Introduction
Coral reefs are threatened ecosystems world-wide due to the increased urban development of coastal areas that promote sedimentation, overfishing, macroalgal growth, low water quality, diseases and direct physical damage (Alvarez-Filip et al., 2011; Monroy-Velázquez et al., 2017). Several approaches have been developed to estimate the health status of coral reefs; one non- destructive method is to survey theabundance and diversity of cryptic fauna from coral rubble and associate them to how conserved or deteriorated the reefis (Linton & Warner, 2003; Takada et al., 2007; Monroy-Velázquez et al., 2017). However, their inclusion in such analyses is dependent upon the availability and completeness of the corresponding inventories (Thomas, 1993).In this paper, we present a list of species of molluscs found in 3 sites within the Puerto Morelos Reef National Park, and 14 new distributional records for the Mexican Caribbean. Further analyses of these data, combined with those of other invertebrate groups recorded in the same samplings, is forthcoming.
Molluscs are a dominant component of the coral reef fauna and their real diversity is still a matter of much controversy. Miloslavich et al. (2010) reported 3,032 species for the whole Caribbean Sea, based on records from major museums and the databases available at the time. González-Vallejo (2011) reported 675 species for the Mexican Caribbean, and 3 years later Castillo-Rodríguez (2014) recorded 985 species for the same area. The large increase in number of species (310) shows that the potential for new distributional records in the area is still quite high. Further, these figures suggest that the Mexican Caribbean could host close to one-third of the total diversity of the region. Other studies that have contributed to the knowledge of the mollusc fauna in the Mexican Caribbean are those of Cruz-Abrego et al. (1995), González-Vallejo (1998) and García-Cubas and Reguero (2004, 2007). In this study, the Mexican Caribbean is considered to be the area between Cape Catoche in the northeastern tip of the Yucatan Peninsula to Xcalak, Quintana Roo, in the border with Belize, a coastline of approximately 418 km.
Materials and methods
The study was conducted in the Puerto Morelos Reef National Park (PNAPM), Quintana Roo, Mexico; which is a section of the Mesoamerican Reef (Fig. 1). Three sites were selected: 1) Bonanza (20°57’58” N, 86°48’27” W), in recovery, located in the northern section of the marine park, now closed to recreational activities after being heavily impacted by snorkeling and diving; 2) Puerto Morelos (20°52’50” N, 86°51’02” W), conserved, in the central portion of the marine park is in good condition although it is adjacent to the town of Puerto Morelos, and 3) Jardines (20°50’20” N, 86°52’41” W), degraded, lies to the south in front of large resorts and golf courses with a heavy sediment load (Monroy-Velázquez &Álvarez, 2016; Monroy-Velázquez et al., 2017; Rodríguez-Martínez et al., 2010; Fig. 1). Using SCUBA equipment, 3 kg of coral rubble were sampled in each site. It is worth mentioning that although no sediment was taken with the coral rubble samples, some micromollusks associated to this substrate were collected. Samplings were conducted in March, May, August, and November 2013, and January 2014, under SAGARPA (Agriculture, Natural Resources and Fisheries Secretariat) collecting permit DGOPA.00008.080113.0006 granted to F. Álvarez.
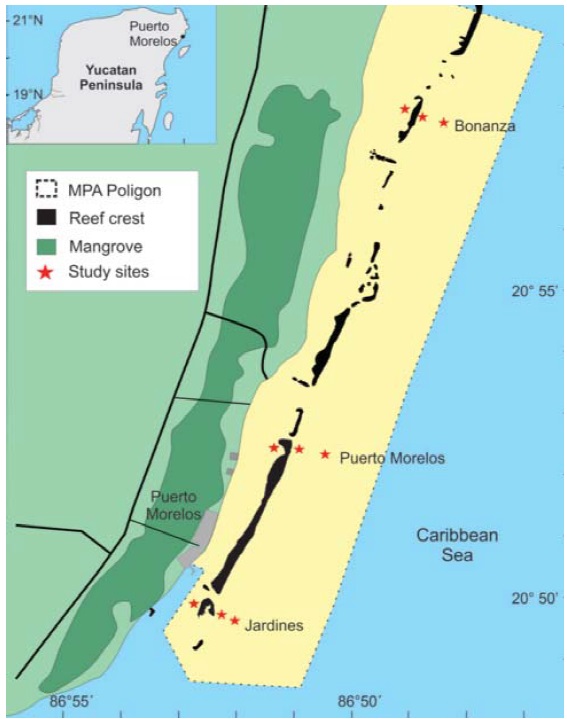
Figure 1 Map of the Puerto Morelos Reef National Park, Quintana Roo, Mexico, showing the collecting sites for this study: Bonanza, Puerto Morelos and Jardines.
Samples were placed in plastic bags and transported to the lab for sorting. All organisms were extracted from the dead coral matrix and preserved in 70% EtOH. The identification guides used were Abbott (1974),Redfern (2001), García-Ríos (2003), Mikkelsen and Bieler (2008) and Tunnell et al. (2010). The synonymies were checked in Abbott and Dance (1982), Tunnell et al. (2010), Redfern (2001, 2013), Rosenberg et al.(2009) and Horton et al. (2018); the last 2 references were used to verify current accepted names. The taxonomic list is presented following Horton et al. (2018) for gastropods; Bieler and Mikkelsen (2006) for bivalves, Kaas and Van Belle (1987) for polyplacophorans, Scarabino (2008) for scaphopods, and Young et al. (2018) for cephalopods.
