Introduction
Seed dispersal by animals is an example of animal-plant mutualism; this interspecific relationship is defined by animals acquiring a benefit from the nutritious pulp of fruits, and in turn ensuring seed transport (González-Varo et al., 2015). Frugivore species play a fundamental role in seed dispersal, increasing the probability of seed germination by separating seeds from pulps and exposing them to acidic conditions through the gut passage, which remove germination inhibitors. At the same time, they transport seeds from parental individuals to sites where the probability of recruitment may increase (Cares et al., 2018). Dispersal of viable seeds increases seedling survival probabilities (Christian & Stanton, 2004; Janzen, 1970) and favors genetic exchange (Escribano-Ávila, 2019; Wehncke et al., 2009).
A systematic review of dispersal ecology in conservation biology concludes that limited knowledge on dispersal often compromises conservation planning, principally in relation to improving connectivity and reversing habitat fragmentation (Driscoll et al., 2014). While there is some progress towards mechanistic representations of seed dispersal by other vectors, such as vertebrates or water (Bullock et al., 2017), an understanding of local population processes may require detailed data on dispersal over a certain distance. Dispersal of an individual seed may be a complex process, through which all the variables (e.g., fruit traits, phenology, seed dispersers, characteristics of the habitat) are potentially relevant to plant-dispersion interactions (Herrera, 1989).
Consumption and dispersal via animals (zoochory) are continuous interactions, and are primary mechanisms for seed exchange between habitat patches, increasing plant recruitment (Kleyheeg et al., 2017). According to Mohammed et al. (2018), the essential components of frugivorous seed dispersal are: 1) the strength of the frugivore-plant interaction, defined as the efficiency of the seed disperser, 2) the abundance and probabilities of seed dispersal, 3) and successful seed germination following dispersion.
Beyond the specifics of the interactions, it is essential to consider the habitat they occur within. Fontúrbel and Medel (2017) show that structural and microclimatic characteristics of a given habitat could affect fruit traits, in relation to dispersal. Accordingly, habitat transformation would change the magnitude, direction, and significance of selection coefficients acting upon them. In this sense, spatial patterns in many populations and communities of desert plants become important, since they derive from the interaction of abiotic factors and biotic interactions (Delmas et al., 2019; Wehncke et al., 2009).
Biotic interactions are crucial for plant community dynamics, especially under environmental change scenarios (Camargo-Sanabria et al., 2015; Simmons et al., 2018). Willson (1993) conducted a review of mammalian seed dispersal, in which carnivores, as opposed to frugivorous species, have many potential taxa as legitimate seed dispersers because they consume/defecate seeds, thus improving their conditions for germination. Particularly, non-specialized frugivores, such as canids, are also habitat generalists, and are highly mobile species, connecting many kinds of habitat states (including fragmented landscapes) providing essential dispersal services for plants and restoration of habitats (Escribano-Ávila, 2019).
Although some palm species are widely recognized as plants with an important ecological role (Wehncke & López-Medellín, 2014; Zona & Henderson, 1989), few studies have focused on vertebrate mediated dispersal mechanisms. Washingtonia is a genus classified in the Trachycarpeae tribe of the Coryphoideae subfamily, within the Arecaceae family, which includes all palm species (Baker & Dransfield, 2016). This genus includes 2 species native to North America, Washingtonia filifera Linden ex André, occurring naturally in southwest Arizona, southern California, and northern Baja California, and Washingtonia robusta H. Wendl, found only in Baja California and Sonora (Felger & Joyal, 1999; Nehdi, 2011), though see Villanueva-Almanza (2018) for discussion of a possible cline between these 2 species in Baja California.
Washingtonia palms show specific traits, such as their patchy geographical distributions, low numbers, and affinity to oases - pockets of tropical environs in arid ecosystems. Fossil evidence supports that during the Late Cretaceous palms were common across North America and extended much further than their current geographic distribution, implying that present populations are relicts of a historically wide distribution (Klimova et al., 2017; Villanueva-Almanza et al., 2018). The biogeographical history of this species and its occurrence limited to oasis habitats offers a fascinating scenario to evaluate patterns and scales of predation/dispersal processes affecting fruits and seeds, a strongly heterogeneous resource that varies among successive spatial and temporal extents (Wehncke et al., 2009).
