Introduction
The Thunnini are unique among bony fishes in having counter-current heat exchange systems that allow them to retain metabolic heat, maintaining a warmer internal temperature than the surrounding water. The big eye tuna, Thunnus obesus (Lowe) is an epipelagic and mesopelagic species present in oceanic waters, that occurs from the surface to a depth of about 250 m, and is found worldwide in tropical and subtropical waters of the Atlantic, Indian and Pacific Oceans, but is absent from the Mediterranean Sea. It is an important commercial species around the world (Collette & Nauem, 1983) and its diet is opportunistic, mainly composed by fish, cephalopod mollusks, and crustaceans (Gorni et al., 2013). According with Madhavi and Ram (2000), the high vagility and endothermy of tuna fishes require high metabolic energy, which is met through foraging on large quantities of food items that serve as intermediate and paratenic hosts for helminth parasites. These species are mainly parasitized by trematodes of the family Didymozoidae that, in severe infections, can reduce the commercial value of the fish. These trematodes are parasites of marine fishes mainly belonging to the families Scombridae, Exocoetidae, Serranidae and Sphyraenidae, found predominantly in tropical and subtropical waters (Nikolaeva, 1985). They represent a different group of parasites by the general morphology highly adapted to different habitats not common to other digenean parasites, as gills, skin, connective, muscular and adipose tissues, bones, nasal, oral and orbital cavities, vascular system, kidneys (Yamaguti, 1971).
Brazil is the most important South American country for fishing of scombrids in the Southwest Atlantic Ocean, but very little is known about the parasites of these species. The aim of this study is to contribute to the increase of the knowledge and expansion of the geographical distribution of didymozoid parasites of tuna in the area of the Southern Atlantic Ocean.
Material and methods
Forty-seven specimens of T. obesus were obtained from fishermen in Cabo Frio, Rio de Janeiro, Brazil (22º52’46” S, 42º01’07” W) and in the municipal market of São Pedro in Niterói, Brazil, being 18 females (44-64 cm standard body length; 1.70-4.55 kg) and 29 males (41-73 cm standard body length; 1.23-6.25kg).
The parasites were released from dissected cysts and fixed with or without compression in AFA (alcohol 93%, formalin 5%, and acetic acid 2%), stained in alcoholic-acid carmine, dehydrated in an alcohol series, cleared in clove oil and mounted in Canada balsam. Measurements are in micrometers, unless otherwise specified, with the mean in parentheses followed by the number of specimens measured in brackets, where applicable. Light micrographs were taken with a digital camera connected to a Zeiss Axioskop microscope. The studied specimens were deposited in the Helminthological Collection of Instituto Oswaldo Cruz (CHIOC), Rio de Janeiro, Brazil.
Results
During a survey of the helminth parasites of Thunnus obesus from Rio de Janeiro coast, 3 adults species of didymozoid trematodes were found: Didymocystis alalongue Yamaguti, 1938 in gill arch and operculum, Didymocystis lamotheargumedoi Kohn & Justo, 2008 associated with 3 different stages of development in the operculum and Platocystis vivipara (Yamaguti, 1970) in the skin of dorsal region.
D. alalongue and D. lamotheargumedoi were allocated in Didymocystis by presenting the posterior region hemispherical, with cup-like concavity on its flattened surface and branched ovary and vitellarium. Platocystis vivipara was identified by the main features of Platocystis Yamaguti, 1938 which are habitat (skin), posterior region semicircular, flattened on one side and arrangement of the vitellarium and ovary. Since the morphology of these species of Didymozoidae has been well described, only a brief description and main measurements are presented.
Didymozoinae Monticelli, 1888
Didymozoini Monticelli, 1888
Didymocystis alalongae Yamaguti, 1938 (Figs. 1-4)
Specimens of D. alalongae were easily observed as yellowish cysts containing 2 hermaphrodite individuals similar in form and size (Fig. 1).
