Introduction
According to Semarnat (2017), the Environmental Management Units (UMA for its acronym in Spanish) are defined as facilities in which owners voluntarily use them for the sustainable management of wild species. The UMA also respond to the need to conserve biodiversity and boost production and socioeconomic development of the country. Among the species protected in the UMA is Boa constrictor Linnaeus, 1758, which is considered as an endangered species (CITES, 2013). However, in spite of the transcendental role of diseases in the wildlife conservation (Daszak et al., 2000), the pathologic effects of the etiological agents that affect it has been neglected. Mostly of the works related to B. constrictor exclusively refer the presence of some parasites; however, to the best of our knowledge, only isolated cases of tissular lesions caused by virus, bacteria, flagellates and amoebas have been described (Ǻgren & Anderson, 2011; Gál & Kiadó, 2004; Souza et al., 2014). Thus, the objectives of this study were to increase the knowledge on the richness of parasites found in B. constrictor in Mexico, as well as to describe their pathological effects.
Materials and methods
The UMA “El Palapo” (Semarnat-UMA-IN-029-COL/2006) is located in Coquimatlán (19°12’ N, 103°49’ W), Colima, Mexico and harbors captured or confiscated snakes, which are conserved outside their wild habitats. Between August 2013 and February 2015, 16 B. constrictor died in the UMA by natural causes and they were submitted for necropsy and parasitological study to the Laboratorio de Patología de la Universidad de Colima, Colima, Mexico. Blood smears were taken for each snake, which were also sent for diagnostics of hemoparasites. Ectoparasites were collected directly from skin and scales with the aid of tweezers and brushes and fixed in 70% alcohol. Damaged organs were immersed in buffered 10% formalin; samples of each tissue were included in paraffin, cut with a thickness of 4 μm and stained with hematoxylin-eosin and Ziehl-Neelsen (Prophet et al., 1995). Helminths were collected with brushes, washed in 0.65% saline solution and fixed with hot 4% formalin; all specimens collected were preserved in 70% alcohol. Cestodes were stained with Mayer´s paracarmin and permanentely mounted with Canada balsam; nematodes and pentastomids were cleared with Amman´s lactophenol (Lamothe-Argumedo, 1997). Voucher specimens of some parasites were deposited at the Colección Nacional de Helmintos (CNHE 11070), housed in the Instituto de Biología, Universidad Nacional Autónoma de México, México City. Prevalence (%) was calculated in all cases; mean intensity (MI) only for helminths and arthropods found in more than 2 hosts. Both parametters follow Bush et al. (1997).
Results
Sixteen B. constrictor (9 females, 7 males, mean body length: 0.963 m, mean body weigth: 1.59 kg) were revised. All snakes examined harbored at least 1 parasite species or had metabolic alterations.
Parasite infections. Etiological agents of the parasitic infections were from 9 taxa belonging to 6 phyla: Actinobacteria (Mycobacterium sp.); Ascomycota (Aspergillus sp.); Apicomplexa (Hepatozoon sp. and Cryptosporidium sp.); Platyhelminthes (Crepidobothrium sp.); Nematoda (Macdonaldius oschei Chabaud et Frank, 1961), and Arthropoda: Ophionyssus natricis (Gervais, 1844), Amblyomma dissimile Koch, 1844 and Porocephalus sp.
Metabolic disease. This was diagnosed as visceral gout, which affected heart, liver, kidneys and lungs (Fig. 1A, B).
