Introduction
Salvia L. (Lamiaceae: Nepetoideae) is the biggest genus of the labiates in terms of the species it comprises, about 1,000 worldwide (Catalogue of Life, 2018; Harley et al., 2004; Hsi-Wen & Hedge, 1994), being almost cosmopolitan in distribution excluding only the higher areas in altitude and latitude (Hedge, 1992; Harley et al., 2004). In addition to its richness and wide distribution, the genus is one of the most charismatic within the family and is frequently used to illustrate its morphology. Salvia stands out due to its distinctive staminal architecture and functionality, the so-called lever mechanism, which consists of a connective attached to the filament by means of a loose junction, allowing the descent of the anterior portion (including the thecae) once the posterior one is pushed back by the pollinator when trying to access the nectar (Claßen-Bockhoff et al., 2003). Through this mechanism, the thecae remain protected under the upper corolla lip until the movement is triggered; then they descend and deposit pollen on the pollinator’s body. The evolution of this characteristic is intricate, involving several evolutionary routes, some of them even consisting of the loss of the mechanism during pollinator transitions from bees/insects to birds (Claßen-Bockhoff et al., 2004; Drew & Sytsma, 2012; Fragoso-Martínez et al., 2018; Kriebel et al., 2019; Wester & Claßen-Bockhoff, 2006, 2007, 2011).
Mexico is the main center of diversity and diversification of Salvia (Hedge, 1992; Martínez-Gordillo, Bedolla-García et al., 2017; Ramamoorthy & Elliott, 1998); the country has even served as a source for the spread of Salvia to South America (Jenks et al., 2013). The genus is emblematic in Mexico because it grows in almost every type of vegetation, from sea level to around 4,000 m, and it is usually a dominant or abundant element in different ecosystems (González- Gallegos et al., 2016; Ramamoorthy & Elliott, 1998). In a recent checklist, 306 Salvia species have been registered for the country (Martínez-Gordillo, Bedolla-García et al., 2017), a number that continues to rise with the addition of new species (Martínez-Gordillo, Sandoval-Gutiérrez et al., 2017). Our present paper is also a contribution to the diversity of the genus: we describe and illustrate a new Salvia species from Sonora, Mexico. It was discovered during the thorough explorations for the inventory of the floristic diversity in the Guaymas region, especially those conducted near San Carlos (Felger et al., 2017a, b).
Materials and methods
The herbarium specimens of the new species were collected while conducting explorations for the botanical inventory of the Guaymas region, Sonora. The specimens were gathered in April and September, 2018, and they were herborized and prepared according to standard procedures (Lot & Chiang, 1986). Photographs of the habitat, habit, leaves and flowers were taken in the field. The samples were morphologically examined with the help of a Zeiss Stemi 508 dissecting microscope and thoroughly analyzed with reference to specialized literature (Epling, 1939, 1940, 1941, 1944, 1947, 1951, 1960; Epling & Játiva, 1963, 1966, 1968; Epling & Mathias, 1957) for identification. Once the Salvia was determined to be a new species, qualitative and quantitative characters were evaluated and recorded to prepare the description of the new taxon.
Description
Salvia palmetorum J.G. González & Carnahan sp. nov. (Figs. 1-3)

Figure 1 Salvia palmetorum J.G. González & Carnahan. A) Habitat; B) general habit; C) leaves; D) flower close up; E), F) floral visitors, Apodemia mejicanus and Campsomeris sp., respectively, note the staminal lever mechanism triggered by the wasp (pictures taken by SD Carnahan).

Figure 2 Salvia palmetorum J.G. González & Carnahan. A) Portion of a flowering branch; B) half portion of a floral node; C) flora bract (left) and bracteole (right); D) dissected calyx, flattened and with the indumentum removed in order to facilitate appreciation of vein arrangement and trimucronate upper lip; E) staminode; F) connective and theca; G) upper section of the style including the stigmatic branches; H) mericarp (illustration made by JG González-Gallegos based on the holotype).
