Introduction
The concept of a guild refers to a group of species that exploit common resources, and may therefore compete among them (Brown et al., 1979; Polis & McCormick, 1986a; Root, 1967; Schoener & Toft, 1983; Waide & Reagan, 1983). Existence of guilds can be assumed when possible members of a guild occur in sympatry (Root, 1967); this is usually the situation with scorpions, especially in deserts and tropical forests (Lira et al., 2013; Polis & McCormick, 1986a). Since scorpions play an important role as predators in the ecosystems in which they live and they can often be sympatric, they could exhibit evident intraguild interactions (Polis, 1990). Those interactions go from mutualism to mutual predation or competition (Polis & McCormick, 1987). Results reported by Warburg (1998) show that “when scorpions encounter another specimen of their species or from other species within the guild, their behavioural patterns were significantly more aggressive during the interspecific encounters than in the intraspecific ones”. Connecting with this thought, Stockman (2013) pointed out that “many species of scorpions that cohabit together could become the prey of the other or even, when food is scarce, they could be eaten by members of their own species”. This type of mutual predation is usually referred to as intraguild predation (IGP), defined as the “killing and eating of species that use similar, potentially limiting resources and are therefore potential competitors” (Polis & McCormick, 1987). Although IGP is known to be widespread among scorpions, it has received very little attention from either theoretical or field biologists (Polis & McCormick, 1986b).
In the tropical forest, many species of scorpions can coexist in the same habitat (Lira et al., 2013; Stockman & Ythier, 2010), possibly competing interspecifically in several ways (McCormick & Polis 1990). In the secondary tropical forest of the Caribbean coast of Costa Rica, Centruroides limbatus (Pocock, 1898) and Tityus oceloteFrancke & Stockwell, 1987 (Buthidae) coexist, a fact that has been known since the original description of T. ocelote. These 2 species share the same forest patches and consequently are part of the same guild. They differ substantially in size: adults of C. limbatus can measure from 50 to 110 mm, whereas adult individuals of T. ocelote do not surpass 40 mm (Fig. 1). Therefore, if extrinsic factors allow them to interact, they may show differential behaviours including competition or even IGP. However, studies on the ecology and habits of scorpions in Costa Rica are very scarce, and intraguild interactions have not been reported. Despite of this fact, there is some information about microhabitat preferences for these 2 species: they are both mainly arboreal and have been found on the trunks of large trees and palms. According to Víquez (1999) and Seiter (2012), T. ocelote prefers palms or medium sized plants, near to the ground and it has never been observed higher than 5 meters above the ground. On the contrary, there are no data about the heights occupied by C. limbatus.
Materials and methods
The study was located at the Pacuare Nature Reserve in Costa Rica (Abellá et al., 2008; Rivas et al., 2016; Fig. 2). Sampling was conducted along the main track of the reserve (Fig. 3A), which was divided into 2 areas due to their vegetational differences, following a similar approach like the one used by Nime et al. (2016): South and north. The southern zone was classified as “disturbed”, since it was nearer to the main camp of the reserve and it had a lot of allochthonous vegetation such as the palms and other plants that were mostly introduced. In contrast, the more distant northern zone was classified as “secondary forest”, since it had better conserved forest patches of native vegetation that was also denser and with a wider coverage than the one in the south. The study was carried out during 7 nights, from the 12th to the 18th of May, 2019. We established 4 sampling transects in the south and 3 sampling transects in the north that are represented in Figure 2; each transect was 350 m long. During that week, the moon was in crescent phase and it reached the full moon on the 18th. This is something important to take into consideration, since scorpions are nocturnal and the lunar cycle may have influenced negatively their activity as some authors have reported for other species (Ahsan et al., 2016; Castilla et al., 2010; Polis, 1990; Tigar & Osborne, 1999).

Figure 2. Map of the Pacuare Nature Reserve. All transects sampled along the main track are represented.

Figure 3. A) Main track of the Pacuare Nature Reserve (photograph by A. Calatayud Mascarell). B) Female specimen of Tityus ocelote under the UV light (JBA, in situ photograph). C) One of the marked trees with vines in which we found scorpion specimens (photograph by JBA).
Field work took place at night and the observations were made during 2 consecutive hours per day, distributed between 18:30 h and 00:00 h (randomly selected during the week) for covering the peaks of abundance and activity of scorpions (Polis, 1990). The following time intervals were used and repeated twice each: 18:30-20:30, 20:00-22:00 and 22:00-00:00. For the last day, we did our sampling in a different time interval (19:30-21:30) trying to avoid the maximum overlap within the hours of the other intervals and also because the moon was full and at that time it had already risen over the horizon but had not reached its maximum altitude (Table 1).
