Introduction
Cacti (Cactaceae) are a predominant component of arid and semi-arid landscapes in the New World. Their modified stems allow them to perform photosynthesis and store humidity in environments where moisture is usually a limited resource. For this reason, cacti in process of necrosis represent a great opportunity for the reincorporation of humidity back into the ecosystem, providing, for a brief period, the chance for the sustainment of a wide variety of invertebrates from different guilds (Ferro et al., 2013). Water scavenger beetles (Coleoptera: Hydrophiloidea: Hydrophilidae) are typically associated to a variety of water bodies (Hansen, 1991). Nevertheless, members of Sphaeridiinae, the most derived and probably most speciose subfamily in Hydrophilidae (Hansen, 1991; Short & Fikáček, 2013) are primarily found outside of water, almost wherever there is organic material in process of decomposition with enough content of humidity. Among the most common microenvironments where sphaeridiines can be collected are mammal excrements (Archangelsky, 1997; Arriaga-Varela, Seidel et al., 2018; Sowig et al., 1997), humid leaf litter (Fikáček & Short, 2006), inflorescences of Araceae and Heliconiaceae (Arriaga-Varela, Wong et al., 2018) and debris accumulated by social insects in or near their nests (Fikáček et al., 2013; Spangler, 1962). Few sphaeridiine representatives have colonized arid zones and are associated to necrotic tissues of succulent plants. Among the New World hydrophilids associated to rotten cacti we can find representatives of 4 genera: Dactylosternum Wollaston, 1854, Cryptopleurum Mulsant, 1844, Pelosoma Mulsant, 1844 and Agna Smetana, 1978. Agna stands as the only sphaeridiine genus presumably strictly associated to necrotic tissue of succulent plants, mainly Cactaceae (Smetana, 1978). Its 2 closely similar Nearctic species were classified as members of Pelosoma until Smetana (1978) revealed that they differ from the latter by few crucial characters, namely the elevated middle portion of the prosternum, absence of male maxillary suckers and the concave metaventrite in males. Based on these differences, Smetana (1978) transfered both species into a new genus, Agna Smetana, 1978. In this paper we review the genus, redescribe its morphology and describe a new species collected in the semi-arid zones of Hidalgo, Oaxaca, and Puebla, Mexico. Additionally, we summarize the known data on the other New World Sphaeridiinae which have been found in rotten cacti.
Materials and methods
Acronyms for the collections where the examined specimens are deposited are as follows: BMNH, Natural History Museum, London, United Kingdom (M.V. L. Barclay); CAS, California Academy of Science, San Francisco, United States of America (D. Kavanaugh, V. Lee); CNIN, Colección Nacional de Insectos, Instituto de Biología, Universidad Nacional Autónoma de México, México City, Mexico (S. Zaragoza-Caballero); CZUG, Colección Entomológica del Centro de Estudios en Zoología, Universidad de Guadalajara, Zapopan, Mexico (J. L. Navarrete-Heredia); FMNH, Field Museum of Natural History, Chicago, United States of America (C. Maier, A. F. Newton, M. Thayer); FSCA, Florida State Collection of Arthropods, Gainesville, USA (P. Skelley); NHMW, Naturhistorisches Museum, Wien, Austria (M. A. Jäch); NMPC, National Museum, Prague, Czech Republic (M. Fikáček).
Specimens were dissected, with genitalia embedded in a drop of alcohol-soluble Euparal resin on a piece of glass glued to a small piece of cardboard attached below the respective specimen. Habitus photographs were taken using a Canon D-550 digital camera with attached Canon MP-E65mm f/2.8 1-5 macro lens. Pictures of genitalia were taken using a Canon D1100 digital camera attached to an Olympus BX41 compound microscope; pictures of different foci were combined using Helicon Focus software. Scanning electron micrographs were taken using Hitachi S-3700N environmental electron microscope at the Department of Paleontology, National Museum in Prague. Pictures used for plates were adapted in Adobe Photoshop CS6. Distribution map, based on the examined material and records given by Smetana (1978), was prepared using QGIS software and freely available GLOBE altitude data and DIVA-GIS country borders data. All original pictures including additional views not presented in this paper are included in the dataset submitted to the Zenodo archive under doi: 10.5281/zenodo.1473568.