Specimens representing the new records of less than 2 mm in total length were photographed in an Axio Zoom V16 Zeiss microscope, and larger specimens in a Z16 APO-A Leica microscope. No taxonomic comments are included since all the species agree well with the original descriptions. All specimens representing the new distributional records are deposited in the “Colección Malacológica Dr. Antonio García-Cubas” (COMA) of the Instituto de Ciencias del Mar y Limnología, Universidad Nacional Autónoma de Mexico (UNAM). All the remaining specimens are deposited in the “Colección Nacional de Moluscos” (CNMO) of the Institutode Biología, UNAM.
A rarefaction curve was obtained based on the observed data using EstimateS 9.1 software and Chao 1 estimator (Colwell, 2016). The curve was plotted in Statistica v10.0.1011 with the model S = (a*v1)/(1+(b*v1)), where “a” is the rate at which new species are incorporated at the beginning of the sampling and “b” a parameter related to the shape of the curve (Jiménez-Valverde & Hortal, 2003). The asymptote of the curve, the theoretical maximum number of species, is equal to a/b.
Results
A total of 653 organisms from 120 species were collected. Gastropoda was represented by 80 species in 60 genera and 38 families, Bivalvia by 26 species in 24 genera and 16 families, Polyplacophora by 12 species in 5 genera and 3 families, and Scaphopoda and Cephalopoda by 1 species each (Table 1).
Table 1 Abundance of molluscs’species collected in the Puerto Morelos Reef National Park by date (month/year). Species in bold represent new distributional records for the Mexican Caribbean.
| Family | Genus | Species | 03/2013 | 05/2013 | 08/2013 | 11/2013 | 01/2014 | 05/2014 |
| Class Gastropoda | ||||||||
| Eoacmaeidae | Eoacmaea | pustulata (Helbling, 1779) | 1 | 2 | 2 | 2 | ||
| Fissurellidae | Diodora | dysoni (Reeve, 1850) | 1 | 1 | 3 | |||
| minuta (Lamarck, 1822) | 2 | 2 | ||||||
| variegata (G. B. Sowerby II, 1862) | 1 | |||||||
| Fissurella | angusta (Gmelin, 1791) | 1 | ||||||
| Lucapina | philippiana (Finlay, 1930) | 2 | ||||||
| Chilodontaidae | Euchelus | guttarosea Dall, 1889 | 3 | 1 | ||||
| Trochidae | Synaptocochlea | picta (d’Orbigny,1847) | 3 | |||||
| Pseudostomatella | erythrocoma (Dall, 1889) | 1 | ||||||
| Tegulidae | Tegula | fasciata (Born, 1778) | 4 | |||||
| gruneri (Philippi, 1849) | 1 | 1 | ||||||
| Areneidae | Arene | cruentata (Megerle von Mühlfeld, 1824) | 1 | 1 | 1 | |||
| Phasianellidae | Eulithidium | adamsi (Philippi, 1853) | 1 | 7 | 24 | 41 | ||
| bellum (M. Smith, 1937) | 5 | 43 | 13 | 2 | 30 | 29 | ||
| thalassicola (Robertson, 1958) | 2 | 2 | ||||||
| Turbinidae | Lithopoma | americanum (Gmelin, 1791) | 2 | |||||
| Neritidae | Smaragdia | viridis (Linnaeus, 1758) | 2 | |||||
| Cerithidae | Cerithium | atratum (Born, 1778) | 1 | 2 | 4 | 2 | 6 | |
| eburneum Bruguière, 1792 | 1 | 9 | 3 | 3 | ||||
| litteratum (Born, 1778) | 6 | 3 | 4 | 3 | 5 | 4 | ||
| Planaxidae | Fossarus | orbignyi P. Fisher, 1864 | 1 | |||||
| Rissoidae | Alvania | faberi De Jong & Coomans, 1988 | 2 | |||||
| Simulamerelina | caribaea (d’Orbigny, 1842) | 1 | 1 | 1 | ||||
| Rissoinidae | Ailinzebina | elegantissima (d’ Orbigny, 1842) | 1 | |||||
| Phosinella | cancellata (Philippi, 1847) | 1 | 1 | |||||