Table 1. Moonrise and moonset data from the days of the study. From 12th to 17th, the moon was in crescent phase, and on the 18th it reached the full moon.
| Moonrise/Moonset | Meridian passing | Behaviour of the specimens | ||||
| Date | Moonrise | Moonset | Time (max. ºAltitude) | Distance (km) | Illumination | “Hidden” |
| 12/05/2019 | 12:19 | 00:13 | 18:42 (86.8º) | 369.256 | 61.3% | 1 |
| 13/05/2019 | 13:16 | 01:04 | 19:35 (88.1º) | 369.017 | 72.5% | 2 |
| 14/05/2019 | 14:11 | 01:52 | 20:26 (82.6º) | 369.514 | 82.4% | 4 |
| 15/05/2019 | 15:05 | 02:39 | 21:16 (77.0º) | 370.862 | 90.5% | 0 |
| 16/05/2019 | 16:00 | 03:25 | 22:07 (71.5º) | 373.127 | 96.2% | 0 |
| 17/05/2019 | 16:54 | 04:12 | 22:58 (66.6º) | 376.295 | 99.3% | 0 |
| 18/05/2019○ | 17:50 | 05:00 | 23:50 (62.5º) | 380.242 | 99.7% | 5 |
Scorpions fluoresce under ultraviolet light, so we used blacklight lanterns to locate the specimens along the track (Ali et al., 2001; Gaffin et al., 2012) (Fig. 3B). When an individual was found, we took data on time of observation, species, sex, height from the ground, microhabitat in which it was located and the behavioural activity it was showing (classified as: “exposed”, “moving”, “hidden” and “feeding”) (Table 2).
Table 2. Behavioural activity of both species during the sampling.
| Species | Exposed | Hidden | Moving | Feeding |
| Centruroides limbatus | 11 | 6 | 6 | 1 |
| Tityus ocelote | 26 | 6 | 6 | 1 |
| Total | 37 | 12 | 12 | 2 |
To avoid pseudo-replicates, we marked the end of our 2-hour walk every night and that became the starting point the next day (Ranstam, 2012). We also marked every microhabitat in which an individual of scorpion was found with a red ribbon and gave it a number and a code (Fig. 3C). The microhabitats were classified by vegetation type or structure (Goodman & Esposito, 2020; Lira et al., 2013; Nime et al., 2016): “bamboo”, “calathea”, “heliconia”, “palm”, “plant litter”, “rotten trunk”, “vine”, and “tree with vines” (Table 3).
Table 3. Total data collected along the seven days of sampling. *This female individual was carrying its nymphs (Fig. 11).
| Zone | Date | Sampling interval | Exact hour | Species | Habitat | Sex | Activity | Height |
| South | 12/05/2019 | 20:00-22:00 | 20:23 | Tityus ocelote | Palm 1 | ♂ | Hidden | 50 cm |
| South | 12/05/2019 | 20:00-22:00 | 20:30 | Tityus ocelote | Plant litter1 | ♀ | Exposed | 0 cm |
| South | 12/05/2019 | 20:00-22:00 | 21:00 | Centruroides limbatus | Rotten trunk 1 | N/S (juv.) | Exposed | 60 cm |
| South | 12/05/2019 | 20:00-22:00 | 21:18 | Tityus ocelote | Palm 2 | ♀ | Exposed | 160 cm |
| South | 12/05/2019 | 20:00-22:00 | 21:18 | Tityus ocelote | Palm 2 | ♂ | Exposed | 140 cm |
| South | 12/05/2019 | 20:00-22:00 | 21:18 | Tityus ocelote | Palm 2 | ♀ | Exposed | 130 cm |
| South | 12/05/2019 | 20:00-22:00 | 21:18 | Tityus ocelote | Palm 2 | ♀ | Exposed | 140 cm |
| South | 12/05/2019 | 20:00-22:00 | 21:18 | Tityus ocelote | Palm 2 | ♂ | Exposed | 120 cm |
| South | 12/05/2019 | 20:00-22:00 | 21:23 | Tityus ocelote | Palm 3 | ♀ | Moving | 160 cm |
| South | 12/05/2019 | 20:00-22:00 | 21:23 | Tityus ocelote | Palm 3 | ♀ | Exposed | 130 cm |
| South | 12/05/2019 | 20:00-22:00 | 21:23 | Tityus ocelote | Palm 3 | ♂ | Exposed | 150 cm |
| South | 12/05/2019 | 20:00-22:00 | 21:36 | Tityus ocelote | Plant litter 2 | ♀ | Moving | 0 cm |
| South | 12/05/2019 | 20:00-22:00 | 21:46 | Tityus ocelote* | Palm 4 | ♀ | Exposed | 140 cm |
| South | 12/05/2019 | 20:00-22:00 | 21:56 | Centruroides limbatus | Palm 4 | ♀ | Exposed | 210 cm |
| South | 13/05/2019 | 18:30-20:30 | 18:39 | Tityus ocelote | Heliconia 1 | ♂ | Moving | 50 cm |
| South | 13/05/2019 | 18:30-20:30 | 18:58 | Tityus ocelote | Plant litter 3 | ♂ | Exposed | 0 cm |
