Introduction
The whale shark (Rhincodon typus) is the world’s largest filter feeding shark, with individuals sighted in numerous regions across the globe (Compagno, 2001; Rowat & Brooks, 2012). The species is mainly solitary, spending the majority of its life migrating from tropical to sub-tropical waters seemingly navigating along scent trails that lead them to areas of intense productivity, such as coastal upwelling zones, continental shelves and submerged seamounts (Beckley et al., 1997; Ketchum et al., 2013). In spite of this solitary lifestyle, these sharks are known to regularly form almost entirely sex-segregated aggregations in coastal waters (Colman, 1997; Rowat et al., 2011). These aggregations are usually on a seasonal basis, but at times can be sporadic, and typically lie between latitudes 30° N, 35°S (Compagno, 2001). From current research, it seems to be clear that these aggregations are often linked to certain productivity events such as fish spawning (Heyman et al., 2001) or blooms of zooplanktonic species (Clark & Nelson, 1997; Hacohen-Domené et al., 2006; Hernández-Nava & Álvarez-Borrego, 2013; Lavaniegos et al., 2012; Wilson et al., 2001). Bahia de La Paz is located in the southern part of Baja California peninsula and is an almost entirely enclosed bay with high levels of both primary (Reyes-Salinas et al., 2003) and secondary productivity (De Silva-Dávila & Palomares-García, 1998) supporting an array of filter feeding marine fauna such as: sardines, manta rays and whale sharks (De Silva-Dávila & Palomares-García, 1998; Ketchum et al., 2013). The oceanographic circulation within this enormous bay is distributed by a central mesoscale cyclonic eddy, which influences the complete horizontal distribution of all trophic groups of zooplankton in the bay (Durán-Campos et al., 2015). Additionally, the bay is known to have seasonal tendencies, which rotate this eddy in an anti-clockwise direction during the winter months (November- March) and in the summer months (May- August) in a clockwise direction. The bay also demonstrates transitional periods throughout the year, where environmental conditions are shifting between these seasonal tendencies (Durán-Campos et al., 2015; Ketchum et al., 2013; Robles-Pacheco & Marinone, 1987). The area to the southern part of the bay, encompasses a protruding sandbar attached at one end to the mainland and stretching out approximately 12 km parallel to the mouth of the bay, known as the El Mogote sandbar (León-de la Luz et al., 2006). The natural formation of this sandbar has created almost 2 separate bodies of water: the main bay itself and the inlet or lagoon known as the Ensenada de la Paz (León-de la Luz et al., 2006). It has approximately 16 km2 of surface vegetation comprised mainly of a sandy coastal environment with periodic patches of mangroves on its inlet side (León-de la Luz et al., 2006). This region of Bahía de La Paz is thought to be influenced dramatically by its orientation to the mouth of the bay and changing environmental factors which occur throughout the year, such as seasonal changes in wind direction and magnitude (Durán-Campos et al., 2015). This highly productive coastal zone is known as the principal area for seasonally aggregating whale sharks (Ketchum et al., 2013; Ramírez-Macías et al., 2012; Whitehead et al., 2019). The size of these annual aggregations of juvenile whale sharks is suspected to be around 70 individuals (Ramírez-Macías et al., 2012), but this number seems to vary among seasons (Whitehead et al., 2019). Eco-tourism activities involving whale sharks have existed for more than a decade in Bahia de La Paz and concerns are now growing with regards to the effect it may be having on this internationally endangered species. In recent years, pressures from uncontrolled tourism have motivated authorities to implement an area of protection and site-specific regulations for these activities, as a means to regulate this ever-growing industry (Semarnat, 2017; Whitehead et al., 2019). A better understanding of the environmental conditions and productivity of available food that drive whale shark foraging events may help to improve the conservation and management of the species in this region and ensure that whale sharks have access to this vital food source without disturbance.