| Rissoina | pulchra (C. B. Adams, 1850) | 1 | ||||||
| Zebinella | decussata (Montagu, 1803) | 1 | ||||||
| princeps (C. B. Adams, 1850) | 1 | |||||||
| Zebinidae | Schwartziella | bouryi (Desjardin, 1949) | 1 | |||||
| bryerea (Montagu, 1803) | 1 | 1 | 1 | 2 | 1 | |||
| Stosicia | aberrans (C. B. Adams, 1850) | 2 | 2 | |||||
| Zebina | browniana (d’Orbigny, 1842) | 1 | ||||||
| vitrea (C. B. Adams, 1850) | 3 | 1 | ||||||
| Vitrinnellidae | Circulus | semisculptus (Olsson & McGinty, 1958) | 1 | |||||
| Tornidae | Parviturboides | interruptus (C. B. Adams, 1850) | 1 | |||||
| Eratoidae | Hespererato | maugeriae (J. E. Gray, 1832) | 1 | |||||
| Epitoniidae | Epitonium | turritellula (Mörch, 1875) | 2 | |||||
| Eulimidae | Melanella | eburnea (Megerle von Mühlfeld, 1824) | 1 | |||||
| Cerithiopsidae | Cerithiopsis | flava (C. B. Adams, 1850) | 2 | |||||
| fusiformis (C. B. Adams, 1850) | 1 | |||||||
| lata (C. B. Adams, 1850) | 1 | |||||||
| Seila | adamsii (H. C. Lea, 1845) | 1 | 2 | 1 | 2 | 3 | ||
| Newtoniellidae | Retilaskeya | bicolor (C. B. Adams, 1845) | 1 | |||||
| Triphoridae | Cosmotriphora | ornata (Deshayes, 1832) | 1 | |||||
| Iniforis | turristhomae (Holten, 1802) | 1 | 3 | |||||
| Triphora | elvirae De Jong & Coomans, 1988 | 1 | ||||||
| Muricidae | Coralliophila | galea (Dillwyn, 1823) | 1 | |||||
| Dermomurex | pauperculus (C. B. Adams, 1850) | 2 | ||||||
| Columbellidae | Astyris | lunata (Say, 1826) | 1 | 1 | ||||
| Columbella | mercatoria (Linnaeus, 1758) | 2 | 3 | 6 | 3 | 4 | 4 | |
| Mitrella | nycteis (Duclos, 1846) | 1 | ||||||
| Steironepion | maculatum (C. B. Adams, 1850) | 1 | ||||||
| moniliferum (G. B. Sowerby I, 1844) | 2 | 1 | ||||||
| Nassariidae | Phrontis | alba (Say, 1826) | 1 | 1 | ||||
| antillarum (d'Orbigny, 1847) | 1 | |||||||
| Bellolividae | Jaspidella | blanesi (Ford, 1898) | 1 | |||||
| Olividae | Olivella | acteocina Olsson, 1956 | 1 | |||||
| exilis (Marrat, 1871) | 1 | 5 | ||||||
| bullula (Reeve, 1850) | 2 | |||||||
| Cystiscidae | Gibberula | lavalleeana (d’Orbigny, 1842) | 10 | 1 | ||||
| Costellariidae | Vexillum | moniliferum (C. B. Adams, 1850) | 1 | |||||
| Mitromica | foveata (G. B. Sowerby II, 1874) | 1 | ||||||
| Pseudomelatomidae | Crassispira | fuscescens (Reeve, 1843) | 1 | |||||
| Marginellidae | Hyalina | pallida (Linnaeus, 1758) | 1 | 1 | ||||
| Volvarina | ceciliae Espinosa & Ortea, 1999 | 1 | 1 | 1 | ||||
| avena (Kiener, 1834) | 1 | |||||||
| subtriplicata (d’Orbigny, 1842) | 1 | 2 | ||||||
| Mangeliidae | Tenaturris | inepta (E. A. Smith, 1882) | 1 | 1 | 2 | |||
| dysoni (Reeve, 1846) | 1 | |||||||
| Granoturris | padolina Fargo, 1953 | 1 | ||||||
| Rissoellidae | Rissoella | caribaea Rehder, 1943 | 1 | 3 | ||||
| Pyramidellidae | Chrysallida | nioba (Dall & Bartsch, 1911) | 1 | |||||
| Odostomia | laevigata (d’Orbigny, 1841) | 1 | ||||||
| Triptychus | niveus (Mörch, 1875) | 1 | 1 | |||||
| Bullidae | Bulla | occidentalis A. Adams, 1850 | 1 | |||||
| Haminoeidae | Atys | sharpi Vanatta, 1901 | 1 | |||||
| Tornatinidae | Acteocina | candei (d’Orbigny, 1841) | 3 | 1 | ||||
| liratispira (E. A. Smith, 1872) | 1 | 2 | ||||||
| Volvatellidae | Ascobulla | ulla(Er. Marcus & Ev. Marcus, 1970) | 1 | |||||