| South | 13/05/2019 | 18:30-20:30 | 19:01 | Tityus ocelote | Palm 5 | ♀ | Hidden | 150 cm |
| South | 13/05/2019 | 18:30-20:30 | 19:01 | Tityus ocelote | Palm 5 | ♀ | Exposed | 200 cm |
| South | 13/05/2019 | 18:30-20:30 | 19:05 | Tityus ocelote | Palm 5 | N/S (juv.) | Exposed | 260 cm |
| South | 13/05/2019 | 18:30-20:30 | 19:13 | Tityus ocelote | Calathea 1 | ♀ | Exposed | 60 cm |
| South | 13/05/2019 | 18:30-20:30 | 19:17 | Tityus ocelote | Tree with vines 1 | ♀ | Exposed | 190 cm |
| South | 13/05/2019 | 18:30-20:30 | 19:17 | Tityus ocelote | Tree with vines 1 | ♀ | Moving | 190 cm |
| South | 13/05/2019 | 18:30-20:30 | 19:25 | Tityus ocelote | Tree with vines 2 | ♀ | Exposed | 180 cm |
| South | 13/05/2019 | 18:30-20:30 | 19:25 | Tityus ocelote | Tree with vines 2 | ♀ | Exposed | 185 cm |
| South | 13/05/2019 | 18:30-20:30 | 19:26 | Centruroides limbatus | Tree with vines 2 | ♂ | Hidden | 200 cm |
| South | 13/05/2019 | 18:30-20:30 | 19:46 | Tityus ocelote | Tree with vines 3 | ♀ | Exposed | 200 cm |
| South | 13/05/2019 | 18:30-20:30 | 19:46 | Tityus ocelote | Tree with vines 3 | N/S (juv.) | Exposed | 230 cm |
| South | 13/05/2019 | 18:30-20:30 | 19:46 | Tityus ocelote | Tree with vines 3 | ♀ | Exposed | 200 cm |
| South | 13/05/2019 | 18:30-20:30 | 20:00 | Tityus ocelote | Tree with vines 4 | ♀ | Exposed | 190 cm |
| South | 13/05/2019 | 18:30-20:30 | 20:00 | Tityus ocelote | Tree with vines 4 | ♀ | Exposed | 20 cm |
| South | 14/05/2019 | 22:00-00:00 | 22:05 | Centruroides limbatus | Tree with vines 5 | ♀ | Feeding | 190 cm |
| South | 14/05/2019 | 22:00-00:00 | 22:10 | Tityus ocelote | Tree with vines 6 | ♂ | Hidden | 160 cm |
| South | 14/05/2019 | 22:00-00:00 | 22:10 | Tityus ocelote | Tree with vines 6 | ♀ | Exposed | 50 cm |
| South | 14/05/2019 | 22:00-00:00 | 22:10 | Tityus ocelote | Tree with vines 6 | ♂ | Feeding | 60 cm |
| South | 14/05/2019 | 22:00-00:00 | 22:15 | Tityus ocelote | Tree with vines 7 | ♀ | Moving | 60 cm |
| South | 14/05/2019 | 22:00-00:00 | 22:22 | Centruroides limbatus | Palm 6 | ♀ | Exposed | 350 cm |
| South | 14/05/2019 | 22:00-00:00 | 22:37 | Tityus ocelote | Vine | ♂ | Exposed | 20 cm |
| South | 14/05/2019 | 22:00-00:00 | 22:43 | Tityus ocelote | Vine 2 | ♀ | Hidden | 30 cm |
| South | 14/05/2019 | 22:00-00:00 | 22:43 | Tityus ocelote | Vine 2 | ♀ | Hidden | 40 cm |
| South | 14/05/2019 | 22:00-00:00 | 23:05 | Tityus ocelote | Tree with vines 8 | N/S | Hidden | 310 cm |
| South | 15/05/2019 | 20:00-22:00 | 20:18 | Tityus ocelote | Tree with vines 9 | ♀ | Exposed | 200 cm |
| South | 15/05/2019 | 20:00-22:00 | 20:39 | Centruroides limbatus | Tree with vines 10 | ♂ | Exposed | 300 cm |
| South | 15/05/2019 | 20:00-22:00 | 20:52 | Tityus ocelote | Tree with vines 11 | ♀ | Exposed | 250 cm |
| South | 15/05/2019 | 20:00-22:00 | 21:00 | Centruroides limbatus | Bamboo 1 | ♀ | Exposed | 10 cm |
| South | 15/05/2019 | 20:00-22:00 | 21:02 | Centruroides limbatus | Bamboo 2 | ♀ | Exposed | 10 cm |
| North | 16/05/2019 | 18:30-20:30 | 18:59 | Tityus ocelote | Vine 3 | ♀ | Exposed | 20 cm |
| North | 17/05/2019 | 22:00-00:00 | 22:00 | Centruroides limbatus | Tree with vines 12 | ♀ | Exposed | 150 cm |
| North | 17/05/2019 | 22:00-00:00 | 22:10 | Centruroides limbatus | Rotten trunk 2 | ♀ | Exposed | 30 cm |
| North | 17/05/2019 | 22:00-00:00 | 22:32 | Centruroides limbatus | Tree with vines 13 | N/S | Exposed | 600 cm |
| North | 17/05/2019 | 22:00-00:00 | 22:40 | Centruroides limbatus | Tree with vines 14 | ♀ | Moving | 220 cm |
| North | 17/05/2019 | 22:00-00:00 | 22:42 | Tityus ocelote | Palm 7 | ♀ | Exposed | 150 cm |
| North | 17/05/2019 | 22:00-00:00 | 22:47 | Centruroides limbatus | Tree with vines 15 | ♀ | Exposed | 160 cm |