To study the whole seed dispersal process by animals, an approach used has been to divide it into 3 different phases, namely a predispersal, dispersal, and a postdispersal phase (Wehncke & Reyes-Amaya, 2019). The aim of this study is to discern the essential components of the seed dispersal of W. robusta by gray fox and coyote. The gray fox is considered ecologically and economically important for the development of forests, promoting its optimal sustainable yield, its home range allows it to travel long distances; its home range varies according to habitat quality and food availability, from 0.8 to 27.6 km2 (Fleming et al., 2017; Fritzell & Haroldson, 1982; Haroldson & Fritzell, 1984). The coyote is able to consume a total of 33 genera of fleshy fruit plants in North America, where it is abundant and has a wide distribution (Wilson, 1993). It has been reported that various seeds in feces of the coyote have germinated (Silverstein, 2005), which makes it a potential seed disperser. Like the gray fox, it inhabits almost any ecosystem and travels long distances, from 0.8 to 80 km2 (Bekoff, 1982). It is important to note that the coyote reproduces between the months of January to March and the gray fox from January to April (Sillero-Zubiri, 2004).
For Bullock (1980) and Wehncke and López-Medellín (2014), canids (and other mammals) use desert oases as biological corridors and transport seeds of palms to distant places, favoring genetic exchange between palms, since small-sized fruits can be dispersed over longer distances than big fruits. Therefore, determining whether the gray fox and coyote are dispersers of W. robusta seeds at Las Barajitas Canyon will allow the identification of ecological interactions in these ecosystems.
To define the importance of the consumption and dispersal and other factors that could be involved in shaping the distribution of this endemic Sonoran Desert palms, we: 1) explored the attributes of the frugivore-plant interaction by investigating whether W. robusta has traits that benefit its consumption and thus increase its likelihood of being dispersed, 2) addressed the abundance and probabilities of seed dispersal by questioning if the zoochory by gray fox and coyote favor plant establishment in certain microhabitats, and 3) tested if there is successful probabilities for seed germination and seedling establishment following seed dispersal.
Materials and methods
The Barajitas Canyon is one of several canyons found in the Sierra El Aguaje just north of San Carlos, Sonora, Mexico (28°3'24.60"- 28°2'17.64" N, 111°12'31.61"-111°9'24.80" W). It is located in the Central Gulf Coast subdivision of the Sonoran Desert (Shreve, 1951), an area with uncertain rainfall between 50 and 400 mm (with a mean of 218 mm), where maritime influence supports plants with exaggerated stems and trunk diameters. A few species are thought to have become locally restricted to isolated permanent water bodies such as oases and canyons, often with lush relictual tropical vegetation, isolated from one another by volcanic cliffs and vast expanses of Sonoran Desert scrub vegetation (Felger et al., 2017). The study area is vulnerable to natural phenomena, especially to semi-annual tropical storms and hurricanes, which modify the structure of the landscape and the deposition sites of the dispersed seeds.
From the coast to the canyon bottom, we identified 3 different conditions of palm canopy exposure: covered (C), exposed (E), and partially-exposed (Pe). In each site, we made the following local scale analyses, which also incorporate biotic variables: W. robusta seed deposition by canids, gray fox and coyote relative abundance index (RAI), and abiotic variables: soil organic matter, nitrogen, phosphorous, and potassium content. All analyses were measured within a 3km transect in the canyon bottom, which is the area were the palms are found before entering closed and high ridge areas (Fig. 1).

Figure 1 Study area at Las Barajitas Canyon, Sonora ( ). The 3 sampling sites C = covered (28°03'24.6"-111°09'24.8"), E = exposed (28°03'16.8"-111°10'17.2"), and Pe = partially exposed (28°02'56.4"-111°12'02.9").