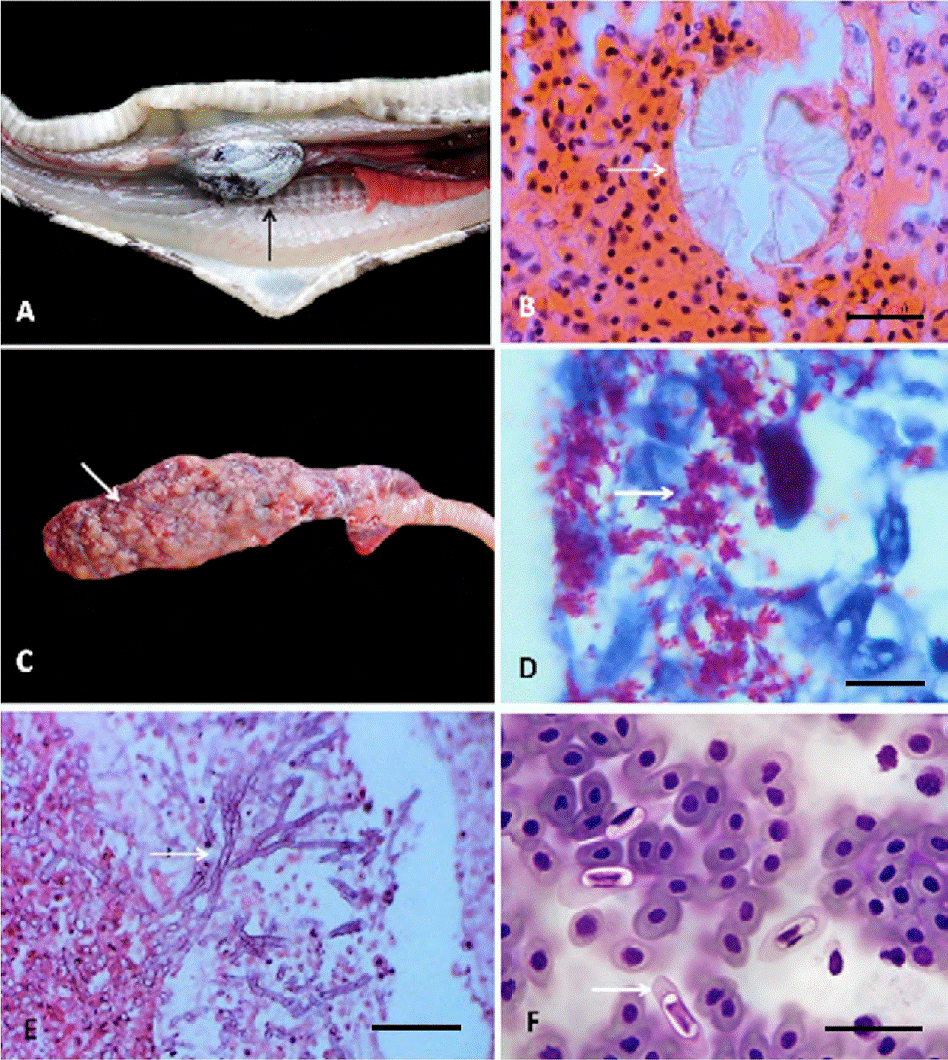
Figure 1 A) Visceral gout in heart, showing diffuse whitish granular material on the epicardium (arrow); B) rosettes of crystals of uric acid (arrow), hematoxyline-eosin; C) lung with multifocal nodules of 1-3 mm of length (arrow); D) granulomatous pneumonia with alcohol-acid resistant bacilli of Mycobacterium sp. (arrow), Ziehl-Neelsen; E) granulomatous pneumonia with hyphae of Aspergillus sp. (arrow), hematoxyline-eosin; F) blood smear with presence of Hepatozoon sp. (arrow), Giemsa-Wrigth. Scale bar: 25 μm.
The most common disease was visceral gout in 5 snakes (31.2%), followed by hepatozoonosis and acariosis in 4 boas (25%, respectively), cryptosporidiosis in 3 (18.7%), tick infestation in 2 (12.5%), as well as 1 case mycobacteriosis, aspergillosis, cestodosis, nematodosis, and porocephalosis (6.25% each).
In the lungs, Mycobacterium sp. and Aspergillus sp. produced multifocal granulomatous pneumonias, with intralesional bacilli resistant to alcohol and septate hyphae, respectively, represented by nodular lesions, whitish and firm, 1 × 3 mm in diameter with caseous necrosis (Fig. 1 C-E).