| North | 17/05/2019 | 22:00-00:00 | 23:05 | Centruroides limbatus | Tree with vines 16 | ♀ | Exposed | 60 cm |
| North | 18/05/2019 | 19:30-21:30 | 20:00 | Centruroides limbatus | Tree with vines 16 | ♂ | Hidden | 170 cm |
| North | 18/05/2019 | 19:30-21:30 | 20:10 | Centruroides limbatus | Calathea 2 | ♀ | Exposed | 60 cm |
| North | 18/05/2019 | 19:30-21:30 | 20:17 | Centruroides limbatus | Tree with vines 17 | N/S | Hidden | 460 cm |
| North | 18/05/2019 | 19:30-21:30 | 20:17 | Centruroides limbatus | Tree with vines 17 | N/S | Hidden | 410 cm |
| North | 18/05/2019 | 19:30-21:30 | 20:34 | Centruroides limbatus | Tree with vines 18 | N/S | Exposed | 650 cm |
| North | 18/05/2019 | 19:30-21:30 | 20:43 | Centruroides limbatus | Tree with vines 19 | ♀ | Hidden | 320 cm |
| North | 18/05/2019 | 19:30-21:30 | 20:53 | Centruroides limbatus | Tree with vines 20 | ♂ | Moving | 320 cm |
| North | 18/05/2019 | 19:30-21:30 | 20:57 | Centruroides limbatus | Tree with vines 21 | ♀ | Hidden | 300 cm |
| North | 18/05/2019 | 19:30-21:30 | 21:04 | Centruroides limbatus | Tree with vines 22 | ♀ | Exposed | 50 cm |
| North | 18/05/2019 | 19:30-21:30 | 21:09 | Centruroides limbatus | Tree with vines 23 | ♀ | Exposed | 210 cm |
As both species show marked sexual dimorphism as adults, sexing mature specimens in the field was no problem; however, as juveniles are not sexually dimorphic, they could not be sexed without capturing and disturbing them; therefore, we only sexed adults of both species.
All analyses were performed with the R environment (R Core Team, 2018). For testing differences in height location above ground we first analysed which factors were influencing our data the most. Following the R Studio procedure, we fitted a linear model to investigate the effect of zone (north, south), species (C. limbatus and T. ocelote), and their interaction on the height at which every individual was found. Then, we used a sequential sum of squares (type I, ANOVA) to test the effect of each predictor, including the zone first -as a surrogate of human disturbance and vegetation structure- and then species. Normality and homoscedasticity of model residuals were tested with the Shapiro-Wilk and the Levene tests, respectively. We square root transformed the response variable to account for lack of normality. For the final analysis, we used a t-test for evaluating if the height location above the ground had statistical differences depending on the zone (north and south). Additionally, the Morisita index was chosen to calculate intraspecific aggregation of each species in both zones and niche overlap among both species in the 2 zones (Amaral et al., 2015; Mueller & Altenberg, 1985). We used the function ‘agg_index’ from package ‘epiphy’ v0.3.4 (Gigot, 2018) to analyze intraspecific aggregation (where an index < 1 indicates an uniform pattern; an index = 1 indicates a random pattern; and an index > 1 indicates an aggregated pattern) and the function ‘niche. overlap’ from package ‘spaa’ v0.2.2 (Zhang, 2016) for niche overlap (where index = 0 indicates no niche overlap, and index = 1 indicates full niche overlap).
The map of the area where the study was carried out was created using QGIS v3.8 (QGIS Development Team, 2019).
Results
Patterns of activity are quite similar in both species. Centruroides limbatus shows its maximum activity between 20:15 and 21:00, and T. ocelote between 21:00 and 21:45; however, any real differences are obscured by the apparently discontinuous patterns of activity exhibited by these 2 species (Fig. 4). There are differences in the number of active individuals in any given time span, always higher in T. ocelote.