| Class Bivalvia | ||||||||
| Nuculidae | Ennucula | delphinodonta (Mighels & C. B. Adams, 1842) | 1 | |||||
| Solemyidae | Solemya | occidentalis Deshayes, 1857 | 1 | 12 | ||||
| Arcidae | Barbatia | domingensis (Lamarck, 1819) | 1 | 3 | 1 | |||
| cancellaria (Lamarck, 1819) | 5 | 1 | 11 | 11 | ||||
| Noetiidae | Arcopsis | adamsi (Dall, 1886) | 1 | |||||
| Mytilidae | Lithophaga | antillarum (d’Orbigny, 1853) | 1 | |||||
| nigra (d’Orbigny, 1853) | 1 | |||||||
| Leiosolenus | bisulcatus (d’Orbigny, 1853) | 2 | 1 | 1 | ||||
| Musculus | lateralis (Say, 1822) | 1 | ||||||
| Botula | fusca (Gmelin, 1791) | 1 | ||||||
| Pteriidae | Pinctada | longisquamosa (Dunker, 1852) | 1 | |||||
| Limidae | Lima | caribaea d’Orbigny, 1853 | 1 | 5 | 1 | 1 | ||
| Pectinidae | Caribachlamys | ornata (Lamarck, 1819) | 2 | |||||
| Lucinidae | Ctena | orbiculata (Montagu, 1808) | 1 | |||||
| Lucina | pensylvanica (Linnaeus, 1758) | 1 | ||||||
| Condylocardiidae | Carditopsis | smithii (Dall, 1896) | 1 | 1 | ||||
| Ungulinidae | Diplodonta | notata (Dall & Simpson, 1901) | 1 | |||||
| Phlyctiderma | semiaspera (Philippi, 1836) | 1 | 1 | |||||
| Chamidae | Pseudochama | cristella (Lamarck, 1819) | 1 | |||||
| Cardiidae | Americardia | guppyi (Thiele, 1910) | 2 | 1 | ||||
| Laevicardium | mortoni Conrad, 1831) | 1 | ||||||
| Veneridae | Chioneryx | pygmaea (Lamarck, 1818) | 2 | 1 | ||||
| Petricola | lapicida (Gmelin, 1791) | 2 | 1 | |||||
| Gastrochaenidae | Lamychaena | hians (Gmelin, 1791) | 1 | |||||
| Spengleria | rostrata (Spengler, 1783) | 2 | 4 | |||||
| Thraciidae | Bushia | elegans (Dall, 1886) | 1 | |||||
| Class Polyplacophora | ||||||||
| Ischnochitonidae | Ischnochiton | erythronotus(C.B. Adams, 1845) | 1 | 6 | 11 | 6 | 1 | |
| hartmeyeri Thiele, 1910 | 3 | 1 | 1 | |||||
| Stenoplax | bahamensis Kaas & Van Belle, 1987 | 4 | 2 | 5 | 3 | 1 | ||
| boogii (Haddon, 1886) | 1 | 3 | ||||||
| floridana (Pilsbry, 1892) | 1 | |||||||
| Lepidochitonidae | Lepidochitona | liozonis (Dall & Simpson, 1901) | 1 | 2 | 5 | 2 | 3 | |
| rosea Kass, 1972 | 1 | |||||||
| Acanthochitonidae | Acanthochitona | lineata Lyons, 1988 | 1 | 1 | ||||
| roseojugum Lyons, 1988 | 1 | 2 | 5 | 2 | ||||
| zebra Lyons, 1988 | 5 | 15 | 8 | 1 | ||||
| andersoni Watters, 1981 | 1 | 2 | ||||||
| Cryptoconchus | floridanus (Dall, 1889) | 1 | ||||||
| Class Cephalopoda | ||||||||
| Octopodidae | Octopus | maya Voss & Solís, 1966 | 3 | |||||
| Class Scaphopoda | ||||||||
| Dentaliidae | Antalis | antillaris (d’Orbigny, 1853) | 3 | 1 | ||||
| Total | 24 | 85 | 133 | 73 | 154 | 184 | ||
The values obtained for the model used to construct the rarefaction curve (a = 0.397, b = 0.0018) result in a theoretical maximum number of species of a/b = 220.55 (Fig. 2; curve obtained with Chao 1 estimator), 100 more than what was obtained (Fig. 2; curve constructed with the observed values). This result suggests that the number of rare species, or species that occur with low numbers, can make up for more than half of the mollusc community in the area.
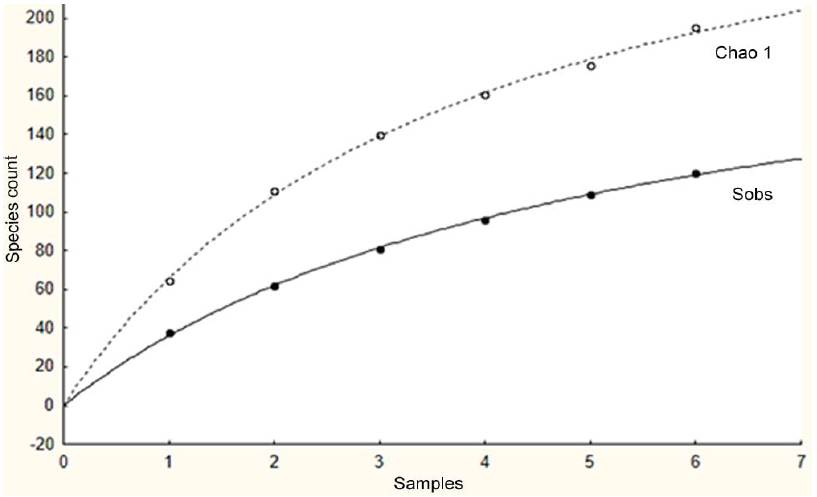
Figure 2 Rarefaction curve based on the Puerto Morelos mollusc data using Chao 1 estimator (Sobs = number of species observed; Chao1 = projected number of species under this estimator).