The infection for Hepatozoon sp. (more than 4 merozoites by field) occasioned cellular hypertrophy and cytoplasmic pallor in the erythrocytes, as well as nuclear displacement towards the periphery (Fig. 1F).Trophozoites of Cryptosporidium sp. caused infiltration of heterophilic and mononuclear inflammatory cells in the surface of the gastric and intestinal epithelial membranes, leading to moderate focal heterophilic gastroenteritis (Fig. 2A).
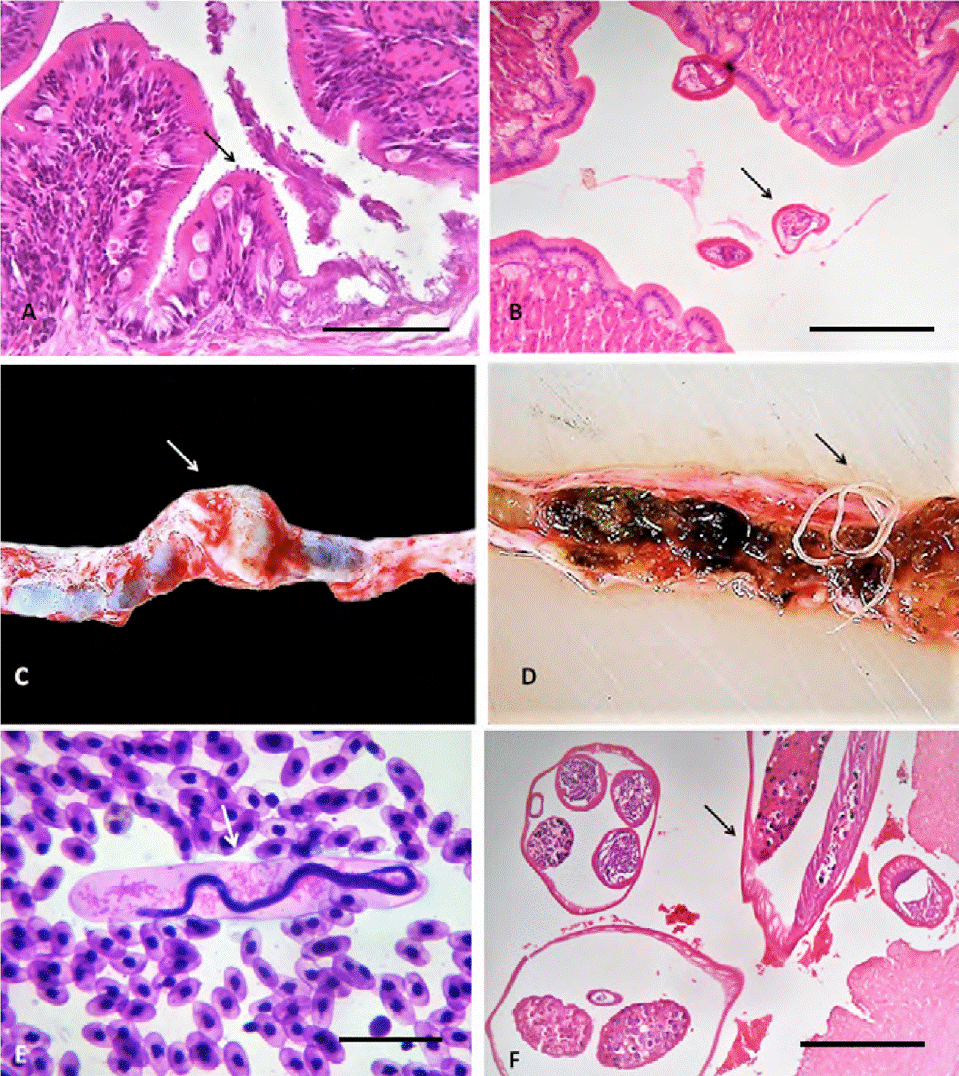
Figure 2 A) Heterophilic gastritis by Cryptosporidium sp. in the apex of enterocytes (arrow), hematoxyline-eosin. Scale bar: 200 μm; B) heterophilic enteritis by Crepidobothrium sp. (arrow), hematoxyline-eosin. Scale bar: 500 μm; C-D) aneurysm in the caudal renal vein (arrow) caused by Macdonaldius oschei (arrow), hematoxyline-eosin; E) blood smear with presence of M. oschei microfilariae (arrow), Giemsa-Wrigth. Scale bar: 25 μm; F) caudal renail vein with M. oschei adults forming a thrombus (arrow), hematoxyline-eosin. Scale bar: 25 μm
The presence of 1 adult cestode (Crepidobothrium sp.) determined the increase of mucus on the intestinal surface, as well as the infiltration of heterophilic inflammatory cells on the mucosa and lamina propria, with hyperplasia of goblet cells, i.e., a moderate focal heterophilic catarrhal enteritis (Fig. 2B). The adults of M. oschei were found in the caudal renal vein of 1 snake, close to the kidneys, resulting in a 1.5 × 1 cm aneurysm with 35 parasites (Fig. 2C, D, respectively); its microfilariae (with a mild parasitemia of less than 200 individuals per cm3) were observed in peripheral blood surrounded by a broadly dilated sheath. These larvae were located in kidneys and lungs, which involved a discrete heterophilic inflammatory infiltration with focal interstitial nephritis and pneumonitis, respectively (Fig. 2E). Adults worms induced a thrombus with a focal heterophilic vasculitis (Fig. 2F).
A total of 270 acarine Ophionyssus natricis infecting 4 snakes (MI = 67.5 individuals per infected host) and 17 A. dissimile in 2 (MI = 8.5) were located between the scales and the skin (Fig. 3A, B, respectively), causing loss of scales with infiltration of heterophilic cells to the dermis resulting in moderate heterophilic focal superficial dermatitis (Fig. 3C). The only pentastomid collected (Porocephalus sp.) also inhabited the lung tissue of the snakes, producing a discrete interstitial heterophilic inflammatory infiltration, with muscular hyperplasia (Fig. 3D).
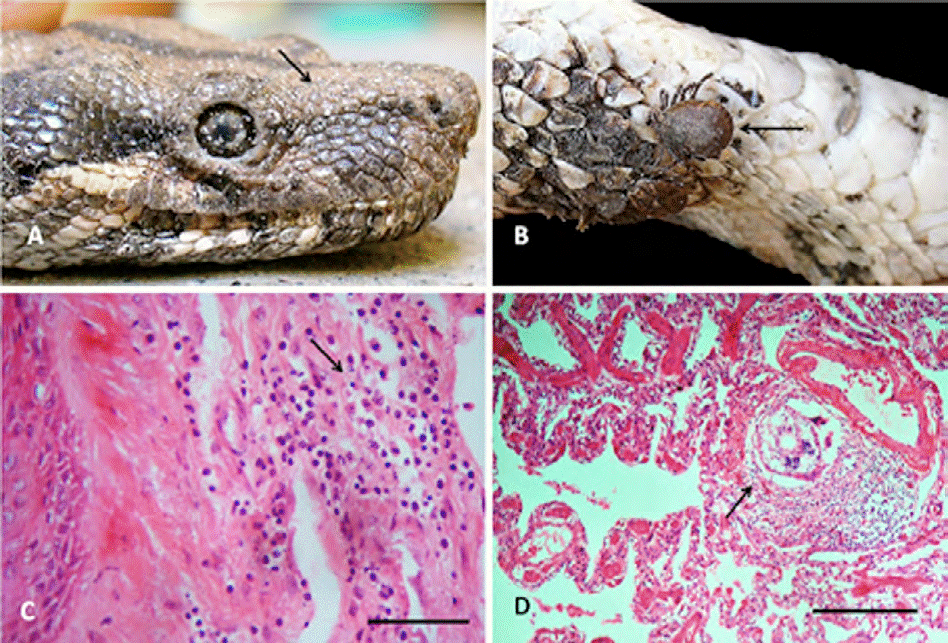
Figure 3 A) Head of Boa constrictor with numerous individuals of the acari Ophionyssus natricis; B) Amblyomma dissimile attached to the cervical region of B. constrictor (arrow); C) heterophilic dermatitis by O. natricis (arrow), hematoxyline-eosin. Scale bar: 25 μm; D) focal pneumonitis by Porocephalus sp. (arrow), hematoxyline-eosin. Scale bar: 200 μm.