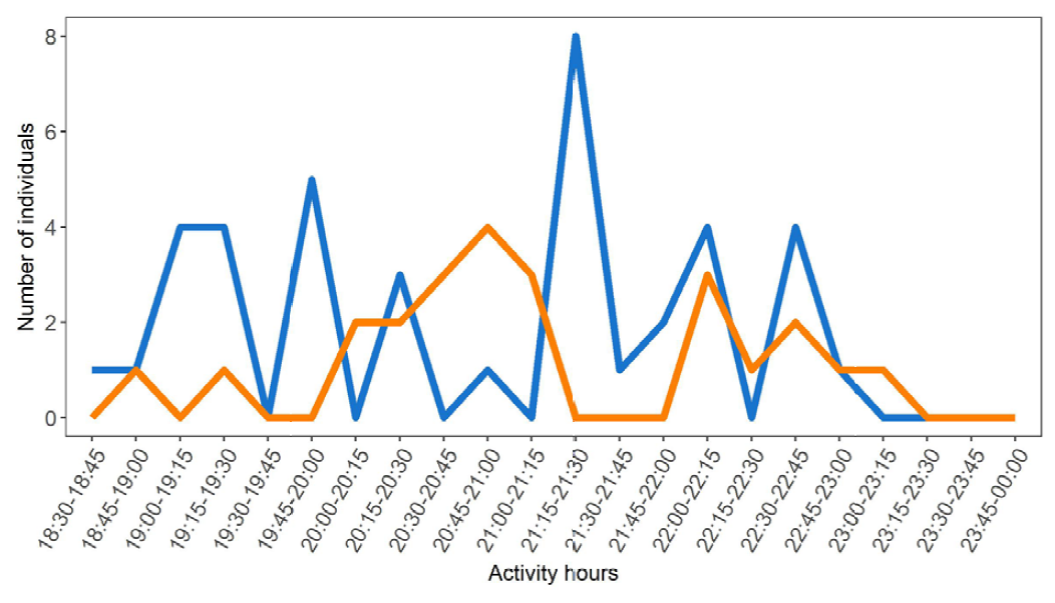
Figure 4. Peaks of activity of the 2 scorpion species present in Pacuare Nature Reserve. The orange line represents Centruroides limbatus and the blue line corresponds to Tityus ocelote.
The total N of the study was 63, of which 24 individuals belonged to C. limbatus and 39 to T. ocelote. The 2 species also differ in abundance between the areas. Figure 5 shows that C. limbatus is more likely to be seen in the northern zone (north = 16, south = 8), whereas T. ocelote only seems to be well represented in the southern zone (north = 2, South = 37).
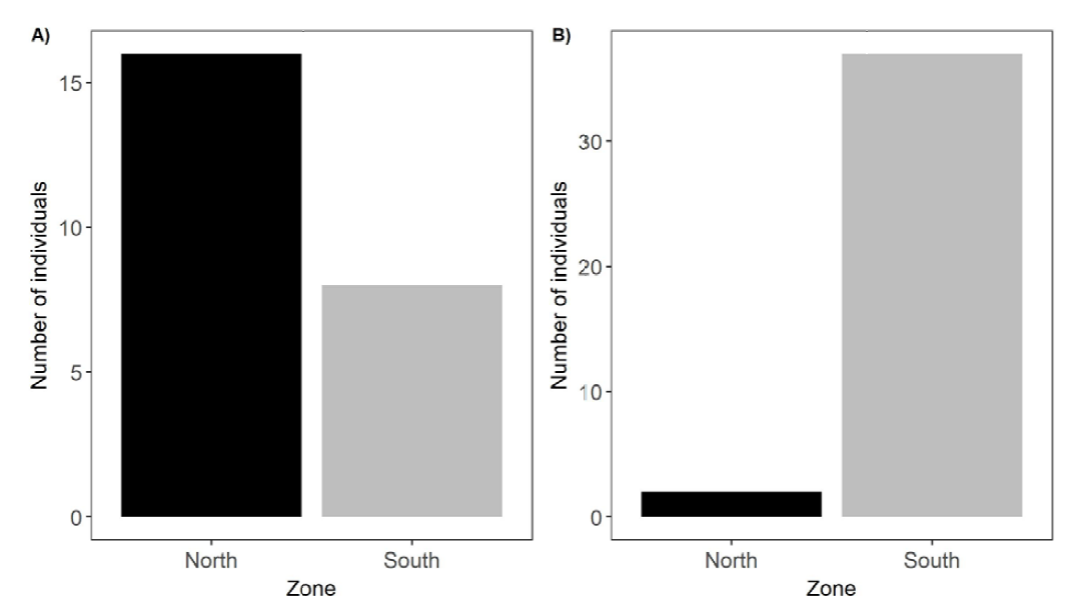
Figure 5. Distribution North-South of the scorpions in Pacuare Nature Reserve. A) Centruroides limbatus specimens within the 2 zones. B) Tityus ocelote specimens within the 2 zones.