The feeding mechanism of whale sharks is designed to capture their food supply by the filtering of water through special filter pads (Motta et al., 2010). An early dissection of a whale shark in South Africa revealed that there is a transverse band of a spongy filtration tissue within each gill slit of the species (Beckley et al., 1997). Each pore in this uniquely designed tissue is effectively a tiny canal that is connected to another directly under the surface to form a much larger passage, which eventually opens into the branchial cavity underneath the branchial arches (Motta et al., 2010). The overall function of these filtration pads is based upon this pore structure, allowing smaller items of prey to pass through with the water as it is swept over the gills which are then discharged through the gill slits, while larger prey items are trapped and ultimately directed down the small central gullet (Paig-Tran et al., 2011). The filtration of food particles in whale sharks is believed to be different dependant on the maturity of the species due to the development of the filtration system in the species (Paig-Tran & Summers, 2014), with adult whale sharks reported to feed primarily through a form of cross-flow filtration (Motta et al., 2010; Paig-Tran et al., 2011) and smaller neonate sharks through the collection of particles in their esophagus, given the lack of development (Paig-Tran et al., 2011, 2014).
Whale sharks are well-known to display multiple types of foraging behaviours presumably related to differing concentrations of available food sources (Hacohen-Domené et al., 2006; Ketchum et al., 2013; Motta et al., 2010). In many feeding aggregations, the most frequently observed feeding behaviour is active surface feeding (Clark & Nelson, 1997). During this behaviour the species may swim almost at the surface with its mouth open and slightly lifted out of the water, repeatedly opening and closing its mouth driving water and food items over its filtering apparatus (Clark & Nelson, 1997; Heyman et al., 2001; Motta et al., 2010). Other occasions when food concentrations are higher, suction or vertical feeding might occur, where the species could be observed in a diagonal or an almost vertical position, while generating a suction in its pharynx drawing in large volumes of water and prey (Motta et al., 2010; Nelson & Eckert, 2007). Last of all, passive feeding can be witnessed within the species and may be simply described as the animal swimming slowly through the water with their mouth marginally open, presumably filtering the scarce prey items, closing its mouth every few minutes with the shark appearing to swallow (Heyman et al., 2001). Examinations of the composition of zooplanktonic organisms present in Bahia de la Paz have shown that a number of species show a higher level of dominance during certain times of the year (Clark & Nelson, 1997; Hacohen-Domené et al., 2006). An early study by Clark and Nelson (1997) observed the foraging of juvenile whale sharks in Bahia de La Paz. From this early observational study of whale sharks, feeding behaviours linked to high concentrations of copepod species comprised mainly of Acartia clausi and Acartia sp. Ketchum et al. (2013) examined the foraging ecology of whale sharks in Bahía de La Paz and observed that the zooplankton biomass varies seasonally, with the lowest being observed in May and June and peaks of biomass occurring in November and February. Furthermore, juvenile sharks were observed foraging on dense copepod swarms in shallow waters compared to adult sharks foraging in deeper waters on euphausiids. Previous work on the foraging ecology of the species (Ketchum et al., 2013) and composition of prey items in the presence and absence of sharks (Hacohen-Domené et al., 2006) have been successful in achieving specific objectives, but have focused sampling both inside and outside of the bay and not exclusively in the coastal waters of the El Mogote where the main aggregation area for juvenile whale sharks exists. The aim of this study was to investigate whether or not whale shark foraging behaviours are related to the density or concentration of available zooplanktonic prey items specifically in the El Mogote region of Bahia de La Paz and provide the first documented evidence which will offer important information on the feeding habitats of the species in this coastal zone for the conservation and management of this resource.
Materials and methods
Zooplankton samples were obtained monthly from October 2016 to March 2017 from Bahia de La Paz in both, the absence and presence of foraging juvenile whale sharks. A total of 32 surface plankton tows were obtained in the absence of feeding whale sharks from 4 sampling stations (8 at each station) and registered by the global positioning system (GPS). The sampling stations were distributed throughout the known aggregation area along the coastal waters of the El Mogote sandbar where whale sharks are reported during the season (Fig.1). Zooplankton samples were collected using a 505 μm mesh plankton net, which was towed for 5 minutes behind the boat in a circular motion at approximately 1-1.5 km h−1. Upon the net being removed from the water, collected zooplankton were washed down into the collecting container at the end, fixed with 4% formalin solution, labelled for identification and stored in plastic screw top containers.