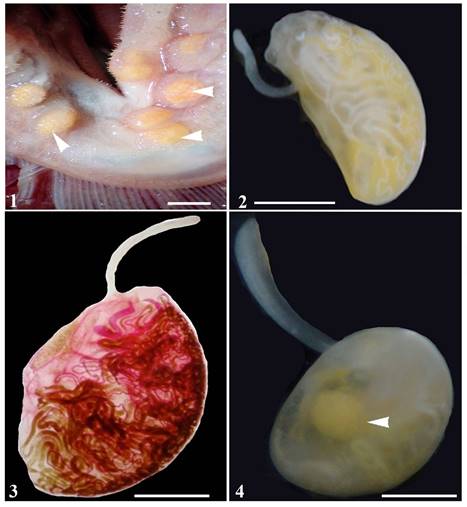
Figures 1-4 Photomicrographs of Didymocystis alalongae Yamaguti, 1938. 1, Cysts in gill arch (arrowhead). Scale bar: 0.5mm; 2, total, without compression. Scale bar: 2 mm; 3, total, compressed specimen, stained with alcoholic-acid carmine. Scale bar: 2 mm; 4, total, without compression, showing egg reservoir (arrowhead). Scale bar: 2 mm.
Description based on 19 specimens. Forebody slender, attached to near anterior end of hindbody on ventral side, measuring 1.1-3.7 (2.1) mm long by 0.1-0.5 (0.3) mm wide. Hindbody half-oval, flattened on ventral side and convex dorsally, varying greatly in size, 1.1-7.3 (3.3) mm long by 0.9-4.6 (2.7) mm wide (Figs. 2-4). Oral sucker 50-75 (63) long by 22-47 (36); pharynx muscular 40-87 (60) long by 32-67 (48); esophagus 180-350 (270) [n = 11] long. Tubular testes paired and very long, 1 on each side, in anterior part of hindbody 400-1,750 (900) [n = 7] long by 40-90 (64) [n = 13] wide. Genital pore ventral to oral sucker. Ovary tubular divided into 2 branches that extend through all fields of hindbody, ranging from 27 to 65 (45) wide. Vitelline gland consists of a short system which divides 3 times near the genital junction into 4 very long slender branches (Fig. 3). Uterus coiled irregularly and occupying most central area of hindbody, forming an egg reservoir (Fig. 4);metraterm muscular, sinuous in forebody. Eggs embryonated, 15-17 (16) long by 7-13 (9) wide.
Taxonomic summary
Host: Thunnus obesus (Lowe) (Thunnini, Scombridae, Perciformes).
Site: gill arches and operculum.
Locality: littoral of Rio de Janeiro State, municipality of Cabo Frio.
Number of specimens: 160 specimens (observed as yellowish cysts containing 2 individuals similar in form and size).
Prevalence: 23.4% (11 out of 47 T. obesus examined).
Deposited specimens: CHIOC n°: 37106 a-b, 37107 a-b, 37108, 37109, 37110, 37111, 37112, 37113 a-h, 37114 a-b.
Didymocystis lamotheargumedoi Kohn & Justo, 2008 (Figs. 5-10)
Specimens of D. lamotheargumedoi were observed in rounded cysts containing 2 hermaphrodite individuals similar in form and size (Fig. 5). Three different stages were found freely associated in the same site (operculum) among the adults forms (Figs. 6): an early stage of the development of the similar to structural type anacetabulum Kurochkin & Nikolaeva, 1978 (Fig. 7) and more advanced stages (adult juveniles), 1 in initial transformation for the original form of the adults (Fig. 8) and 1 in the initial stage of maturation where the formation of the reproductive organs can already be visualized (Fig. 9).
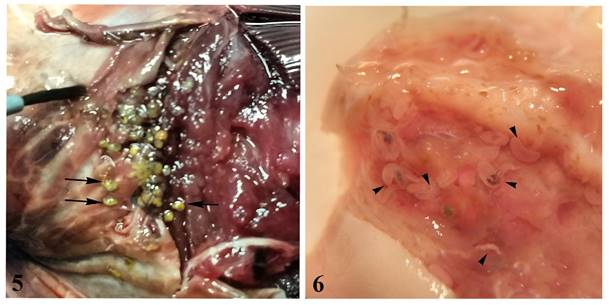
Figures 5-6 Photomicrographs of Didymocystis lamotheargumedoi Kohn & Justo, 2008. 5, Cysts (black arrow) in palate of Thunnus obesus; 6, cysts in operculum (arrowhead) of T. obesus.