The scheduled times of our sampling scheme show clearly that time of the night does not affect the level of activity observed in the field: 18:30-20:30 (n = 2 nights) 17 scorpions; 20:00-22:00 (n = 2) 19 scorpions; and 22:00-00:00 (n = 2) 17 scorpions. On the last night, with the full moon, we sampled from 19:30 to 21:30 and observed 10 scorpions (n = 1), which doubled for another sample would be 20 in total (we only did one sampling effort during this time interval, whereas 2 sampling efforts were done in the others). Obviously, the time of night at which we sampled had no effect on the number of scorpions observed on the surface (17, 19, 17, “20”) (Table 3).
Centruroides limbatus and T. ocelote specimens observed and sexed during the time of the study were mostly females (Fig. 6).

Figure 6. Sex frequency of the scorpions sampled in Pacuare. A) Sex frequency of Centruroides limbatus. B) Sex frequency of Tityus ocelote.
The presence of 2 or more specimens at the same location (i.e., trunk, leaf, vine…) was more frequent (64.1%) in T. ocelote; 43.6% of the individuals of T. ocelote were found in little groups of 3 or more specimens, 20.5% in groups/pairs of 2 specimens, and 35.9% were found alone (Table 4); we counted a maximum of 5 T. ocelote individuals on the same plant (one of the palm trees). Results of the Morisita index for the spatial distribution and intraspecific aggregation (M-SDIA from now on) showed an aggregate pattern of the individuals of T. ocelote in the south (M-SDIA = 1.201), whereas it reported an uniform pattern in the north (M-SDIA = 0; there were just 2 separate specimens). In contrast, individuals of C. limbatus were found mostly alone (83.3% were solitary, and 16.7% were in pairs, Table 3). The M-SDIA showed an uniform pattern of this species in the south (M-SDIA 0.714), whereas it reported an aggregated pattern in the north (M-SDIA = 1.5; where the abundance of C. limbatus is higher). During a previous visit, 2 specimens of C. limbatus were also found together, but it was precisely a case of intraspecific predation between specimens of different size (Fig. 7; F. Díaz Béjar pers. obs., April 2017). Cohabitation between species (C. limbatus, T. ocelote) was observed in just 2 occasions in the southern zone (6.4% of the total N). These individuals of each species were sharing the same microhabitat (tree with vines, palm) separated in height (i.e., a few centimeters). No co-occurrence was observed in the northern zone; although T. ocelote was so scarce in that zone (N = 2), that this observation lacks significance.As stated by the abundance of C. limbatus and T. ocelote in each transect (Table 5), Morisita index for niche overlap (M-NO from now on) -the analysis of the coexistence between them in both zones- reported a strong niche overlap between both species in the south (M-NO = 0.686); whereas in the north there was a weak niche overlap (M-NO = 0.364), probably related to the poor representation of T. ocelote in this section of the transect.
Table 4. Number of specimens of each species found alone, in pairs or in groups. *Specimens that are not classified in these groups were too high for sexing or determining their life stage without disturbing them.
| Species | Specimens found alone | Specimens found in pairs |
Specimens found in groups of three or more |
Total of specimens |
| Centruroides limbatus | 20 (14♀, 3♂, 1 juv.)* | 4 (1♀, 1♂)* | 0 | 24 |
| Tityus ocelote | 14 (9♀, 4 ♂)* | 8 (8♀) | 17 (10♀, 5 ♂, 2 juv.) | 39 |

Figure 7. Adult male of Centruroides limbatus preying on a smaller individual of the same species at the Pacuare Nature Reserve (F. Díaz Béjar, in situ photograph).
Table 5. Number of individuals of each species in every transect along the main track of the Pacuare Nature Reserve.
| Species | T1 (south) | T2 (south) | T3 (south) | T4 (south) | T5 (north) | T6 (north) | T7 (north) |
| Centruroides limbatus | 2 | 1 | 2 | 3 | 0 | 6 | 10 |
| Tityus ocelote | 12 | 15 | 8 | 2 | 1 | 1 | 0 |
Trees with vines were the primary habitat for both species (C. limbatus = 17/24, T. ocelote = 16/39). The secondary habitats in which both species were found were palm leaves and trunks (C. limbatus = 2/24 , T. ocelote = 14/39). Other microhabitats are recorded in Figure 8.

Figure 8. Microhabitat preference of the scorpions in Pacuare Nature Reserve. The orange bars represent Centruroides limbatus and the blue bars correspond to Tityus ocelote.