DNA was extracted from the holotype of the new species of Agna collected in a recent expedition in Mexico using a Qiagen Blood and Tissue DNA extraction kit following the manufacturer’s instructions. The highly variable 5’ region of the mitochondrial cytochrome c oxidase subunit I gene (COI) was amplified using LCO1490 (5’GGTCAACAA ATCATAAAGATATTGG-3’) and HCO2198 (5’TAAACTTCAGGGTGACCAAAAAATCA-3’) primers (Folmer et al., 1994). Each 10 µl PCR reaction contained 6.7 µl H2O, 0.4 µl of MgCl2 (25 mM), 0.2 µl of dNTPs (10 mM), 0.3 µl of each forward and reverse primer (10 µM), 0.1 µl of Taq polymerase (5 u/µl), 1.0 µl of 10x Taq buffer, and 1.0 µl of DNA template. The PCR conditions consisted of 3 min at 94 °C + 35 cycles of 30 s at 94 °C, 45 s at 48 °C and 1 min at 72 °C + 8 min at 72 °C. 5 µl of each PCR product were purified by adding 0.5µl (20 u) Exonuclease I (Exo1) and 1µl (1 u) Thermosensitive Alkaline Phosphatase (FastAP) (Thermo Fisher Scientific) and incubating the mixture for 15 min at 37 °C, followed by 15 min at 80 °C. Sanger sequencing was performed by Macrogen Europe (Amsterdam, The Netherlands) on a capillary DNA sequencer.
Descriptions
Agna Smetana, 1978
Agna Smetana, 1978: 173 (type species: Cercyon capillatum LeConte, 1855, by original designation).
Diagnosis. Agna can be differentiated from other megasternines by the following characters: maxilla of both sexes without sucking disc (Figs. 2d, 3b); prosternum strongly raised medialy, with a strong median carina, raised part clearly demarcated from the from lateral portions of prosternum (Fig. 3d); mesoventral plate hexagonal, slightly longer than broad, posterior margin broadly connected with metaventrite (Figs. 2g, 3g-i); male sternite IX horse-shoe shaped, without sclerotized median process (Fig. 1i).

Figure 1 Agna spp. habitus and genitalia. a-c, g-i: A. capillata (LeConte); d-f, j, k: A. zaragozai sp. nov. a, Dorsal habitus. White arrow pointing to series of punctuation, scale bar = 1mm; b, ventral habitus; c, lateral habitus; d, dorsal habitus, scale bar = 1mm; e, ventral habitus; f, lateral habitus; g, tegmen of aedeagus; h, median lobe of aedeagus; i sternite IX; j, tegmen of aedeagus; k, median lobe of aedeagus.
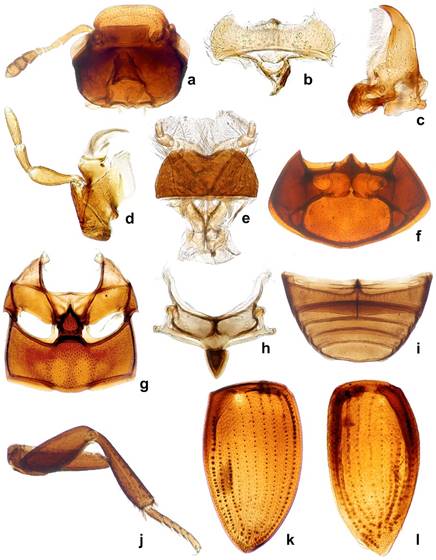
Figure 2 Agna spp. general morphology. a-k: A. capillata (LeConte); l: A. zaragozai sp. nov. a, Ventral view of head; b, labrum; c, mandible; d, maxila; e, labium; f, ventral view of prothorax; g, meso- and metathoracic ventrites; h, scutellum; I, abdominal ventrites; j, hind leg; k, elytron in dorsal view; l, elytron in ventral view.
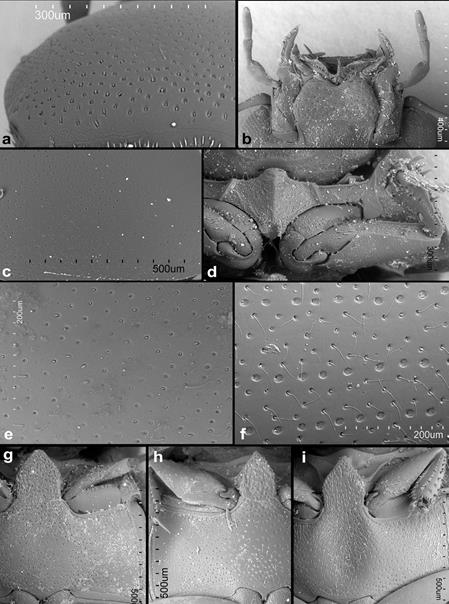
Figure 3 Agna spp. SEmicrographs. a-e, g, h: A. zaragozai sp. nov.; f, i, A.capillata (LeConte). a, Dorsal surface of head; b, ventral view of mouthparts; c, dorsal surface of pronotum; d, prosternum, e, dorsal surface of elytron in horizontal position; f, dorsal surface of elytron in horizontal position; g, meso- and metaventrites of male; h, meso- and metaventrites of female; i, meso- and metaventrites of male.