Figures 7-10 Photomicrographs of Didymocystis lamotheargumedoi Kohn & Justo, 2008. 7, Total, larval stage anacetabulum Kurochkin e Nikolaeva, 1978 stained with alcoholic-acid carmine. Scale bar: 1mm; 8, total, juveniles (intermediate stage between anacetabulum and adults juveniles) showing caeca twisted with dark ingest (arrowhead). Scale bar: 0.8 mm; 9, total, adult juvenile showing testes filiform, paired (black arrowhead), and initial formation of the genital junction, showing the definitive pattern of arrangement of the reproductive organs (white arrowhead). Scale bar: 0.8 mm; 10, total, adult, compressed specimen, stained with alcoholic-acid carmine. Scale bar: 0.8 mm
Anacetabulum Kurochkin & Nikolaeva, 1978 (Fig. 7)
Description based on 5 specimens: body lanceolate, 0.34-4.85 (4.00) mm long by 0.25-0.37 (0.29) mm wide; oral sucker 40-62 (52) long by 30-37 (35) wide, directly followed by pharynx 29-30 (30) long by 30-35 (33) wide; caeca twisted, inflated and constricted at many places, containing dark ingest.
Juveniles (intermediate stage between anacetabulum and adult juveniles) (Fig. 8)
Description based on 5 specimens: body begins to take the adult shape, already differentiating into 2 distinct regions: forebody subcylindrical measuring 0.69-1.43 (1.14) mm long by 0.24-0.30 (0.28) mm wide and hindbody oval 1.05-1.45 (1.28) mm long by 0.46-0.80 (0.65) mm wide. Oral sucker 35-48 (42) long by 23-35 (28) wide; pharynx 21-32 (26) long by 25-35 (30) wide; caeca inflated and constricted at places, containing dark ingest.
Adult juveniles (Fig. 9)
Description based on 6 specimens: forebody slender 0.97-1.30 (1.10) mm by 0.22-0.32 (0.28) mm wide. Hindbody globular to oval 1.27-1.57 (1.48) mm long by 0.60-0.92 (0.77) mm wide. Oral sucker 52-57 (56) long by 25-37 (34) wide directly followed by pharynx, 35-37 (35) in diameter. Caeca twisted, inflated and constricted at many places. Testes filiform, paired. Female reproductive organs show their definitive pattern of arrangement, except by the absence of eggs in the uterus that is a characteristic of adults. Genital pore lateral to oral sucker.
Adults (Fig. 10)
Description based on 10 adult specimens: Forebody slender, attached to near anterior end of hindbody on its ventral side, measuring 0.80-1.67 (1.31) mm long by 0.30-0.48 (0.37) wide; hindbody ventrally concave, 1.62-2.80 (2.29) mm long by 1.02-1.95 (1.55) mm wide. Oral sucker terminal, pyriform, 35-62 (54) long by 22-37 (34) wide; pharynx globular 30-41 (36) in diameter. Esophagus 500-950 (689). Testes tubular extending along anterior margins of hindbody, 490-1,175 (889) long by 100-275 (155) [n = 7] wide. Ovary divided into 5-6 tubular branches lacking ramifications; branches extend through lateral fields of hindbody, ending at different levels, 40-80 (63) wide. Vitelline gland consists of 5-7 tubules, without ramifications, extending along convex side to posterior extremity of hindbody. Eggs bean-shaped 12-20 (16) long by 7-12 (9) wide.
Taxonomic summary
Host: Thunnus obesus (Lowe) (Thunnini, Scombridae, Perciformes), new host record
Locality: littoral of Rio de Janeiro State, municipality of Cabo Frio
Site: operculum
Number of specimen adults: 434 specimens (observed in cysts containing 2 hermaphrodite individuals similar in form and size).
Prevalence: 8.5% (4 out of 47 T. obesus examined).
Deposited specimens: CHIOC n°: 40087 a-f, 40088 a-d.
Platocystis vivipara (Yamaguti, 1970) Pozdnyakov, 1987 (Figs. 11-12)
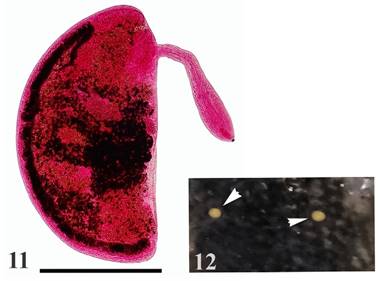
Figures 11-12 Photomicrographs of Platocystis vivipara (Yamaguti, 1970) Pozdnyakov, 1987. 11, Total, compressed specimen, stained with alcoholic-acid carmine. Scale bar: 0.5 mm; 12, cysts in tegument of dorsal region (arrowhead).
Specimens of P. vivipara were easily observed as yellowish cysts containing 2 hermaphrodite individuals similar in form and size.