The fit of the lineal model reported a strong normality and homoscedasticity after the transformation of our response variable (F = 6.09, df = 1, R² = 0.076). Results of the analysis of variance showed a significant effect of “Zone” (p = 0.016; Table 6) in the height at which both species were on the vegetation. The t-test showed that there also were significant differences (p = 0.037) between the heights at which specimens of both species were observed determined by the 2 zones (Fig. 9A), with higher ranges of height in the north. The mean height at which every individual of each species was located depending on the zone is recorded in Table 7.
Table 6. Results of the ANOVA test analysis for our model.
| Df | F | p | |
| Height zone | 1 | 6.1223 | 0.01624 |
| Species | 1 | 1.5964 | 0.21138 |
| Zone X species | 1 | 1.5531 | 0.21761 |

Figure 9. A) Height location above the ground of both scorpion species depending on the zone (North and South). B) Height location above the ground depending on the species. Discontinuous lines in both graphs represent the mean height of each zone (A) or species (B). Height is square root transformed in both graphs.
Discussion
The species of our study are well distributed along disturbed environments of Costa Rica such as Pacuare Nature Reserve, and are even associated with human settlements (Santoro et al., 2008; Víquez, 1999). As some authors have addressed, this could be a response to their ecological requirements, and in this case, C. limbatus and T. ocelote seemed to fit in the “opportunistic” ecological group (Lourenço & Cuellar, 1995; Polis, 1990). The observations made for C. limbatus and T. ocelote fit well with other observations on members of the genera Centruroides and Tityus as occupying disturbed environments and being broadly distributed (Lourenço, 1991; 1994); although the abundances of these species in Pacuare seemed to be low compared to other species from drier environments. We found a mean of only 16 (15.75) individuals per 350 m long transect.
Activity peaks for both species were quite similar; they followed a discontinuous pattern along the night. The maximum peaks for C. limbatus occurred between 20:15 and 21:00 h, whereas T. ocelote was more often seen between 21:00 and 21:45h. Nevertheless, these maximums could be determined by the overlap in hours produced by some of the time intervals used in the sampling (18:30-20:30, 20:00-22:00, 19:30-21:30), in which we, consequently, increased the sampling effort. Another fact that we took into consideration during the sampling was the lunar cycle. During that week, the moon was in crescent phase, reaching a full moon on the final day of our study. This might have conditioned the activity of both species, as it has been previously tested in other scorpions (Castilla et al., 2010; Nime et al., 2013; Polis, 1990; Tigar & Osborne, 1999), but other studies have also proved that the lunar cycle had no influence in some species (Ahsan et al., 2016; Hadley & Williams, 1968). In this study, we found that neither species showed a change in surface presence related to the time of night during the sampling, and that there was no apparent effect of the moon on surface activity, except for individuals of C. limbatus in the last northern transect (Table 3).
Through our data, we see that the “exposed” behaviour dominance in the scorpions from Pacuare (58.7% “exposed”) together with the “moving” and “feeding” behaviours (19% and 3.2%, respectively), against the percentages for “hidden” behaviour (19%) reflect that the lunar cycle seemed to have had no influence in their activity (Table 2). Only during the last day (May 18th, full moon) it seems to have been a direct relationship with the moon state and the behavioural activity of the scorpions, since half of the specimens observed that night were in “hidden” behaviour (5 out of 10) (Tables 1, 2). Notwithstanding, the other behaviours represented during that night were “exposed” and “moving”.
However, the ecological aspects of the 2 species differ on some points. Whereas specimens of C. limbatus were mostly solitary, we observed gregarious behaviour in T. ocelote, as previously reported for other species of scorpions (Buskirk, 1981; Shivashankar, 1994; Kaltsas et al., 2009). M-SDIA showed an aggregated pattern for T. ocelote individuals in the south (M-SDIA = 1.201), but a uniform pattern in the north (M-SDIA = 0). Our data hint that this might be a reflection of the abundances of this species in each zone (Fig. 5, Table 5), since T. ocelote “dominates” the southern transects and it is practically absent in the northern ones (just 2 specimens). Overall, pairs and groups of 3 or more individuals were more frequently observed than finding the specimens alone (64.1% vs 35.9%, respectively). Something similar occurs with C. limbatus: the M-SDIA reported a uniform pattern in the south (M-SDIA = 0.714), whereas it showed an aggregated pattern in the north (M-SDIA = 1.5). In this case, we can see that C. limbatus was well represented in the North although its presence in the south was limited (Fig. 5, Table 5). Despite of these results, overall, finding the individuals alone was more frequent than finding them in pairs (83.3% vs. 16.7%). It is possible that being solitary confers C. limbatus a more aggressive and territorial behaviour against members of its own species, as suggested by the presence of intraspecific cannibalism (Fig. 7). This finding proved that IGP was occurring between members of the same species within the guild as reported in other species (Polis & McCormick, 1987). We do not have enough observations to test Warburg’s (1998) hypothesis, but in our case the only intraguild predatory event observed occurred within the same species. Nevertheless, these results might be also indicative of the influence one species has on the other. As suggested by studies such as Lisboa et al. (2017), size and feeding behaviour might be the main factors responsible for interactions of this kind (Figs. 1, 10).