Differential diagnosis. Before the establishment of Agna, its species were classified as members of Pelosoma. Smetana (1978) distinguished Agna from Pelosoma (see above for diagnostic characters), but supposed them to be closely related on the basis of the mesoventral plate broadly connected to the metaventrite. The male sternite IX assignes Agna to the Oosternum group of genera (“Gondwanan genera” sensu Fikáček, 2010 and Fikáček & Short, 2010), in contrast to Pelosoma, that bears a tongue- like male sternite IX and is a part of the Cercyon group of genera. This phylogenetic placement is corroborated by molecular data (Arriaga-Varela, in prep.), therefore Agna and Pelosoma are not closely related as previously hypothesized. Whitin the Oosternum group of genera, Agna resembles some representatives Oosternum by the form of the prosternal plate and the mesoventral elevation (e.g., some undescribed Neotropical species of the O. sahlbergi group). It can be, however, very easily distinguished from all Oosternum by the absence of the ridge demarcating anterolateral portion of the metaventrite and by the elytra without impressed elytral striae. Among the genera without that ridge, Agna is superficially similar to the Neotropical species of the genus Australocyon which also bear widely triangular to pentagonal mesoventral plate and smooth elytra with elytral series not impressed; it can be diagnosed from Australocyon by the prosternal elevation demarcated by a simple lateral ridge (rather than double V-shaped ridge as in Australocyon) and by the grooves for reception of the procoxae (procoxal rests) distinctly demarcated posteriorly and not reaching mesocoxal cavities (not demarcated posteriorly and reaching mesocoxae in Australocyon).
Description. Head: anterior margin of clypeus with very fine bead, not emarginate medially, anterolateral corners rounded; antennal bases exposed; frontoclypeal suture distinct laterally, absent in medial third; transverse ridges absent. Median portion of frons and clypeus not elevated above remaining surface. Whole dorsal surface with relatively coarse setiferous punctures; setae fine and decumbent (Fig. 3a); area between punctures without visible microsculpture. Eyes small, situated on lateral angular portions of head (Fig. 3a), with dorsally-visible portion slightly smaller than ventral one, separated by 6-7 × the width of 1 eye in dorsal view. Labrum ca. 0.4 × as wide as head, membranous, largely retracted under clypeus, markedly bisinuate on anterior margin, with moderately dense pubescence dorsally, setae almost of same lenght all over (Fig. 2b). Mandible with apex simple, curved, with external margin simple; prostheca with anterior half covered by long thin setae (Fig. 2c). Maxilla of male without sucking disc on galea (Fig. 2d); maxillary palps with basal palpomere minute, palpomere 2 large, widened at apical half, 1.8 × as long as palpomere 3, palpomere 3 slightly widened apicad, palpomere 4 fusiform, 1.5 × as long as palpomere 3, without digitiform sensilla. Mentum (Fig. 2e) transverse, more than twice as wide as long, lateral margins with few sparse setae, anterior margin emarginate; labial palps trimerous, basal palpomere transverse, palpomere 2 as wide as the basal one, ca. 1.4 × as long as wide, terminal palpomere very narrow, elongate, almost as long as the previous ca. 2.4 × as long as wide. Submentum with moderately dense setiferous punctures, gular sutures vaguely developed, rather widely separated from each other, tentorial pits small, shallow. Antenna (Fig. 2a) with 9 antennomeres; scape long, cylindrical, constricted medially; pedicel rather short, narrowing apically; antennomeres 3-5 short, subequal in length, continuously widening apically; cupule weakly asymmetrical and long then antennomere 5; antennomeres 7-9 forming elongate pubescent club (ca. 3 × longer than wide), antennomere 7 ca. 1.3 × as long as 8, antennomere 9 1.5 × longer than the previous one, acuminate at apex; special sensorial antennal fields absent. Genal ridge absent. Prothorax: pronotum transverse, moderately convex, nearly as wide as bases of both elytra combined; lateral margins very minutely beaded; punctation dense, composed of comparatively large setiferous punctures. Prosternum (Figs. 2f, 3d) strongly raised medially, with strong median carina; raised part clearly demarcated from lateral portions of prosternum by a simple oblique ridge; prosternal process short, almost reaching half of lenght of procoxal cavities, weakly bifurcate; prosternal portion anterior of procoxae very narrow. Procoxal cavities large, open posteriorly. Notosternal suture very short. Antennal grooves present, very small, defined laterally by a faint ridge parallel to lateral prosternal margin, vanishing posteriad. Mesothorax: mesoventrite completely fused