Three species in the family Phasianellidae were collected, of which Eulithidium bellum (M. Smith, 1937) was the most abundant gastropod with 122 (18%) individuals. The gastropods Columbella mercatoria (Linnaeus, 1758), Cerithium litteratum (Born, 1778) and Eulithidium bellum were present in all samples. The gastropod family Rissoini- dae was the most diverse group in the sampling with 5 species. Barbatia cancellaria (Lamarck, 1819) was the most abundant bivalve with 27 individuals. The polyplacophoran genus Acanthochitona was the most diverse with 4 species. Acanthochitona zebra Lyons, 1988 was the most abundant chiton species with 29 individuals, followed by Ischnochiton erythronotus (C.B. Adams, 1845) with 25 individuals. The classes Scaphopoda and Cephalopoda were represented by 1 species each (Table 1).
Class Gastropoda Cuvier, 1795.
OrderLittorinimorpha Golikov & Starobogatov, 1975.
Superfamily Truncatelloidea Gray, 1840
FamilyVitrinellidae Bush, 1897
Genus Circulus Jeffreys, 1865
Circulus semisculptus (Olsson & McGinty, 1958) (Fig.3a)
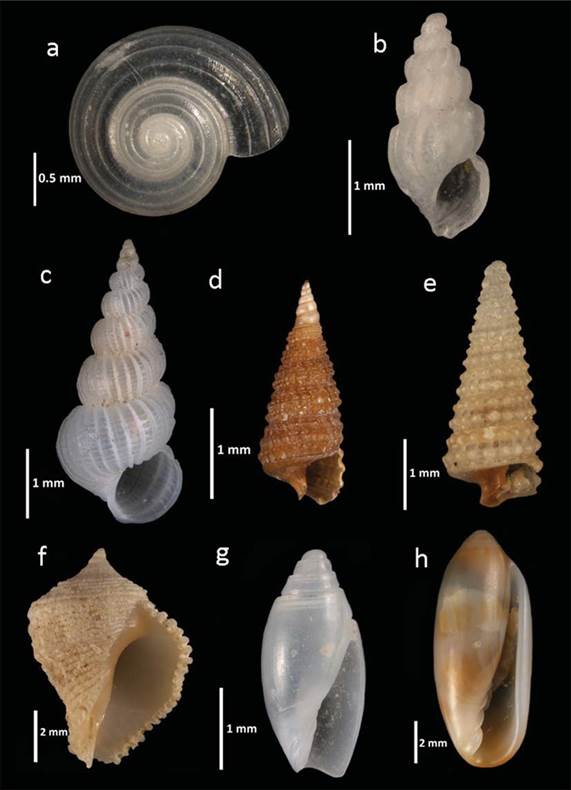
Figure 3 New records of molluscs from the PNAPM: a, Circulus semisculptus; b, Schwartziella bouryi; c, Epitonium turritellula; d, Cerithiopsis flava; e, Retilaskeya bicolor; f, Coralliophila galea; g, Olivella acteocina; h, Volvarina ceciliae.
Taxonomic summary
Synonymy:Vitrinella semisculpta Olsson & McGinty, 1958.
Material examined: 1 individual; Jardines, PNAPM; 14-I-2014; COMA-0000001771.
Distribution:USA (Florida), throughout the Caribbean including the Greater Antilles, Costa Rica, Panama and Colombia (Abbott, 1974; Miloslavich et al., 2010; Rosenberg, 2009; Rosenberg et al., 2009).
FamilyZebinidae Coan, 1964
Genus SchwartziellaG. Nevill, 1881
Schwartziella bouryi (Desjardin, 1949) (Taxon inquirendum) (Fig.3b).
Taxonomic summary
Original name:Rissoina bouryi Desjardin, 1949
Material examined:1 individual; Puerto Morelos, PNAPM; 14-I-2014; COMA-0000001832.
Distribution:USA(Florida), Bahamas and Cuba (Miloslavich et al., 2010; Rosenberg, 2009; Rosenberg et al., 2009).
Order Caenogastropoda Cox, 1960
Superfamily Epitonioidea Berry, 1910
Family Epitoniidae Berry, 1910 (1812)
Genus EpitoniumRöding, 1798
Epitonium turritellula (Mörch, 1875) (Fig. 3c)
Taxonomic summary
Synonymy:Scala turritellula var. riisei Mörch, 1875; Scala turritellula Mörch, 1875; Scala stylina Dall, 1889; Scala rushi var stylina Dall, 1889; Epitonium turritellulum (Mörch, 1874); Epitonium (Asperiscala) turritellulum.
Material examined: 2 individuals, Puerto Morelos, PNAPM; 14-I-2014; COMA-0000001826.
Distribution:USA (Texas), Cuba, Jamaica, Dominican Republic, Puerto Rico,U.S. Virgin Islands, Aruba, Bonaire, Curaçao, Venezuela and Brazil (Miloslavich et al., 2010; Rosenberg, 2009; Tunnell et al., 2010; Warmke & Abbott, 1962).