Description based on 12 specimens. Cysts rounded, flattened containing 2 hermaphrodite worms (Fig. 12). Forebody slender visibly spatulate in its anterior third, 375-725 (543) long by 75-125 (98) wide [n = 11] connected to the anterior end of the flat margin in the posterior region. Hindbody flattened, semicircular 825-1,275 (1,139) long by 450-800 (652) wide [n = 11]. Oral sucker terminal, piriform, weakly muscular 37-47 (44) long by 22-27 (25) directly followed by rudimentary pharynx 17-26 (21) long by 20-27 (21) wide. Esophagus narrow 85-205 (134) [n = 9] long. Testes tubular, paired, close to flat margin near anterior end of hindbody, 152-380 (294) long by 45-120 (78) wide [n = 8]. Genital pore ventrolateral to oral sucker. Ovary tubular, 22-40 (29) [n = 4] wide, extending from near distal end of vitelline gland to near base of forebody. Vitelline gland single, extending along convex edge of hindbody from posterior extremity 1.02-2.06 (1.64) mm long by 0.040-0.10 (0.064) mm wide [n = 11]. Uterus occupying all available space of hindbody, not forming egg reservoir. Eggs embryonated 15-16 (15) long by 8-11 (10) wide.
Taxonomic summary
Host: Thunnus obesus (Lowe) (Thunnini, Scombridae, Perciformes)
Locality: littoral of Rio de Janeiro State, municipality of Cabo Frio, new geographical locality
Site: tegument of dorsal region
Number of specimens: 112 specimens (observed as yellowish cysts containing 2 hermaphrodite individuals similar in form and size).
Prevalence: 6.4% (3 out of 47 T. obesus examined). Deposited specimens: CHIOC n°: 40089, 40090 a-e.
Discussion
Didymocystis alalongue was originally described by Yamaguti (1938) parasitizing the operculum, branchial arch and branchial filaments of Thunnus albacares (= Neothunnus macropterus) and gills from Thunnus obesus (= Parathunnus sibi) from Hawaii, Pacific Ocean. In 1970, Yamaguti proposed a new genus, Didymocystoides, allocating the species in the new genus as Didymocystoides alalongue. In a review of the genus Didymocystis, Pozdnyakov (1990) considered Didymocystoides as a synonym of Didymocystis. Murugesh and Madhavi (1995) referred to Didymocystis alalongae Yamaguti, 1938 in the operculum from Thunnus tonggol from the Indian Ocean and considered Didymocystis bifasciatus (Yamaguti, 1970) as synonym of this species. However, Pozdnyakov (1996) did not refer to the synonymy proposed by Murugesh and Madhavi (1995), probably unaware of this paper. This species was also reported as Didymocystoides bifasciatus parasitizing Scomberoides tol (= Chorinemus toe) from Indian Ocean by Hussain et al. (1985) and Mordvinova and Nikolaeva (1990) and from Thunnus thynnus reared in Adriatic cages by Mladineo et al. (2008). Justo and Kohn (2010) reported the occurrence of D. alalongue as D. bifasciatus parasitizing Thunnus albacares, Thunnus atlanticus, Thunnus obesus and Katsuwonus pelamis in the abstract of a scientific meeting in Brazil. In the present paper, the synonymy proposed by Murugesh and Madhavi (1995) was considered and the valid species is D. alalongae.
Didymocystis lamotheargumedoi was originally described by Kohn and Justo (2008) parasitizing the operculum and palate of Thunnus atlanticus, in the palate of Thunnus albacares and operculum of Katsuwonus pelamis. In this study D. lamotheargumedoi is reported parasitizing operculum and palate of a new host, Thunnus obesus. Confirming the findings of the original description, different stages of immature forms were found in the same site infection of the adult forms in T. obesus, showing that this parasite has the same pattern of parasitism in different host species.
Platocystis vivipara was original described from Hawaii by Yamaguti (1970) from tegument of dorsal region of T. albacares (= Neothunnus macropterus) and T. obesus (= Parathunnus sibi) as Dermatodidymocystis vivipara. Pozdnyakov (1987) transferred the species to Platocystis. The current specimens present the same morphometrical features as in the original description and constitute a new geographical report from southwest Atlantic Ocean, Brazil.
Didymozoidae is the family with the largest number of described and reported species in Brazil (Cohen et al., 2018). Most species were found in more than 1 phylogenetically related fish species, which corroborate the specificity of the Didymozoidae to scombrid hosts. The present study contributes to the mapping of host species and biogeographical distribution of this interesting group of trematodes.











 nueva página del texto (beta)
nueva página del texto (beta)