with anepisternum; anterior collar of mesothorax narrow (Fig. 2g). Median portion of mesoventrite elevated forming a subpentagonal elongated plate, slightly longer than wide, posterior margin broadly connected with intercoxal portion of metaventrite, not overlaping it (Fig. 3g-i). Grooves for reception of procoxae well defined posteriorly by a conspicuous carina, short, transverse (Fig. 3i). Mesepimeron moderately narrow, very weakly widening laterad. Mesocoxal cavities broadly separated. Scutellar shield small, triangular, 1.2 × as long as wide (Fig. 2h). Elytra moderately convex, weakly bordered laterally, each elytron with 10 conspicuous series (Fig. 2k) not situated in an impressed sulcus (Fig. 3f), or without series, only bearing irregular ground punctation (Fig. 3e), if present, the series consist of foveate impressions with a setiferous puncture on anterior margin, punctures diameter 1-2 × the diameter of punctures in the interstices, series 1 and 9 reaching apex, enclosing series 2-8 subapically, series 10 reduced both anteriorly and posteriorly; elytral series visible in all species in microscopic slides as composed of dots of more transparent cuticle (Fig. 2k-l); epipleuron oblique, gradually narrowing posteriad to mid-length of elytra, very gradually narrowing more posteriorly, vanishing shortly after the posterior margin of metaventrite, bearing sparse short setae. Metathorax: metaventrite (Fig. 2g) rather homogeneously and densely covered by comparatively long setae (Fig. 3g-i), anterior margin with narrow rim, weakly widening towards intercoxal process; mesal elevate area vaguely pentagonal, rather narrow, about as long as wide, concave in males, slightly convex in females. Femoral lines and anterolateral ridges absent. Metanepisternum ca. 6 × as long as wide, with anterior oblique ridge, metepimeron with minute ventral portion. Metafurca well developed. Metathoracic wings well developed, with transverse vein r4 arising from basal portion of radial cell, RP rather long, reaching ca. halfway to wing base, basal cubito-anal cell small, closed; wedge cell absent, transverse vein mp-cua joining to MP3+4+CuA1+2; anal lobe not defined. Legs: procoxae large, subglobular, transverse, with long setae; meso- and metacoxae broad, transverse. Tronchatero-femoral junction straight. Femora flattened, comparatively short, with very short setae; profemur without impressed parts; metafemur 1.1 × as long as mesofemur (Fig. 2j). Tibiae rather short, triangular, flattened, straight on external margin, weakly convex on internal margin, with short lateral and mesal spines. Tarsi pentamerous, with moderately long tarsomeres (Fig. 2j), tarsomeres sparsely covered with short stiff setae; metatarsomere 1 to tarsomeres 2-3 together, tarsomeres 2-4 gradually getting shorter, tarsomere 5 almost as long as tarsomere 1. Claws simple, arcuate. Abdomen: with 5 ventrites (Fig. 2i). Ventrite 1 about as long as ventrites 2-3 combined, with median carina getting slightly narrower posteriad. Male sternite IX horse-shoe shaped, without sclerotized median process, apophyses narrow (Fig. 1i). Aedeagus (Fig. 1g-h, j-k) simple, median lobe weakly sinuate, phallobase very short, symmetrical, without projected manubrium; parameres simple, sinuate. Female genitalia corresponding to that of Kanala (Fikáček, 2010).
Key to the species of Agna Smetana.
1. Elytra with 10 longitudinal series of punctures visible in dorsal view (Fig. 3f); males with raised area of metaventrite deeply concave at anterior 3/5 (Fig. 3i)……………………………………………………………………………………….2
Elytra without visible longitudinal series of punctures in dorsal view (Fig. 3e); males with raised area of metaventrite weakly concave at anterior 1/3 (Fig. 3g).........................................................................Agna zaragozai sp. nov.
2. Body color predominantly black (Fig. 1d-f); prosternum with median portion projected anteriorly USA: California, Arizona and Texas....................................................................Agna capillata (LeConte)
Body color predominantly brown; prosternum with median portion not projected anteriorly. Mexico: Baja California Sur...........................................................................Agna rufescens (Horn)
Agna zaragozai sp. nov. (Figs. 1d-f, 1j-k; 2l; 3a-e, 3g-h, 4g)
GenBank accession number: MN310536.
Diagnosis. This species can be distinguished from the other 2 congeners by the elytra without conspicuous series of punctures (Fig. 3e) (present in the other known species); metaventrite of males with median raised part just weakly concave anteriorly (Fig. 3g) (distinctly concave at anterior 3/5 in A. capillata); the aedeagus is just slightly sinuate at base (lateral margins slightly more sinuose with basal half briefly broadened in A. capillata (Fig. 1k).