). The 3 sampling sites C = covered (28°03'24.6"-111°09'24.8"), E = exposed (28°03'16.8"-111°10'17.2"), and Pe = partially exposed (28°02'56.4"-111°12'02.9").
We examined certain fruit traits such as nutritional contributions and (in vitro) protein digestibility. The nutritional contributions were obtained from a previous report (Armenta-Méndez et al., 2019), and the (in vitro) protein digestibility was done with the pulp of W. robusta fruits, according to the multienzymes method (Satterlee et al., 1982).
We collected scats from gray foxes and coyotes in winter (December-February) in 2015. The scats were identified as belonging to a canid group based on length and diameter, associated tracks, and bile acid extraction using TLC (Salame-Méndez et al., 2012). To obtain a distribution of the seeds dispersed, scat locations were recorded using a Garmin Map 78s GPS device (≤ 3 m error). After collection, scat components (hairs, seeds, and others) were separated using mesh sieves. Seeds were identified at the University of Sonora Herbarium and kept in refrigeration until germination tests. The number of W. robusta seeds deposited by canids within each treatment was transformed with Log10 to homogenize the value covariates data.
To determine the effectiveness of gray foxes and coyotes as vectors, we established 3 treatments to compare germination rates of fresh seeds with those that went through the digestive tract of the canids. We sowed: 1) seeds found in the scats, 2) seeds from mature fruits collected in the field and received treatment in purified water for 48 h, and 3) seeds from mature fruits collected without water treatment. All seeds (50 per treatment) were sowed in plastic cones (20 cm × 4 cm) using peat moss as a standard substrate for treatments to reduce any variability due to the use of local substrate, and held at room temperature in a greenhouse at the University of Sonora.
To document gray fox and coyote presence in the different sites (C, E, and Pe), one camera trap per site, separated by 1.5-3 km (Bushnell Trophy Cam HD Aggressor) was set up along the main arroyo in Las Barajitas Canyon, from September 2016 to November 2017. Motion-activated cameras were set to take 3 photographs per second at 1-minute intervals every time motion was detected. Photographs separated by 30 minutes of inactivity between images were considered as separate records (Srbek-Araujo & García-Chiarello, 2005). The value of the Relative Abundance Index (RAI) was the number of separate images divided by the sampling effort per 100 trap-days (Chávez et al., 2013). To determine the presence-absence of 3 groups of carnivores, Felidae (cougar, Puma concolor and bobcat, Lynx rufus), Procyonidae (ringtail, Bassariscus astutus and coati, Nasua narica) and Canidae (gray fox, Urocyon cinereoargenteus and coyote, Canis latrans), the photo captures were analyzed over a year in each photo trapping station, creating a capture history per day, indicating the presence of the species with the number 1 and the absence with the number 0 (White et al., 2001).
Three soil samples per site (C, E, and Pe) were collected in April 2017, 500 g each, at a 0-20 cm depth, separated by at least a 5 m linear distance. Determination of organic matter (OM), nitrogen (N), potassium (K), and phosphorus (F) were conducted according to Álvarez-Sánchez and Marín-Campos (2011). Results were expressed in average and sample standard deviation.
To compare selection coefficients between habitat types, which could be implicated in abundance and probabilities of seed dispersal and successful palm establishment, we performed a covariance analysis, ANCOVA, function AOV conducted in R 3.1.0 (R Core Team, 2013), using sampling site (C, E, and Pe) as factor, plant establishment as response, and biotic (W. robusta seeds deposited by canids and RAI of gray fox and coyote), and abiotic variable OM (organic matter) in the soil along the arroyo as covariates.
Results
Washingtonia robusta pulp is an important source of sugars (71%) and natural antioxidants (phenolic compounds) for gray fox and coyote. The in-vitro protein pulp fruit digestibility was 81%.