Superfamily Triphoroidea Gray, 1847
Family Cerithiopsidae H. Adams & A. Adams, 1853
GenusCerithiopsisForbes & Hanley, 1850
Cerithiopsis flava (C.B. Adams, 1850) (Fig.3d)
Taxonomic summary
Synonymy:Cerithium flavum C. B. Adams, 1850; Cerithiopsis flavum (C. B. Adams, 1850); Bittium flava(C.B. Adams, 1850);Cerithiopsis hero Bartxch, 1911.
Material examined: 2 individuals; Jardines, PNAPM;6-III-2013; COMA-0000001829.1 individual; Puerto Morelos, PNAPM; 7-V-2014; COMA-0000001836.
Distribution:USA (Florida,Louisiana, Texas), Cuba, Belize, Jamaica, U.S. Virgin Islands, Aruba, Bonaire and Curaçao (Miloslavich et al., 2010; Rosenberg, 2009; Rosenberg et al., 2009; Tunnell et al., 2010).
Family Newtoniellidae Korobkov, 1955
GenusRetilaskeyaB.A. Marshall, 1978
Retilaskeya bicolor (C.B. Adams, 1845) (Fig.3e )
Taxonomic summary
Synonymy:Cerithium bicolor C. B. Adams, 1845; Cerithiopsis bicolor (C. B. Adams, 1845); Bittium bicolor (C. B. Adams, 1845); Bittium major Mörch, 1876; Cerithiopsis binoda Usticke, 1969; Cerithiopsis subulata Montagu, 1808; Cerithiopsis emersoni C. B. Adams, 1839; Eumetula emersoni C. B. Adams, 1839.
Material examined: 1 individual; Bonanza, PNAPM; 14-I-2014;COMA-0000001810.
Distribution:USA (Massachusetts, Florida, Texas), Cuba, Jamaica, Puerto Rico, Mexico (Yucatan), Belize, Costa Rica, Panama, Colombia, Venezuela, Lesser Antilles and Brazil (Abbott, 1974; Miloslavich et al., 2010; Rosenberg, 2009; Tunnell et al., 2010).
OrderNeogastropoda Wenz, 1938
Superfamily Muricoidea Rafinesque, 1815
Family Muricidae Rafinesque, 1815
Genus Coralliophila H. Adams & A. Adams, 1853
Coralliophila galea (Dillwyn, 1823) (Fig.3f)
Taxonomic summary
Synonymy:Murex galea Dillwyn, 1823; Coralliophila abbreviata Lamarck, 1816; Rhizochilus abbreviata Lamarck, 1816; Purpura abbreviataLamarck, 1816; Coralliophila deformis Lamarck, 1822; Coralliophila nodulosa H.Adams & A. Adams, 1864; Coralliophila undosa H. Adams & A. Adams, 1864; Purpura miocenica Guppy, 1873.
Material examined: 1 individual; Jardines, PNAPM;6-V-2013;COMA-0000001805.
Distribution:Bermuda, USA (North Carolina, Florida, Texas), Mexico (Yucatan), Belize, Cuba, Jamaica,Cayman Islands, Costa Rica, Panama, Colombia, Aruba, Bonaire, Curaçao and Brazil(Miloslavich et al., 2010; Rosenberg, 2009; Rosenberg et al., 2009).
Superfamily Olivoidea Latreille, 1825
Family Olividae Latreille, 1825
Genus Olivella Swainson, 1831
Olivella acteocina Olsson, 1956 (Fig.3g)
Taxonomic summary
Material examined: 1 individual; Puerto Morelos, PNAPM; 14-I-2014; COMA-0000001831.
Distribution: Mexico(Yucatan),Cuba, Puerto Rico, Panama, Bahamas(Grand Bahama Island, New Providence), St. Vincent and the Grenadines, Colombia, Aruba, Bonaire and Curaçao (Abbott, 1974; Miloslavich et al., 2010; Rosenberg, 2009; Rosenberg et al., 2009; Vokes & Vokes, 1983; Warmke & Abbott, 1962).
Family Marginellidae Fleming, 1828
Genus VolvarinaHinds, 1844
Volvarina ceciliae Espinosa & Ortea, 1999 (Fig.3h)
Taxonomic summary
Material examined:1 individual; Bonanza, PNAPM; 6-V-2013; COMA-0000001715.1 individual, Puerto Morelos, PNAPM; 7-XI-2013; COMA-0000001751. 1 individual; Puerto Morelos, PNAPM; 6-III-2013; COMA-0000001752.
Distribution: Cubaand Cayman Islands (Miloslavich et al., 2010; Rosenberg, 2009).