Description. Body (Fig. 1d-f): 2.4-2.9 mm long; moderately long-oval, 2.0 × as long as wide, widest at basal fourth of elytra; moderately convex, 2.6 × as long as high. Coloration. dark-brown to piceous black with legs and mouthparts dark reddish-brown, antennae tanned-yellow. Head: frons and clypeus with deep and large round punctures intermixed with few slightly transverse ones; interstices without microsculpture. Eyes small; interocular distance about 6.5 × the width of 1 eye in dorsal view. Mentum (Fig. 3b) subhexagonal, widest at posterior third, about 2.2 × wider than long, 1.4 × wider at widest part than at anterior margin, almost completely flat; surface glabrous, with few sparse small punctures, surface rugged. Antenna with scapus ca. 1.5 × as long as antennomeres 2-6 combined; antennal club moderately elongate, about twice as long as wide, about 1.2 × as long as scapus; antennomere 9 acuminate at apex. Prothorax. Pronotum transverse, widest at base 1.9-2.0 × wider than long; 1.6-1.7 × wider at base than between anterior angles, 1.7 × wider than head including eyes, as convex as elytra in lateral view. Punctuation dense and comparatively deep, consisting of rounded setiferous punctures intermixed with denser, smaller and rather transverse non-setiferous punctures (Fig. 3c). Elevated median portion of prosternum 1.4 wider than long, with anteriorly projecting anterior margin. Pterothorax: elytra widest at anterior fourth, 1.2 × as long as wide, 2.5-2.6 × as long as pronotum, 1.1 × as wide as pronotum. Surface (Fig. 3c) shortly pubescent; interstices without microsculpture. Mesoventral plate deeply punctuate, 1.3-1.6 × as long as wide (Fig. 3g). Metaventrite with raised area vaguely pentagonal, about as long as wide, pubescent, slightly more sparsely punctate than lateral areas, slightly convex in females, weakly concave at center in anterior 1/3 in males (Fig. 3g-h); lateral parts of metaventrite covered by short pubescence. Hind wings present. Legs: metafemora with setiferous punctures shallow. Metatibiae 0.3 × as long as elytra. Metatarsus 0.8 × as long as metatibia. Male genitalia: sternite 9 subtruncate apically, without median projection. Phallobase (Fig.1j) very short. Parameres long, almost 9 × as long as phallobase, narrowing towards apical 3/4, then weakly broadening towards apex, apex slightly hooked. Median lobe (Fig. 1k) narrow, almost parallel- sided in basal half, slightly constricted in apical 3/4, apex acuminate, gonopore moderately large, situated subapically; basal portion truncate.
Taxonomic summary
Type locality. Mexico: Hidalgo, Mezquititlán, 20°31.8’ N, 98°38.5’ W.
Type material. Holotype: male: Mexico: Hidalgo: 4.5 km SSW of Cacalomé on rd.105; 20°25’ N, 98°41.3’ W; 1,785 m; 13.ix.2016; Arriaga, Cortés, Fikáček & Seidel lgt. (2016-MX20) in rotten stems of Stenocereus cacti (partly fermented) (CNIN). Paratypes. Mexico: Puebla: Tehuacán, Pue. ca. 5,500 ft. Alt. VII:6:41 // Col. By H. Dybas (2 males, 1 female: FMNH, 1 male: NMPC); Oaxaca: Mexico: Oaxaca, 5 mi W Tequilistlán [Tequisistlán], 1,100 ft, viii.23-ix-5.73 // on rotting cacti, columnar/Opuntia, A. Newton (1 male: FMNH); Mexico: Oaxaca, 2.1 mi NW Totolapam [Totolapa], 3,500 ft, x.6.1973 // on rotting cacti, columnar/Opuntia, A. Newton (2 females: FMNH).
Etymology. We dedicate this species to Santiago Zaragoza-Caballero (Instituto de Biología, UNAM) as a homage to his work on Mexican Coleoptera.
Distribution. Mexico: Hidalgo, Puebla, Oaxaca.
Biology. Specimens have been collected in decaying parts of Opuntia and Steneocereus spp. cacti (Fig. 4d).
Agna capillata (LeConte) (Figs. 1a-c, 1g-i, 2a-k, 3a-e, 3g-h, 4g)
Cercyon capillatum LeConte, 1855: 374.
Pelosoma capillatum; Horn, 1890: 306.
Agna capillata; Smetana, 1978: 174.