Figure 10. Female specimen of Centruroides limbatus eating the remains of a spider (possibly Cupiennius) at Pacuare Nature Reserve (JBA, in situ photograph).

Figure 11. Female specimen of T. ocelote carrying the newly born nymphs at Pacuare Nature Reserve (J.F. Aldegunde, ex situ photograph).
Centruroides limbatus and T. ocelote are sympatric and they tend to occupy the same primary microhabitat; they were reported together in Finca La Selva, provincia Heredia, by Francke & Stockwell (1987), approximately 90 km inland and northwest of the Pacuare Reserve. We have also seen both species sharing shelter in the same trees with vines and palms, but at different heights. Previous studies have proved that shelter could be one of the most determinant factors in either non-aggressive or aggressive interspecific encounters among scorpions and that the microhabitat selection of scorpions was not randomized (McReynolds, 2008; Warburg, 2000). In Pacuare Nature Reserve it is difficult to determine if this possible “syntopy” is a consequence of non-interacting cohabitation or competition (Kaltsas et al., 2009; Lankau, 2011; Lira et al., 2013; Polis & McCormick, 1987). However, we do not have enough data to support the syntopy hypothesis, because only 6.4% of the total specimens of both species were found on the same plant, and we can only assert that they do occur in sympatry.
The ecological similarities shown by C. limbatus and T. ocelote in relation to microhabitat occupancy are similar to those evidenced by Lira et al. (2013) in their study of scorpion interactions in accordance with microhabitat exploitation in Brazil. Although we did not find evidence for interspecific predation, we found cohabitation in the trees with vines and in palms. Testing the height at which every individual from both species was located at every zone (north and south) showed significant differences (p = 0.037). There were significant differences in heights between zones but not between species. Our hypothesis is that those differences between zones could be due to vegetation structure; whereas the non-significant differences between species might be indicative of either niche partitioning or competition, because they stayed at similar ranges (as shown by Goodman and Esposito [2020] in their study of 3 species of the genus Centruroides in the Los Tuxtlas region, Veracruz, Mexico). In Figure 9B, we can see the different ranges of height between zones depending on the species. As it is shown, T. ocelote seemed to maintain its height location within the same range, whereas C. limbatus stayed at higher ranges. However, we cannot rule out the possibility that T. ocelote occurred as high or higher in the vegetation than C. limbatus did, because of their marked size differential, the later was easier to spot higher than 3 m above in the vegetation than the former. Whereas 4 specimens of C. limbatus were spotted higher than 4 m, and 2 of them where higher than 6 m; only 1 specimen of T. ocelote was spotted higher than 3 m, and 7 others between 2 and 3 m (Table S1). These observations go along with the information provided by Víquez (1999) and Seiter (2012) who indicated that T. ocelote has never been spotted at a height superior to 5 m above the ground. In the north (probably because of the more mature, larger and denser vegetation) the height ranges were higher than in the south. Assuming the dominance of C. limbatus in that zone and with the data in Table 7, we can indicate that this species stayed higher in the north, whereas it had to adapt its niche at lower heights in the south. Consequently, this causes an overlap in the niches of both species in the southern zone, where they are more frequently found coexisting (Table 5; Fig. 5) and staying at similar height ranges (Fig. 9A, B). The M-NO confirmed this hypothesis indicating a strong niche overlap between both species in the south (M-NO = 0.686), whereas it reported a weak niche overlap in the north (M-NO = 0.364). In some species of scorpions there seems to be an avoidance of this spatial overlap in their foraging activities, due to the presence of conspecifics or heterospecifics in the same areas, which would probably lead to competition for resources (Nime et al., 2016). As many authors have noticed, heterospecific individuals coexisting in the same habitat will compete for resources and may be determining habitat selection (Kaltsas et al. 2009; Lankau, 2011; Lira et al., 2013; Polis & McCormick, 1987). Nevertheless, our linear model only explains the 7.6% of the variability (R² = 0.076). Even though the probability holds our conclusions, we must carefully take these assumptions since they might be influenced by the sample size.
The 2-species system we studied in Pacuare Nature Reserve exhibit opposite trends in abundance and distribution (C. limbatus dominates the North and T. ocelote, the South), different behaviour (solitariness, gregariousness), but similar microhabitat preferences - which causes an overlap of their niches- together with a changing environment. It is not possible to predict the future direction this intraguild interaction might take and further studies are necessary to obtain clearer results about the interactions between C. limbatus and T. ocelote in this and other Caribbean forests before those habitats disappear.











 text new page (beta)
text new page (beta)