In 2015, 45 scats were collected along the main arroyo from the coast up the canyon. According to biliary acid determinations, 38 scats were from canids (gray fox and coyote). These contained 1,551 seeds of W. robusta (103.4 ± 94.7, average seeds per scat) and other edible fruits species, Colubrina californica (32%, n = 876), Sabal uresana (4%, n = 120), and Ziziphus amole (7%, n = 177). The W. robusta seeds scattering by canids per different habitat sites was: C, 693 seeds; E, 700 seeds; and Pe, 158 seeds (Fig. 2).

Figure 2 Amount of seeds found of Mexican fan palm W. robusta at the 3 sampling sites at Las Barajitas Canyon, covered = 9, exposed = 700, partially exposed = 158.
The seeds from scats reached a germination of 94%, seeds collected from trees with the water treatment germinated 100% of the time; and seeds collected from trees without water addition reach only a 55% germination.
The frequency of both canids was higher in the covered site (C) than in other sites (Fig. 3). Gray foxes were more frequent than coyotes with RAIs values of 5.7 ± 5 for gray fox and 2.6 ± 3.7 for coyotes (4.2 ± 0.9, average RAI for canids). The analysis of presence-absence of 3 groups of carnivores found that the canids occur in the 3 camera trapping stations, having a greater presence throughout the year, Felidae (13 ± 7.3); Procyonidae (32 ± 7.4); and the Canidae (73 ± 7.3) (Fig. 4).
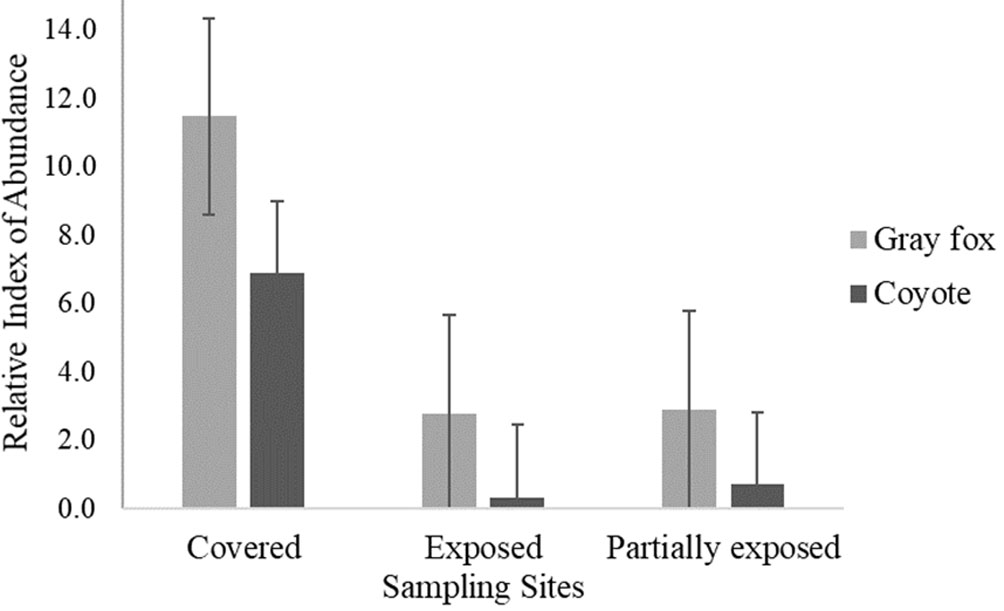
Figure 3 Relative abundance index (RAI) of gray fox and coyote detected at 3 sampling sites at Las Barajitas Canyon.

Figure 4 Presence-absence of the carnivores gray fox (GF), coyote (COYO), bobcat (BOBC), coati (COAT), and ringtail (RING) from the coast to the oasis over a year in Las Barajitas Canyon.
The richest organic matter (OM) detected in the soils sampled was the Partially exposed (Pe) site, but the sample standard deviation does not show significative differences in the characteristics of the chemical soil concentrations (OM, N, P, K) between the 3 sites along the arroyo (10.9 ± 18.9; Table 1).