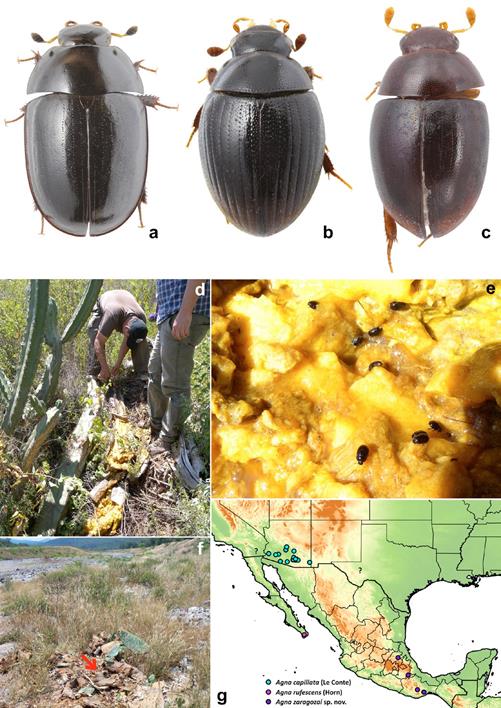
Figure 4 a-c: dorsal habiti of rotten-cacti associated hydrophilids. a, Dactylosternum cacti (Le Conte), length: 6.6 mm; b, Cryptopleurum impressum Sharp, length: 1.6 mm; c, Pelosoma sp., length: 2.6 mm; d-f, habitats of rotten-cacti associated hydrophilids; d, collecting Agna zaragozai sp. nov. and Dactylosternum cacti in rotten Stenocereus in Mezquititlán, Hidalgo; e, Dactylosternum cacti in rotten Stenocereus; f, locality at the river bank in Mezquititlán, Hidalgo were Pelosoma sp. specimens were collected in a pile of rotten Opuntia ficus-indica (indicated by red arrow); g, map with known distribution of Agna species. Cyan circles: A. capillata (Le Conte); interrogation marks: imprecise localities of A. capillata; purple circle: A. rufescens (Horn), blue circles: A. zaragozai sp. nov.
Taxonomic summary
Type locality. United States of America, California, San Diego (based on lectotype designation by Smetana, 1978).
Type material. Not examined. We have studied a series of specimens compared with the lectotype by A. Smetana (see Material examined below).
Material examined. USA: Arizona: Phoenix, Ari. / Liebeck Collection / Agna capillata Smetana det. 1975 (1 male, NMPC); Arizona, Pimo Co. (10 mi. E), 11.Viii.1968 / Saguaro W. Setter leg. (1 female, NMPC); Arizona: Santa Catalina foot hills, Feb. 25 1968, K. Stephen leg (1 male, FSCA); Arizona; Pinal Co. Tortolita Mts. Cottonwood Cyn., Dec. 14 1969, K. Stephen leg. (1 female, FSCA); Arizona: Yuma Kofa Game Reserve, 17-IV-76, Karls Stephan (1 female, FSCA).
Distribution. United States of America: Arizona, California, Texas (Fig. 4g). It is highly likely that the distribution of this species extends to Northwest Mexico.
Biology. This species is commonly found in large rotting cacti like saguaro (Carnegiea gigantea), fishhook barrel cactus (Ferocactus wilizeni) and occasionally Agave spp. (Ferro et al., 2013). It has been reported to be attracted to UV lights (Smetana, 1978).
Diagnosis. This species can be distinguished from A. zaragozai by the elytra with conspicuous series of punctures (Fig. 3f); metaventrite of males with median raised distinctly concave at anterior 3/5 (Fig. 3i) (weakly concave at anterior 1/3 in A. zaragozai); the median lobe with slightly sinuate lateral margins, being briefly broadened at basal half (Fig.1h) (lateral margins subparallel in A. zaragozai [Fig. 1k]). Agna capillata is similar to the holotype of A. rufescens in the presence of elytral series. However, it can be distinguished by the body predominantly black (reddish-brown in A. rufescens holotype) and anterior part of prosternal elevation slightly projecting anteriorly (straight on anterior margin in A. rufescens).