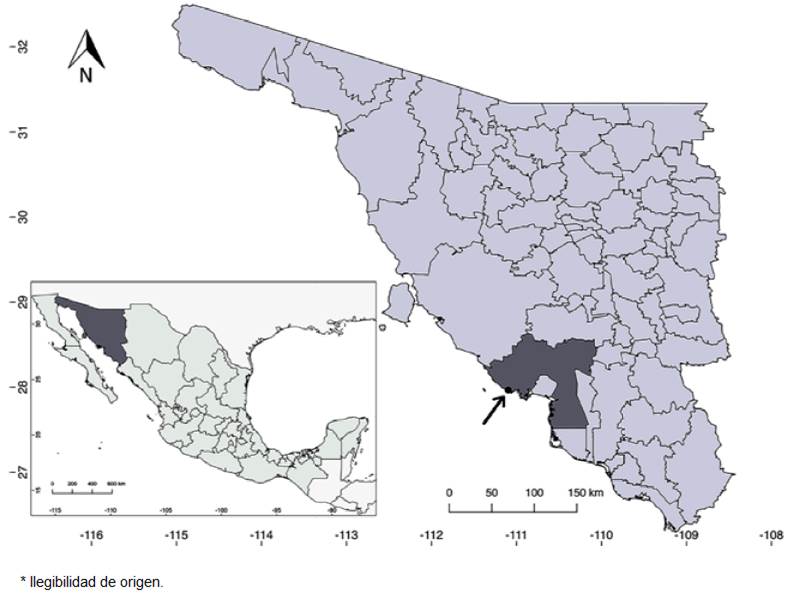
Figure 3 Distribution map of Salvia palmetorum J.G. González & Carnahan (black dot). At bottom left, Sonora is highlighted in darker gray; in the upper right, the state is shown with Guaymas county highlighted in darker gray.
Salvia palmetorum belongs to Salvia subg. Calosphace according to its staminal morphology. It is a very distinctive species within this group due to the combination of densely white-tomentulose stems, petioles, lower surface of the leaf blade and inflorescence axis, flowers arranged in thyrses with peduncles gradually reduced towards the inflorescence apex, presence of bracteoles at the base of each pedicel, trimucronate upper calyx lip, and connective ventrally ornate with an antrorse obtuse tooth (geniculate). It is also ecologically unusual because there are not many Mexican Salvia growing in palm groves.
Shrub, 1.5-2 m tall, profusely branched, stems densely white tomentulose, light amber glandular dots intermixed with the hairs. Leaves with petioles (1.8-) 9-13.3 mm long, covered with the same indumentum as the stems; leaf blade deltoid to rhomboid, (9.1-) 12-24.2 × (4.5-) 10-22 mm, apex acute, base short cuneate, margin serrate (2-3 teeth on each side), puberulent above, densely white-tomentulose beneath and covered with light amber glandular dots; leaves gradually reduced towards the inflorescences and becoming oblong and entire. Inflorescence in thyrses 39- 58 cm long, peduncles mostly ranging from 0.4-4 mm, progressively reduced towards inflorescence apex, 17-32 floral nodes, these 6-12-flowered, the lowermost nodes 2.1-4.5 cm apart from each other, floral axis densely white-tomentulose and covered with light amber glandular dots. Floral bract linear-lanceolate to oblong, 1.5-3.2 (-6.5) × 0.2-0.5 (-0.7) mm, deciduous, apex acute, base truncate, margin entire, indumentum similar to that of the leaves; bracteoles present at the base of each pedicel, linear-lanceolate, 0.8-1.2 × 0.3-0.7 (-1.3) mm, apex acute, base truncate, margin entire, indumentum similar to that of the leaves. Flowers with pedicels (0.8-) 1.5-2.3 mm long, densely white-tomentulose and hirsute, and covered with light amber glandular dots. Calyx 4.2-5.8 × (1.9-) 2.5-2.8 mm, externally hirsute with simple and glandular- capitate hairs of 2 sizes, 0.1-0.2 mm and 0.4-0.6 mm long, internally short pilose with the hairs concentrated on the veins, lips acute, the upper one 5-veined and trimucronate at apex. Corolla white at the base and progressively blue towards the lips, with white nectar guides on the lower lip, sparsely short pilose, the hairs concentrated in the upper lip and ventral portion of the lower one, those of the upper lip simple and glandular-capitate, tube with light amber glandular dots; tube 4.7-6.4 × 1.8-2.8 mm, non-ventricose, slightly widened towards the throat, straight at base and internally epapillate; upper lip (2.7-) 3.8-4.8 mm long; lower lip 2.7-5.2 × 3.2-4.6 mm. Stamen included; filament 1.2-1.5 mm long; connective (4.5-) 5.7-6.4 mm long, ventrally ornate with an antrorse obtuse tooth (geniculate); theca (0.9-) 1.2-1.5 mm long; a pair of staminodes above and behind filament insertion, 1.1-1.2 mm long, ornate with a tooth near basal portion. Gynobasic horn 0.7-0.8 mm long; style 8.4-10.1 mm long, short pilose towards the apex, the hairs concentrated on ventral portion, lower stigmatic branch acute. Mericarp ovoid, 1.3-1.5 × 1.1-1.3 mm, dark brown, glabrous, smooth.