Table 1 Soil analyses in Las Barajitas Canyon. Covered (C), exposed (E), and partially exposed (Pe). OM: Organic matter, N: Nitrogen, P: Potassium, and K: Phosphorus.
| Characteristics of the soil along the stream | ||||
|---|---|---|---|---|
| Sampling site | % OM | % N total | ppm P | ppm K |
| Covered (C) | 4.78 | 0.38 | 0.98 | 34.84 |
| Exposed (E) | 1.12 | 0.07 | 0.52 | 21.38 |
| Partially exposed (Pe) | 5.06 | 0.36 | 1.21 | 60.45 |
| Mean | 3.65 | 0.27 | 0.91 | 38.89 |
| SD | 2.20 | 0.17 | 0.35 | 19.85 |
The analysis of covariance (ANCOVA) using as covariates W. robusta seeds deposited by canids, RAI of gray fox and coyote, and OM (organic matter) in the soil along the arroyo, did not detect significant differences between sampling sites for W. robusta establishment (α = 0.05, F = 1.104) (Fig. 5).
Discussion
Plant-animal interactions are strong drivers of reciprocal phenotypic evolution, especially at the seed dispersion stage (Fontúrbel & Medel, 2017). Seed dispersion is a food mediated mutualistic relationship, in which the nutritional value of the fruits relays a source of additional nutrients for the animal. The plants in turn have a higher likelihood of deposition in a favorable environment among additional possible advantages (Herrera, 1989). Washingtonia robusta fruits are small but rich in sugars and secondary compounds that increase their consumption by wildlife (Armenta-Méndez et al., 2019). Such fruit characteristics are important for frugivores and may reflect an increase in the number of fruits consumed and consequently the number of seeds dispersed, and swallowed seeds better escape from the grinding of canids that would result in the mutualism becoming frugivory or in the foraging of seeds.
Gray fox and coyote are more active during their reproductive season (late autumn to early spring; Armenta-Méndez et al., 2018; Sillero-Zubiri, 2004), which coincides with the ripeness of the W. robusta fruits. As mentioned by Nuismer et al. (2012) and Kazimierski et al. (2018) seed dispersal patterns and disperser activities are closely related. The abundance of mature fruits at behaviorally critical periods of time for the consumer, such as breeding, can make a plant propagule an important resource and increase the likelihood of its consumption. Demonstrating the viability of ingested seeds, and their dispersion to areas with higher soil humidity, can directly result in better chances for seedling establishment and survival. Pulp removal does not seem to play a relevant role in seed germination and establishment, but the removal of the pulp could prevent that sugars and other nutritious components of the pulp that trigger fruit rotting and serve as clue to pathogens and seed predators (Escribano-Ávila, 2019).
The diets of the Canidae vary widely as some are hyper-carnivorous and specialists, while others are more opportunistic generalists but predominately carnivorous. Other species, such as gray fox and coyote, are primarily omnivorous generalists, which favor frugivory and seed dispersion (Fleming et al., 2017). These canids consume ripe fruits (Colubrina californica, Sabal uresana and Ziziphus amole, mostly) from December to February in Las Barajitas Canyon, but the greatest number of seeds found in their scats were of W. robusta.