Description. Body (Fig. 1a-c): 2.4-2.7 mm long; moderately long-oval, 2.0 × as long as wide, widest at basal fourth of elytra; moderately convex, 2.4 × as long as high. Coloration: dark-brown to piceous black with legs and mouth parts dark reddish-brown, antennae tanned-yellow. Head: frons and clypeus with deep and large punctures, punctuation composed of rounded and transverse impressions; interstices without microsculpture. Eyes small; interocular distance about 6.0 × the width of 1 eye in dorsal view. Mentum subhexagonal, widest at posterior third, about 2.2 × wider than long, 1.4 × wider at widest part than at anterior margin, almost completely flat; surface glabrous, with few sparse small punctures, surface rugged. Antenna with scape ca. 1.5 × as long as antennomeres 2-6 combined; antennal club moderately elongate, about twice as long as wide, about as 1.2 × as long as scape; antennomere 9 acuminate at apex. Prothorax: pronotum transverse, widest at base 2.0-2.2 × wider than long; 1.6-1.7 × wider at base than between anterior angles, 1.7 × wider than head including eyes, as convex as elytra in lateral view. Punctation dense and comparatively deep, consisting of rounded setiferous punctures intermixed with denser, smaller and rather transverse non-setiferous ones. Elevated median portion of prosternum 1.4 wider than long, with anteriorly projecting anterior margin. Pterothorax: elytra widest at anterior fourth, 1.3-1.4 × as long as wide, 2.6-2.9 × as long as pronotum, 1.1-1.2 × as wide as pronotum. Surface glabrous, with 10 series of punctures; serial punctures of same size in all series, their diameter almost twice as that of punctures on intervals; interval punctation composed of rounded setiferous punctures on all intervals; interstices without microsculpture. Mesoventral plate deeply punctuate, 1.2-1.4 × as long as wide (Fig. 3i). Metaventrite with raised area vaguely pentagonal, about as long as wide, pubescent, almost as densely punctuate as lateral areas, slightly convex in females, strongly concave at center in anterior 4/5 in males; lateral parts of metaventrite covered by short pubescence. Hind wings present. Legs: metafemora with setiferous punctures rather deep. Metatibiae 0.3-0.4 × as long as elytra. Metatarsus 0.7 × as long as metatibia. Male genitalia: sternite 9 (Fig. 1i) subtruncate apically, without median projection. Phallobase (Fig. 1g) very short. Parameres long, almost 9 × as long as phallobase, narrowing towards apical 3/4, then weakly broadening towards apex, apex slightly hooked. Median lobe (Fig. 1h) narrow, almost parallel-sided in basal 1/4, then margins sinuated, slightly broadened close to half length, weakly constricted in apical 3/4, apex acuminate, gonopore moderately large, situated subapically; basal portion truncate.
Agna rufescens (Horn) Cercyon rufescens Horn, 1895: 233.
Pelosoma rufescens; Leech, 1948: 457.
Agna rufescens; Smetana, 1978: 174.
Taxonomic summary
Type locality. Mexico: Baja California Sur, Sierra de San Lázaro.
Type material. Holotype examined: unsexed, (likely a female as apices of cerci are apparently visible) (CAS): Sierra / San Lazaro // Horn / Type // HOLOTYPE // rufescens // California Academy / of Sciences / Type No. 7. The holotype was examined by M. Fikáček in 2005.
Distribution. Mexico: Baja California Sur.
Diagnosis. The holotype of A. rufescences is very similar to the examined specimens of A. capillata, and we found only the following differences: body is much paler in general coloration (thought this may be caused by a possible teneral condition of the), body is slightly smaller than in the known specimens of the other 2 species, median elevated portion of prosternum is not projecting anteriad. Redescription. Body: 2.1 mm long; moderately long-oval, 1.7 × as long as wide; moderately convex. Coloration: reddish, elytralapices slightly paler. Head: frons andclypeus with moderately deep and large punctures; interstices without microsculpture. Eyes small; interocular distance about 5.0 × the width of 1 eye in dorsal view. Mentum subhexagonal, widest at posterior third, about 2.3 × wider than long; surface glabrous, with moderately dense small punctures, surface with distinct rugged microsculpture. Prothorax: pronotum transverse, widest at base 2.0 × wider than long, as convex as elytra in lateral view. Punctation dense and comparatively deep, consisting of rounded setiferous punctures, smaller intermixed punctures not observed. Elevated median portion of prosternum 1.7 wider than long, without anteriorly projecting anterior margin. Pterothorax: elytral surface glabrous, with 10 series of punctures clearly visible in dorsal view; serial punctures of same size in all series, their diameter only slightly larger than punctures on intervals; interval punctation composed of rounded setiferous punctures on all intervals; interstices without microsculpture. Mesoventral plate deeply and densely punctate, 1.3 × as long as wide. Metaventrite with raised area vaguely pentagonal; lateral parts of metaventrite covered by short pubescence.
Remarks
Since no other specimens are available from the Baja California peninsula, and taking into account the isolated geographic position of the type locality (Fig. 4g), we are treating here A. rufescens as a species separate from A. capillata. The examination of additional specimens from the Baja California peninsula is needed in order to confirm the status of this species.
Other Hydrophilidae occurring in rotten cacti:
Dactylosternum cacti (LeConte) (Fig. 4a)
Cyclonotum cacti LeConte, 1855: 373.
Dactylosternum cacti; Horn, 1890: 284.
Taxonomic summary
Type locality. United States of America: California, San Diego (based on lectotype designation by Smetana, 1978).
Distribution. United States of America: Arizona, California; Guatemala: El Progreso; Mexico: Hidalgo (new country record).