Superfamily Conoidea Fleming, 1822
FamilyMangellidae P. Fischer, 1883
Genus Granoturris Fargo, 1953
Granoturris padolinaFargo, 1953 (Fig.4a )
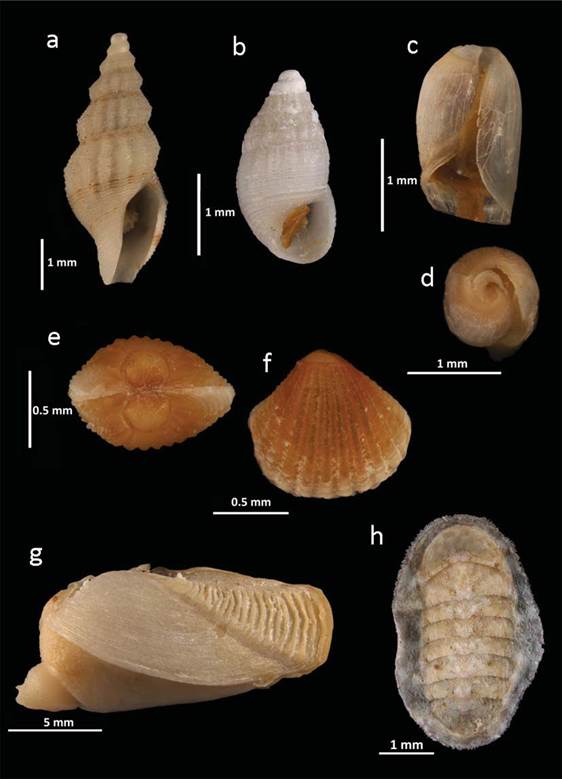
Figure 4 New records of molluscs from the PNAPM: a, Granoturris padolina; b, Chrysallida nioba; c, d, Ascobulla ulla; e, f, Carditopsis smithii; g, Spengleria rostrata; h, Ischnochiton hartmeyeri.
Taxonomic summary
Synonymy:Kurtziella padolina (Fargo, 1953).
Material examined:1 individual; Bonanza, PNAPM; 6-VIII-2013; COMA-0000001812.1 individual, Puerto Morelos, PNAPM; 7-V-2014; COMA-0000001833.
Distribution: USA (Florida, Texas), Bahamas and Brazil (Ríos, 1994; Rosenberg et al., 2009).
Superfamily Pyramidelloidea Gray, 1840
Family Pyramidellidae Gray, 1840
Genus Chrysallida Carpenter, 1857
Chrysallida nioba (Dall & Bartsch, 1911) (Fig.4b)
Taxonomic summary
Synonymy:Odostomia niobaDall & Bartsch, 1911; Menestho nioba (Dall & Bartsch, 1911).
Material examined: 1 individual, Jardines, PNAPM; 6-III-2013; COMA-0000001830.
Distribution:Bermuda,USA (Florida, Texas) and Cuba (Miloslavich et al., 2010; Rosenberg, 2009; Rosenberg et al., 2009).
Order Sacoglossa Ihering, 1876
Family Volvatellidae Pilsbry, 1895
Genus Ascobulla Ev. Marcus, 1972
Ascobulla ulla (Er. Marcus & Ev. Marcus, 1970) (Fig.4c, d)
Taxonomic summary
Synonymy:Cylindrobulla ullaEr. Marcus & Ev. Marcus, 1970
Material examined: 1 individual;Puerto Morelos, PNAPM; 6-VIII-2013; COMA-0000001827.
Distribution:Bermuda, Bahamas (Grand Bahama Island), USA(Florida), Mexico, Belize, Turks and Caicos, Cayman Islands,U.S. Virgin Islands, Venezuela, Lesser Antilles and Brazil (Pernambuco, Abrolhos Islands, Sao Paulo)(Miloslavich et al., 2010; Rosenberg, 2009; Rosenberg et al., 2009).
Class Bivalvia Linnaeus, 1758
Subclass Heterodonta Neumayr, 1884
Order Carditoida Dall, 1889
Family Condylocardiidae Bernard, 1896
GenusCarditopsis E.A. Smith, 1881
Carditopsis smithii (Dall, 1896) (Fig.4e, f)
Taxonomic summary
Synonymy:Carditella smithii Dall, 1896; Condylocardia floridensis Pilsbry & Olsson, 1946.
Material examined: 1 individual; Puerto Morelos, PNAPM; 7-V-2013; COMA-0000001835. 1 individual; Puerto Morelos, PNAPM; 14-I-2014; COMA-0000001834.
Distribution: Bermuda, USA (Florida, Texas),Bahamas, Mexico (Yucatan), Cuba, Colombia,Aruba, Bonaire, Curacao and Brazil (Abbott,1974; Merlano & Hegedus, 1994; Ríos, 1994; Mikkelsen &Bieler, 2008; Miloslavich et al., 2010; Rosenberg et al., 2009; Tunnell et al.,2010).
Order Veneroida H. Adams & A. Adams, 1856
Family Gastrochaenidae Gray, 1840
Genus Spengleria Tryon, 1861
Spengleria rostrata (Spengler, 1793) (Fig.4g)
Taxonomic summary
Synonymy:Chaena rostrata Spengler, 1793;Gastrochaena callosa Philippi, 1845; Gastrochaena chemnitziana d’Orbigny, 1854.
Material examined: 4 individuals; Puerto Morelos, PNAPM; 14-I-2014; COMA-0000001736.1 individual; Puerto Morelos, PNAPM; 6-VIII- 2013;COMA-0000001765.1 individual; Puerto Morelos, PNAPM; 6-VIII-2013; COMA-0000001801.