Figure 1 Bahía de La Paz in the Gulf of California (inset map) shown in main perimeter. Non-feeding sample stations (▲) and locations of sampled feeding events (O) of whale sharks.
Zooplankton samples were also taken in the presence of foraging whale sharks. A total of 12 samples were taken during the presence of feeding whale sharks in the same area as previously mentioned (Fig.1). Once encountering a whale shark, observers on-board confirmed that the animals were either active surface feeding or vertical suction feeding using the description of behavioural characteristics currently available in literature (Clark & Nelson, 1997; Motta et al. 2010; Nelson & Eckert, 2007). Following the confirmation of shark feeding, the research boat approached slowly and the animal’s GPS position was registered. Next, the plankton net was lowered into the water and for vertical feeding events, a tow for 5 minutes was conducted in a circular motion around the animal at a distance of 5-10m. For incidences of active surface feeding the net was towed alongside the sharks for the same amount of time.
The total zooplankton biomass was determined by using the displacement method (Beers, 1976) and standard biomass calculations (Smith & Richards, 1977). Zooplankton was grouped into 5 main taxonomic groups: Copepoda, Chaetognatha, Euphausiidae, Decapoda, and “others”, following previous plankton work from the same region and other sites in Mexican waters (Franco-Gordo et al., 2016; Ketchum et al., 2013). After zooplankton was assembled into groups, individuals were counted to obtain the total number of individuals for each sample and ultimately an overall relative abundance of each group for each sample collected. Qualitative analysis of zooplankton samples was focused on zooplankton biomass (g m-3) and the number of individuals (núm. m-3) present in each taxonomic group within each sample, to gain information on the composition of zooplankton members between present and non-present samples. Differences in zooplankton biomass and number of individuals between the presence and absence of feeding whale sharks were tested using a Welch 2 sample t-test for unequal sample sizes and unequal variances (Zar, 2010). All statistical analyses were conducted in the statistical program R (R Development Core Team, 2018).
Results