Material examined. Mexico: Hidalgo: 4.5 km SSW of Cacalomé on rd.105; 20°25.0’ N, 98°41.3’ W; 1,785 m; 13.ix.2016; Arriaga, Cortés, Fikáček & Seidel lgt. (2016-MX20) in rotten stems of Stenocereus cacti (partly fermented) (38: NMPC; 3: BMNH; 3: NHMW; 3: CNIN, 2: CZUG).
Remarks
Dactylosternum is a worldwide genus with 62 described species (Hansen, 1999). Most species can be collected in decaying plant material, as rotten banana stems, rotten palm trunks etc. Dactylosternum cacti was described from southwestern USA, and as the name suggest it is almost exclusively collected on rotten cacti. Type locality of the species is the same as that of Agna capillata (LeConte): San Diego, California. Besides southern California, this species is known from Arizona, and was also recorded from Guatemala (El Progreso) by Archangelsky (1997), (see Archangelsky et al. 2016) (identification of the Guatemalan specimens was confirmed by examination of male genitalia, Archangelsky, pers. comm). The presence of the species in Mexico is hence not surprising and was also expected by Smetana (1978). Here we confirm that the species occurs in Mexico.
Cryptopleurum impressum Sharp (Fig. 4b)
Cryptopleurum impressum Sharp, 1882: 115.
Cryptopleurum cerei Schwarz, 1899: 8.
Taxonomic summary
Type locality. Mexico: Veracruz, Córdoba (based on lectotype designation by Smetana, 1978).
Material examined. Mexico: Veracruz: 1.7 mi of Teocelo, 3,700 ft, 23-24.vii.1973, berselate dead grass pile, A. Newton (21: NMPC; 30: FMNH); Jalisco: Puerto los Mazos, 10 mi. SW Autlán 4,400 ft, 25.ix.1973, refuse deposit Atta mexicana, A. Newton (12: NMPC; 50: FMNH); San Luis Potosí: 15 mi. N Tamazunchale, 500 ft, 26.vi.1973, ex refuse deposit Atta mexicana, A. Newton (7: NMPC; 15: FMNH); Oaxaca: 9 mi. NE Oaxaca, Km 9.5 Mex 175, 6,100 ft, 25.viii.1973, refuse deposit Atta mexicana, A. Newton [larvae vial #20] (3: NMPC; 10: FMNH).
Remarks
Cryptopleurum is a globally distributed genus with 24 described species, 7 of which are distributed in the New World, either as native species or accidentally introduced through human activities (Hansen, 1999). In USA, Cryptopleurum impressum is commonly found in decaying large cacti of the genus Cereus and other rotten cacti (Smetana, 1978). In Mexico, it has been most commonly found associated with refuse piles of Atta ants (Hinton & Ancona, 1934; this paper). In Mexico, C. impressum has been reported from Baja California, México, Morelos, Oaxaca, Veracruz (Arce-Pérez & Morón, 2011). Here we record its presence from Jalisco and San Luis Potosí states from the first time. Most likely this species is more widely distributed in Mexico than presently known.
Distribution. Mexico: Baja California, México, Morelos, Jalisco, Oaxaca, San Luis Potosí, Veracruz. United States of America: Arizona.
Pelosoma sp. (Fig. 4c)
Taxonomic summary
Material examined. 1 male, 5 spec. (NMPC): Mexico: Hidalgo: Mezquititlán, river bank at the bridge 20°31.8’ N, 98°38.5’ W; 1,350 m; 16-17.ix.2016; Arriaga, Cortés, Fikáček & Seidel lgt. 016-MX2 // in pile of decaying parts of Opuntia ficus-indica.
Remarks
Pelosoma is a New World genus currently including 17 described species. Nonetheless, our observation indicates that the diversity is highly understimated. Specimens are commonly found in flowers or rotten tissue of plants. In humid tropical forests they are collected in interior parts of inflorescences of families Araceae and Heliconiaceae or other Zingiberales, or in rotten parts of these plants, like stems and bases of leaves, or in leaf litter. In seasonally dry or semi-arid zones they have been found in rotten succulent plants. We collected a few specimens in a pile of rotting cladodes of Opuntia ficus-indica cactus in an area with xerophytic vegetation (Fig. 4f). Comparison of the specimens with the descriptions and illustrations of species known from USA and Central America (Sharp, 1882; Smetana, 1978) suggests that they represent an undescribed species. Additional observations are needed to reveal whether this species occurs specifically in rotten cactus tissues, or if it may inhabit a wider spectrum of microhabitats. A different unidentified species of Pelosoma was collected by one of us (JCA) in rotten Agave valencia (Asparagaceae), a succulent plant species of a diferent family than Cactaceae, in the ecotone of tropical dry forest and a seasonally dry Quercus forest, in El Coamil, Mascota, in Jalisco, Mexico.











 nueva página del texto (beta)
nueva página del texto (beta)