The visceral gout found in several organs produced external and internal lesions of 1 to 3 mm in diameter, firm, whitish, multifocal and of nodular appearance. The lesions correspond to granulomas formed by an outer capsule of fibrous tissue and infiltration of inflammatory cells: histiocytes, epithelioids, foreign-body giant cells and to the center a necrosis with deposits of crystals of uric acid forming rosettes (tophi).
Discussion
According to González-Solís et al. (2014), the parasitological record of B. constrictor in Mexico is constituted by 15 taxa: 3 platyhelminths [Crepidobothrium gerrardi (Baird, 1860) Monticelli, 1900, Styphlodora horridum (Leidy, 1850) Odhner, Telorchis corti Stunkard, 1916], 1 acanthocephalan (Acanthocephla gen. sp.), 8 nematodes (Contracaecum sp., Cruzia sp., Gnathostoma binucleatum Almeyda-Artigas, 1991, Gnathostoma sp., Kalicephalus subulatus Molin, 1861, Macdonaldius colimensis Telford, 1965, Macdonaldius oschei Chabaud and Frank, 1961, Physocephalus sp.), and 3 arthropods [A. dissimile, O. natricis, Porocephalus clavatus (Wyman, 1845)]. Our study increased the number of parasites infecting this reptile species, since we found 1 Actinobateria (Mycobacterium sp.), 1 Ascomycota (Aspergillus sp.) and 2 Apicomplexa (Hepatozoon sp. and Cryptosporidium sp.). Most likely the finding of these species had not occurred because the previous works conducted on this host did not carry out histological studies.Thus, we can conclude that the parasitic record of this reptile species is scarce and fragmentary, since most of the information comes from punctual collections in 12 localities belonging to 7 Mexican states (González-Solís et al., 2014; Paredes-León et al. 2008). This is the first study describing the etiological agents and lesions they cause in B. constrictor. However, the reduced number of individuals of most parasites precluded their specific identification, particularly in the case of the cestode and the pentastomid, since the study of their pathologic effects was preferred.
In general, one of the most common pathologies observed in captive B. constrictor is pneumonia. Its prevalence increases when the reptile is stressed, due to inappropriate temperature, humidity, diet, transportation, and parasites (Timmerman & Doolen, 1994). However, in the present study, pneumonia was only observed in 2 specimens, 1 infected with Aspergillus sp. and the other with Mycobacterium sp. Pneumonia and subcutaneous nodules caused by Mycobacterium thamnopheos Aronson, 1929 have been reported previously in a B. constrictor purchased in a pet shop in USA (Kiel, 1977). Moreover, fungal pneumonia with presence of intralesion hyphae of Aspergillus has been described from several reptiles species, causing granulomatous lesiones (Girling & Fraser, 2009), similar to those described in our study.
Hepatozoonosis was the most common parasitosis, occurring both in asymptomatic and diseased snakes; this condition has been previously reported in B. constrictor from Mexico (Gordon et al., 1969) and Brazil (Lopes et al., 2010). Its prevalence can be explained based on its transmission, which involves 1 arthropod as vector (ticks, mites, mosquitoes, etc.). Intraerythrocytic hepatozoonosis is considered asymptomatic; however, it can cause hemolytic anemia (subclinical infections), immunosuppression and predisposition to secondary infections (Lopes et al., 2010; Luz et al., 2012). In Brazil, the prevalence of infection ranged 16.4-38.9% in captive B. constrictor, but may be in latent period with low percentages of parasitemia (0.15-0.52%). The infection recorded in the Colima boas (more than 4 merozoites by field) is grave in accordance with the classification of De Biase et al. (1989), that established this level based in the presence of more than 3 parasites per field. The injuries observed in boas parasitized by Hepatozoon sp. included erythrocyte hypertrophy; however, no fusiform erythrocytes, nor clinical, hematological alterations and hemolytic anemia were observed, as was reported by Lopes et al. (2010), probably due to differences in post-infection time, wich by the way, is unknown in both studies.