Taxonomic summary
Type: Mexico. Sonora, Guaymas county, San Carlos, El Palmar, sabal palm grove, between Puente El Palmar and dunes at Playa Los Algodones, 27.966 N 111.097 W, 5 m elevation, September 30, 2018 (fl, fr), S. Carnahan 3444 (Holotype: CIIDIR; isotypes: ARIZ, IBUG, MEXU, USON).
Additional specimens examined (paratypes): Mexico. Sonora, Guaymas county, San Carlos, El Palmar, between Puente El Palmar and dunes at Playa Los Algodones, 3 km NNW of Cerro Tetas de Cabra peak, 27.966 N 111.097 W, 5 m elevation, April 7, 2018 (fl, fr), S. Carnahan 2966 (ARIZ, CIIDIR, USON).
Distribution, habitat and phenology: Salvia palmetorum is known from only 1 locality near the town of San Carlos, in Guaymas County, Sonora (Fig. 3). It grows about 500 m from the coastline, in a palmetto grove in silty soil. It shares habitat with Abutilon incanum (Link) Sweet, Bebbia juncea (Benth.) Greene, Bonellia macrocarpa (Cav.) B. Ståhl & Källersjö, Cenchrus ciliaris L., Cocculus diversifolius DC., Cynodon dactylon (L.) Pers., Encelia farinosa A. Gray ex Torr., Forchhammeria watsonii Rose, Guaiacum coulteri A. Gray, Melinis repens (Willd.) Zizka, Pisonia capitata (S. Watson) Standl., Prosopis glandulosa Torr., Sabal uresana Trel., Struthanthus palmeri Kuijt, Tamarix aphylla (L.) H. Karst., Tephrosia tenella A. Gray, and Tournefortia hartwegiana Steud. It has been observed in flower and fruit in April, June and September. It is not known if flowering also occurs during the winter (i.e., between October and March); perhaps it is able to flower whenever the humidity is adequate. The flowers have been visited by Apodemia mejicanus Behr, 1865, (Mexican metalmark) and a wasp of the genus Campsomeris Lepeletier, 1838 (Fig. 1E, F).
Conservation status: based on the current information available and IUCN criteria (2012), Salvia palmetorum should be classified as critically endangered because the area of occupancy is less than 4 km2, the plant is known only from 1 location, and there are fewer than 50 mature individuals. In addition, real estate growth driven by the tourism in San Carlos might threaten the habitat of the species in the near future. However, it is strange that, although the area has been extensively explored, no other plants have been discovered, which raises the possibility that this locality is marginal and that more plants would eventually be found at higher elevations in the nearby mountains. Unfortunately, these peaks have not been botanically explored, and they are steep and rugged, with few or no trails into the high country. It is crucial to encourage the exploration of these areas in order to know if Salvia palmetorum has a wider distribution and additional populations.
Etymology: the specific epithet refers to the known habitat of the species; it is translated as the sage of the palm groves.
Remarks
Salvia palmetorum fits better within the circumscription of Salvia sect. Tomentellae (Epling) Epling than within any of the other sections in Salvia subg. Calosphace (Benth.) Epling (Epling, 1939). This section includes shrubs and subshrubs, generally tomentose with branched hairs, with deltoid-ovate or oblong leaves, sometimes persistent floral bracts, calyces covered with glandular dots, frequently obscurely trimucronate upper calyx lip, blue corollas, ventricose corolla tube, internally naked or ornate with 2 folds, obscurely invaginated, pubescent upper corolla lip, connective ornate with a deltoid tooth, pilose or glabrous style, much longer upper stigmatic branch, and frequently pubescent mericarps. Salvia palmetorum differs a little because of the absence of branched hairs (although Salvia ballotiflora Benth., also lacks these) and the non-ventricose corolla tubes. It is worth noting that most of the sections traditionally recognized in Salvia subg. Calosphace are artificial, so their use is just provisional; for example, the species of Tomentellae included in phylogenetic analyses do not form a monophyletic group (Fragoso-Martínez et al., 2018; Jenks et al., 2013).
