . . . . . . . . . . . . . . 5 |
| 4b. | Caudal region with a single papilla . . . . . . . . . . . . . . . . . . . . . . | . . . . . . . . . . . . . . . . . . . . . . 6 |
| 5a. | Caudal region with 10 single and 1 double papillae . . . . . . . . . . . . . . . | . . . . . . . . . . . . . . .. A. versteraeHugot, 1980 |
| 5b. | Caudal region with 10 single and 2 double papillae . . . . . . . . . . . . . . . | . . . . . . . . . . . . . . . A. tetraptera (Nitzch, 1821) |
| 6a. | Palearctic distribution . . . . . . . . . . . . . . . . . . . . . . . . . . . | . . . . . . . . . . . . . . . 7 |
| 6b. | Nearctic distribution . . . . . . . . . . . . . . . . . . . . . . . . . . . . . | A. huascaensisFalcón-Ordaz et al. 2010 |
| 7a. | Caudal region with 10 papillae . . . . . . . . . . . . . . . . . . . . . . . . . | . . . . . . . . . . . . . . . 8 |
| 7b. | Caudal region with 11 papillae . . . . . . . . . . . . . . . . . . . . . . . . . | . . A. arianicaKotrla and Daniel, 1970 |
| 8a. | Egg size ranges 0.07 - 0.08 × 0.03 - 0.04 mm . . . . . . . . . . . . . . . . . . . | . . . . . . . . . . . . . . . 9 |
| 8b. | Eggs size 0.11 × 0.05 mm . . . . . . . . . . . . . . . . . . . . . . . . . . . | . . . . . . . . . . . . . . . . A. dinnickiSchulz, 1927 |
| 9a. | Cervical alae ending anterior to esophageal bulb . . . . . . . . . . . . . . . . . . | . . . . . . . . . . . . . . . A. schulzi Popov and Nasarova, 1930 |
| 9b. | Cervical alae ending at middle of esophageal bulb . . . . . . . . . . . . . . . . . | . . . . . . . . . . . . . . . A. lahorica Akhtar, 1955 |
| 10a. | Tail enclosed by caudal alae; Palearctic distribution . . . . . . . . . . . . . . . . | . . . . . . . . . . . . . . . A. tschertkovi Tarjymanova, 1959 |
| 10b. | Tail not enclosed by caudal alae; Neotropical distribution . . . . . . . . . . . . . | . . . . . . . . . . . . . . . A. mexicana n. sp. |
| 11a. | Body size of female > 6 mm; Palearctic distribution . . . . . . . . . . . . . . . . | . . . . . . . . . . . . . . . 12 |
| 11b. | Body size of female < 6 mm; Nearctic distribution . . . . . . . . . . . . . . . . | . . . . . . . . . . . . . . . A. americanaErickson, 1938 |
| 12a. | 11 caudal papillae; caudal alae enclosing tail . . . . . . . . . . . . . . . . . . . | . . . . . . . . . . . . . . . . . A. rysavyiKotrla and Daniel, 1971 |
| 12b. | 12 caudal papillae; caudal alae not enclosing tail . . . . . . . . . . . . . . . . . | . . . . . . . . . . . . . . . . . . . . . . A. tianjinensisLiu et al., 2012 |
| 16a. | Body size of female > 8 mm; Palearctic distribution . . . . . . . . . . . . . . . . | . . . . . . . . . . . . . . . . . . . . . . . . . 17 |
| 16b. | Body size of female < 8 mm; Oriental or Ethiopian distribution . . . . . . . . . . . . . . . . | . . . . . . . . . . . . . . . . . . . . . . . 18 |
| 17a. | Caudal area with 2 raspberry-like structures . . . . . . . . . . . . . . . . . . . | . . . . . . . . . . . . . A. witenbergiQuentin, 1975 |
| 17b. | Caudal area with a double papillae . . . . . . . . . . . . . . . | . . . . . . . . . . . . . A. asiaticaSchulz, 1927 |
| 18a. | Tail not enclosed by caudal alae; Ethiopian distribution . . . . . . . . . . . . . . | . . . . . . . . . . . . . . . . . . . . . . . 19 |
| 18b. | Tail enclosed by caudal alae; Oriental distribution . . . . . . . . . . . . . . . . . | . . . . . . . . . . . . . A. rattiJohnston, 1969 |
| 19a. | Caudal area without raspberry-like structures . . . . . . . . . . . . . . . . . . | . . . . . . . . . . . . . . A. africanaQuentin, 1966 |
| 19b. | Caudal area with 2 raspberry-like structures . . . . . . . . . . . . . . . . | . . . . . . . . . . . . . ..A. shikolouetaInglis et al., 1990 |











 nueva página del texto (beta)
nueva página del texto (beta)