Zooplankton biomass was 1.6 times greater where whale sharks were observed feeding and the mean zooplankton biomass was significantly greater compared to samples where sharks were not feeding (Table 1, t = -3.21, p < 0.05). Individuals for the taxonomic groups of copepods and chaetognaths were predominant in all samples, both in the presence and absence of feeding whale sharks. During the feeding whale shark season, chaetognaths were the most predominant, followed by members of copepods. While in the absence of feeding whale sharks, copepods were the most predominant, followed by chaetognaths (Fig. 2; Table 1). Although the mean number of individuals of all zooplankton taxonomic groups per m3 in the presence of feeding whale sharks was 2.5 times greater than in the absence of feeding, it was not statistically different (Table 1, t = -1.70, p > 0.05).
Table 1 Mean (SE) zooplankton biomass (g m-3) and mean (SE) number of individuals (núm. m-3) present in each taxonomic group of non-feeding stations (stations A-D) and whale shark feeding samples.
| Station | Copepods (núm. m-3) | Chaetognaths (núm. m-3) | Euphausiidae (núm. m-3) | Decapoda (núm. m-3) | Others (núm. m-3) | All taxonomic groups (núm. m-3) | Biomass (g m-3) | n |
| A | 2,441.25 (1,164.03) | 1,252.5 (311.29) | 123.75 (41.44) | 705 (194.67) | 731.25 (225.99) | 5,253.75 (1,068.92) | 65.54 (2.68) | 8 |
| B | 3,022.5 (2,072.86) | 2,583.75 (837.93) | 232.5 (134.13) | 686.25 (248.51) | 915 (347.13) | 7,440 (2,471.5) | 69.41 (3.52) | 8 |
| C | 686.25 (298.73) | 1,136.25 (428.46) | 453.75 (172.19) | 825 (396.7) | 1,155 (155.78) | 4,256.25 (1,172.5) | 62.35 (3.9) | 8 |
| D | 1,976.25 (1,244.36) | 2,433.75 (823.85) | 457.5 (110.77) | 1,218.75 (550.6) | 1,717.5 (475.17) | 7,803.75 (2,018.41) | 58.39 (3.39) | 8 |
| All non-feeding stations | 2,031.56 (659.84) | 1,851.56 (328.42) | 316.87 (64.4) | 858.75 (181.95) | 1,129.68 (168.06) | 6,188.43 (887.55) | 63.92 (1.77) | 32 |
| Whale shark feeding | 5,869.16 (2,011.41) | 6,186.58 (2,525.86) | 336.66 (141.5) | 2,955.08 (1144.73) | 266.33 (141.29) | 15,628.33 (5,454.50) | 103.83 (12.26) | 12 |
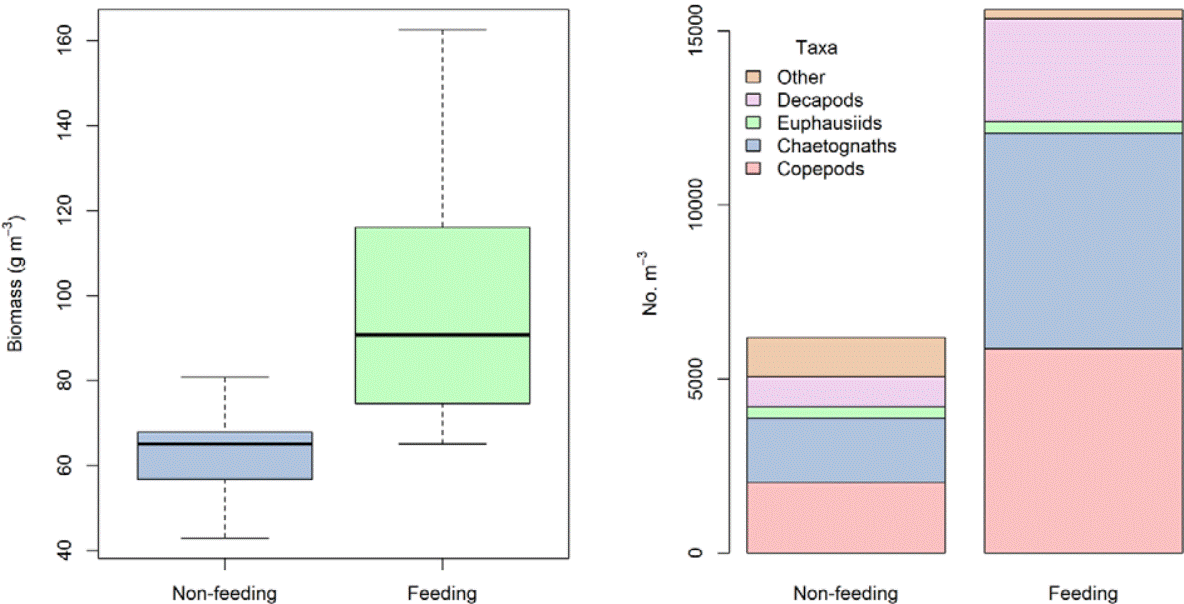
Figure 2 Biomass (left panel) and mean absolute values of the contributions of each taxonomic group to the total number of individuals (right panel) in feeding and non-feeding events of whale sharks.
In the presence of feeding whale sharks the number of copepods, chaetognaths, euphausiids and decapods was 2.8, 3.3, 1.06 and 3.4 times greater than in the absence of feeding whale sharks, respectively. No significant differences were observed in the mean number of individuals of copepods (t = -1.81, p > 0.05), chaetognaths (t = -1.70, p > 0.05), euphausiids (t = -0.12, p > 0.05) and decapods (t = -1.80, p > 0.05) between the presence and absence of feeding whale sharks. Ultimately, the taxonomic group “other” was observed to be 4.2 times greater in absence of feeding whale sharks than in the presence of feeding whale sharks with significant differences in the mean number of individuals (t = 3.93, p < 0.05).