Salvia palmetorum clearly belongs in sect. Tomentellae; however, it does not show a close morphological match to any particular species in the section. It would fall closest to S. ballotiflora because of the absence of branched hairs in both species. Nonetheless, S. palmetorum presents a combination of characters that are unique: densely white-tomentulose pubescence, presence of bracteoles, flowers arranged in thyrses, trimucronate upper calyx lip and ornate connective with an antrorse obtuse tooth (Figs. 1, 2). The dense tomentulose pubescence in the stems, entirely hiding the surface, is present in only another Mexican species, S. cedrosensis Greene [sect. Flocculosae (Epling) Epling]; however, in the latter the hairs are branched instead of simple, and unlike S. palmetorum, its leaves are sparsely pubescent. The tomentose or tomentulose pubescence is more frequently found on the leaves than on the stems in Mexican Salvia. For example, although S. dichlamys Epling (sect. Fulgentes Epling), S. pannosa Fernald (sect. Scorodonia) and S. pruinosa Fernald [sect. Tomentellae (Epling) Epling] have no tomentulose stems, the pubescence in the lower leaf surface of their leaves is similar to that found in S. palmetorum. The bracteoles are known from a few species; all the members of sect. Sigmoideae Epling possess them, along with S. ibugana (sect. Angulatae Epling), S. lasiantha (sect. Mitratae Epling) and S. pringlei B.L. Rob. & Greenm. [sect. Tubiflorae (Epling) Epling] (González-Gallegos, 2015; González-Gallegos & Castro-Castro, 2013; González- Gallegos et al., 2016). However, none of the latter present with a trimucronate upper calyx lip. There are only 2 other Mexican Salvia with inflorescences in thyrses: S. chalarothyrsa Fernald and S. thyrsiflora Benth.; both also belong to sect. Sigmoideae but lack the trimucronate upper calyx lip. This last character is present in most of the species in sect. Uliginosae (Epling) Epling and is also shared with species from other sections, including for example S. axillaris Moc. & Sessé [sect. Axillares (Benth.) Epling], S. concolor Lamb. ex Benth. [sect. Dusenostachys (Epling) Epling], S. purpusii Brandegee (sect. Purpusiana Epling), and several species from sections Blakea Epling, Hastatae (Benth.) Epling, Incarnatae Epling, Microsphace (Briquet) Epling, Standleyana Epling and Tomentellae. Nevertheless, none of the above species has thyrses or densely white-tomentulose stems. Besides, S. palmetorum is distinguished from the species in sect. Uliginosae because it does not have a swollen style at apex. Finally, geniculate connectives are also shared with the species of other sections, for example Sigmoideae and Uliginosae, but these species lack either the thyrses or the trimucronate upper calyx lip.
Additionally, there are not many Mexican Salvia growing at elevations below 100 m, much less in palm groves. Salvia ibugana J.G. González is the only species inhabiting palm groves in Western Mexico, and it occurs at higher elevations (550-660 m) (González-Gallegos et al., 2016). Only about 5% of the Mexican species of Salvia subg. Calosphace have been recorded at elevations below 100 m: S. aliena Greene, S. cedrosensis, S. coccinea Buc’hoz ex Etl., S. herbacea Benth., S. languidula Fernald, S. lasiocephala Hook. & Arn., S. misella Kunth, S. platycheila A. Gray, S. podadena Briq., S. prasiifolia Benth., S. serotina L., S. similis Brandegee, S. tiliifolia Vahl and S. tonalensis Brandegee.
Salvia palmetorum is an interesting addition to the flowering plant diversity in Sonora, and it constitutes the first Salvia endemic to this state. The latter is important considering that endemic plant species are relatively scarce in Sonora, with only 1.85% (68 species) of vascular plants restricted to the state (Van Devender et al., 2010).











 nueva página del texto (beta)
nueva página del texto (beta)