Distribution:Bermuda, USA (North Carolina, Florida, Alabama), Cuba, Puerto Rico, Jamaica, Cayman Islands, Lesser Antilles, Costa Rica, Colombia and Brazil (Abbott, 1974; Abbott & Dance, 1982; Mikkelsen & Bieler, 2008; Miloslavich et al., 2010; Rosenberg, 2009; Rosenberg et al., 2009; Warmke & Abbott, 1962).
Class Polyplacophora Gray, 1821
Order Chitonida Thiele, 1909
Family Ischnochitonidae Dall, 1889
Genus Ischnochiton Gray, 1847
Ischnochiton hartmeyeri Thiele, 1910 (Fig.4h)
Taxonomic summary
Material examined:1 individual; Jardines, PNAPM; 7-XI-2013; COMA-0000001792. 1 individual; Puerto Morelos, PNAPM;14-I-2014;COMA-0000001793. 3 individuals;Puerto Morelos, PNAPM; 6-VIII-2013; COMA-0000001828.
Distribution:USA (Florida),San Salvador, Jamaica, Cayman Islands, Belize, Puerto Rico, U.S. Virgin Islands, British Virgin Islands, Aruba, Curaçao and Brazil (Lagoas, Isla Guataquaz) (Ferreira, 1987; García-Ríos, 2003; Kaas, 1972; Lyons,1980, 1989; Lyons & Moretzsohn, 2009).
Discussion
In spite of the abundance and diversity of molluscs in coastal and reef environments, relatively few studies have been conducted in the Mesoamerican Reef, especially in the Mexican portion of it. Castillo-Rodríguez (2014) estimated at 2,067 the number of species of molluscs for the Mexican coast of the Gulf of Mexico and Mexican Caribbean, while 2,567 species are known for the Pacific coast. If the projections of number of species to be found in the Mexican Caribbean are realistic, then the total number of species for the eastern coast of Mexico will be greater than that for the Pacific coast. Indirect support for this idea could be the estimation of Miloslavich et al. (2010) who placed the “western Caribbean” region, which includes Mexico, as contributing with only 10% of the total mollusc diversity for the whole Caribbean Sea, suggesting that the regional diversity in this area is still underestimated.
Although a total of 653 molluscs were collected, belonging to 120 species, all the species presented here as new distributional records were represented by 1 to 6 individuals only, indicating that they are naturally rare in this area. Nine of the 14 species were collected only once, 2 were collected twice and 3 were collected 3 times; only 4 species were collected in more than 1 site (Table 1). Further, it is relevant to note that most of the new records of species (10 out of 14, 71%) and the highest number of its organisms (21 out of 30, 70%) came from the Puerto Morelos site, the most conserved one (Monroy-Velázquez &Álvarez, 2016; Monroy-Velázquez et al., 2017; Rodríguez-Martínez et al., 2010; Table 1). Taxonomically, the new records are dominated by the gastropods with 11 species, followed by bivalves with 2 species and 1 polyplacophoran. The “degraded” and “in recovery” localities had 5 and 3 of the new records, respectively (Table 2).
Table 2 Collection site and number of specimens collected of each of the species representing a new distributional record for the Mexican Caribbean.
| Jardines Degraded | Puerto Morelos Conserved | Bonanza In recovery | |
|---|---|---|---|
| Circulus semisculptus | 1 | ||
| Schwartziella bouryi | 1 | ||
| Epitonium turritellula | 2 | ||
| Cerithiopsis flava | 2 | 1 | |
| Retilaskeya bicolor | 1 | ||
| Coralliophila galea | 1 | ||
| Olivella acteocina | 1 | ||
| Volvarina ceciliae | 2 | 1 | |
| Granoturris padolina | 1 | 1 | |
| Chrysallida nioba | 1 | ||
| Ascobulla ulla | 1 | ||
| Carditopsis smithii | 2 | ||
| Spengleria rostrata | 6 | ||
| Ischnochiton hartmeyeri | 1 | 4 |
Overall, mollusc diversity of the PNAPM is underestimated, as intensive sediment sampling, or sampling with several different methods has not been conducted yet. Other studies that have used several sampling techniques, including suction sampling on hard and soft bottoms, have found thousands of species of molluscs in one reef (Bouchet et al., 2002). When the sampling in a reef environment focuses on micromolluscs, the results can yield several hundred species, with a large proportion of them undescribed (Albano et al., 2011). Future efforts along the Caribbean coast of Mexico should focus on this particular component of the mollusc fauna using several collecting techniques.
Many species of marine molluscs in Mexico are being impacted by: urban wastewater, hydrocarbons, pesticides, competition with introduced species and overexploitation; factors that are modifying their distribution patterns and abundance. The IUCN has assessed the conservation status of about 10% of the described species of molluscs, and one- fourth of those are threatened (IUCN, 2019). In Mexico, the NOM-059 (the Mexican Red List of Endangered Species) includes only 17 species, a figure that clearly shows the lack of studies on this fauna.











 nueva página del texto (beta)
nueva página del texto (beta)