Gray fox and coyote are wide ranging and mobile species (Sillero-Zubiri, 2004) that frequent Las Barajitas Canyon (Armenta-Méndez et al., 2018). Their home-range size varies and can be influenced by diverse factors like social organization and habitat features, improving fidelity to the home range area that may persist for many years. Dispersal seems to be voluntary as social and nutritional pressures intensify during winter when food becomes limited, dispersal by juveniles usually occurs during autumn and early winter and male foxes also may increase their ranges during spring, probably in response to increased food requirements of more sedentary females and newborn pups (Sillero-Zubiri, 2004). Our findings suggest that a high RAI of gray foxes and coyotes in the covered habitat of the oasis is correlated with greater chances of W. robusta fruit consumption and its subsequent dispersion, according to the biology of the dispersal behavior of these canids as pointed out by Sillero-Zubiri (2004). Within the canyon study site there is a 10 km gradient from the coast inland (Fig. 1). Along this habitat gradient there is a shift in vegetation communities, from open desert scrub to subtropical species, and tropical deciduous forest in the wetter and shaded habitats, which helps hape the nature of the biotic interactions. The more tropical habitats have increased competition between resources and plants (Wehncke & Reyes-Amaya, 2019). Metabolic compounds -e.g., sugars in seeds- have been demonstrated to play a central role in drought protection by increasing tolerance to desiccation. As shown here, Mexican fan palm seeds have 73% sugars in its composition and contain secondary metabolites (high contents of alkaloids and flavonoids, in seeds), which are important in plant resistance against biotic and abiotic stressors faced by seedlings (Armenta-Méndez et al., 2019; Ingram & Bartels, 1996). These attributes in plants are an important characteristic for their establishment (Dirzo, 1985).
The sites for suitable germination of the Mexican fan palm are dynamic. The 3 primary habitats covered (C, with perennial water availability), exposed (E, with no water flow outside of floods), and partially-exposed (Pe, with an intermittent flow for several weeks or months after the monsoon storms) frequently change in their exact location. Heavy storm flows are a core driver of the ecological dynamics of this canyon. Historical records are sparse, though the frequency of great precipitation events (tropical storms and hurricanes in the late summer and fall months) have become recently more frequent from the decade of the 2000s to a recent string of tropical storms and hurricanes that have hit the coast of Sonora and impacted in the Sierra El Aguaje: Lowell and Norbert in 2008, Jimena in 2009, Georgette in 2010, Norman in 2012, Newton in 2016, and Lidia in 2017, with an average in precipitation of 163 mm and winds of 97 km/h (REMAS, 2018). When the flood events occur, the main arroyo swells and changes the landscape and location of the habitats, moving the seeds to different places (Fig. 6). In addition, these occasional water pulses create positive conditions for seed germination, and soil denitrification due to increased soil moisture (Wehncke et al., 2013). It is estimated that 95% of the lower canyon water availability is from such ephemeral events (Bogan, 2017 personal communication).
The Mexican fan palm is restricted to seeps, springs, and arroyos with persistent surface or subsurface flow. The scattering of this species' seeds by gray foxes and coyotes along the arroyo increases the chances of establishment as well as allowing the colonization of new sites. These species also disperse the palm seeds away from their parental neighborhood, where both intraspecific competition (with siblings and parent) and external attack (from species-specific predators, parasites, and pathogens) are prevalent (Franklin et al., 2016). For any given plant, a variety of visitors is likely to include pollinators or dispersers, which differ in effectiveness; we observed that white-nosed coati (Nasua narica) are abundant in the palm oases. Coatis consume W. robusta fruits and defecate the seeds near parent trees and play a role in palm recruitment within oases. These processes, within and between oases, are of great demographic importance for palm populations and their genetic diversity.
Mexican fan palm is a keystone species in the oases where they thrive. They are the dominant vegetation, and centers forbirds and mammals through providing shelter and resource concentration (Felger et al., 2017). Washingtonia palm oases can be considered an archipelago habitat with specific environmental conditions surrounded by desert scrub (Villanueva-Almanza et al., 2018). Many species of mammals, reptiles, insects, migratory and resident birds, including raptors, have been observed to use canyons as sources of water and food, taking seeds in their movements from lowlands to uplands and connecting isolated palm populations (Wehncke et al., 2009; and records of camera traps from this study).
Mutualisms play a key role in biodiversity (Franklin et al., 2016). Seed dispersal by canids of W. robusta is likely critical in increasing the possibilities for the successful establishment of new stands of this relict native Sonoran Desert palm. This dependable interaction, use, and subsequent dispersion of the palm fruits during periods of reproduction, helps maintain the population structure of this species in an inherently dynamic ecosystem.











 text new page (beta)
text new page (beta)