Discussion
From the majority of work focused on the prey preference of whale sharks, it is correct to assume that seasonal aggregations of whale sharks are often linked or related to productivity and the availability of zooplanktonic organisms (Clark & Nelson, 1997; Hacohen-Domené et al., 2006; Heyman et al., 2001; Ketchum et al., 2013; Motta et al., 2010). In our results, we observed that the total zooplankton biomass was notably greater with as much as 1.6 times during feeding events, in relation to non-feeding events. This confirms the theory that whale shark feeding events are related to the densities of available food and theoretically the species search out patches of high concentration of prey to ensure optimal feeding. In a high number of sites, aggregations of whale sharks have been linked to (or somewhat in response to) zooplankton blooms or fish spawning events such as in the Seychelles (Rowat et al., 2011), Belize (Heyman et al., 2001), Australia (Meekan et al., 2009), Tanzania (Rohner et al., 2015), Qatar (Robinson et al., 2013) as well as the Mexican Caribbean (De la Parra-Venegas et al., 2011). From earlier work on the diet of whale sharks, we know that foraging events may, to some extent, be related to concentrations of prey items and feeding behaviours are intriguingly different with varying densities of planktonic food sources (Clark & Nelson, 1997; Motta et al., 2010; Nelson & Eckert, 2007; Rohner et al., 2015).
Composition of the different groups of prey items in our results for both samples where sharks were observed in feeding and non-feeding activity, showed the dominance of 2 taxonomic groups (copepods and chaetognaths) in all collected samples. This may provide more evidence that verifies that in Bahia de La Paz for the most part, 2 main taxonomic groups of zooplankton are the most abundant on a seasonal basis and an important dietary item for whale sharks. Previous work of the foraging behaviors of the species in Bahia de la Paz (Hacohen-Domené et al., 2006; Ketchum et al., 2013) and studies in the other regions of the bay away from the El Mogote (Clark & Nelson, 1997) also observed seasonal aggregations of whale sharks foraging on copepods. Furthermore, studies from other sampling sites in the Gulf of California, where whale sharks were also witnessed foraging, similarly confirms the species to be drawn to dense patches of plankton, primarily composed of copepods (Nelson & Eckert, 2007).
A detailed examination of the mean values for the number of individuals of all zooplankton taxonomic groups during the presence of feeding whale sharks demonstrated that although the biomass was 2.5 times greater during the presence than in the absence of feeding whale sharks, it was not significantly different (Table 1, t = -1.70, p > 0.05). This may simply indicate that in such a focused small sample area with sample stations separated by only a short distance, environmental factors such as sea surface current and wind direction may influence the biomass and composition tendencies of available prey items. Previous work that examined environmental factors present in this region, demonstrated that its connectivity with the Gulf of California and an important central mesoscale cyclonic eddy in the bay itself, can influence the complete horizontal distribution of all trophic groups of zooplankton (Durán-Campos et al., 2015; Monreal-Gómez et al., 2001). We also observed that in the presence of feeding whale sharks the number of copepods, chaetognaths, euphausiids and decapods species were 2.8, 3.3, 1.06 and 3.4 times greater than in the absence of feeding whale sharks, supporting the existing evidence that whale sharks opt to forage on certain taxonomic groups (Hacohen-Domené et al., 2006; Ketchum et al., 2013; Nelson & Clark, 1997; Rohner et al., 2015). While our study confirms the findings of previous studies (Hacohen et al., 2006; Ketchum et al., 2013; Rohner et al., 2015) we observed that whale sharks clearly feed on patches of food dominated by a certain food source, but this evidence must be taken with caution. It is probable that the species do not show prey selectivity, but rather foraging habits that solely target high concentrations of available food in areas where the composition changes on a seasonal or even daily basis, due to constantly changing environmental factors. In recent years, whale shark related tourism in the entire Gulf of California has increased government authorities´ motivation to provide site specific regulations and management plans for this thriving industry that relies on a number of variables that must be adequately managed, ensuring that the interactions between participants and the species are beneficial for both. Given the limited information available for this coastal region and the lack of focused studies in the El Mogote zone, this work provides important information on the presence of certain food sources and its relation to the foraging behaviors of the species and a platform for management recommendations to ensure that the species are not disturbed during these important foraging events.
In conclusion, we can report that within the El Mogote region of Bahia de La Paz whale shark foraging behaviours occur during higher concentrations of zooplankton biomass and that the species may potentially target dense patches of both copepods and chaetognaths species as a primary prey source.











 nova página do texto(beta)
nova página do texto(beta)