Cryptosporidiosis has previously been recorded in the gastrointestinal epithelium of Boa sp., causing epithelial hyperplasia of goblet cells and lymphoid tissue (Karazawa et al., 2002; Ruggiero et al., 2011). These lesions are compatible with those found in this study. However, it is unknown if the symptoms reported by these authors (weight loss, regurgitations or diarrhea) were presented by the Colima snakes because these were not registered in the UMA. In addition, according to Upton et al. (1989), this disease commonly occurs in a subclinical manner.
The heterophilic catarrhal enteritis derived from the presence of the cestode Crepidobothrium sp., may be considered mild, similar to that ocasionated by one unidentified cestode species in Bothrops jararaca (Wied-Neuwied, 1824) from Brazil responsible for the congestion of the intestine of this host (Grego et al., 2004).
The parasitemia by M. oschei recorded in this study (less than 200 individuals per cm3) was lower than that reported by Telford (1965) for B. constrictor also in Colima (10,000 microfilariae per cm3). The lesions produced by filariae in the vena cava were less severe than those described by Telford (1965), particularly in terms of the number of aneurysms found (1 in the present study and 1-3 in Telford’s study [1965]).
The presence of O. natricis and A. dissimile is common in captive reptiles, including B. constrictor (Paredes-León et al., 2008). These arthropods caused simple mechanical action (ulcerations in the dermis) and dermatitis in 6 specimens. These lesions predispose the host to secondary infections by bacteria, fungi or larvae of insects (Carrascal et al., 2009). In addition, they can produce severe anemia, immunosuppression and act as vectors of Hepatozoon spp., filariasis and retroviruses. In massive infestations, occasionally can kill the host (Chang & Jacobson, 2010; Guglielmone & Nava, 2006). Their presence in the snakes suggest a role as vectors of the infections produced by Hepatozoon sp. and M. oschei in Colima, as previously reported for this host in Mexico (Gordon et al., 1969) and Brazil (Lopes et al., 2010).
Injuries caused by Porocephalus sp. (interstitial heterophilic inflammatory infiltration, with muscle hyperplasia) are similar to those reported by Grego et al. (2004) in the pit viper B. jararaca from Brazil, differing only in the degree of the lesions, since in our study we found slight effects in only 1 boa sampled, while in B. jararaca they reported them as subacute throughout the year of sampling. Probably, such differences can be attributed to the number of parasites involved (1 in our study and numerous but not specified, in the pit vipers). Porocephalus spp. are potentially zoonotic; people may be exposed to their ova, particularly workers of snake-farms, snake keepers in zoos and pet shops, veterinarians, etc. (Tappe & Büttner, 2009).
In addition to the parasites found, we also detected a metabolic alteration (visceral gout), which is one of the most serious and common pathologies in reptiles. Several risk factors contribute to the presence of this disease, such as dehydration, excess of protein in diet, renal tubular damage, and the application of nephrotoxic drugs, causing a state of hyperuriciemia (Mader, 2006). The granulomatous lesions observed in several organs in our study are due to the deposits of uric acid crystals, as previously described by Nasoori et al. (2015) for the cobra Naja oxiana. The relatively high prevalence of snakes with visceral gout found by us (31.2%) can be attributed to changes in diet and/or dehydration suffered during their captivity. Both factors reduced the function of renal tubules, as was reported for one captive alligator from Edimburg (Appleby & Siller, 1960).
Based on the results obtained in the present work, it is relevant to recommend the establishment of diagnostic protocols (at least one blood smear, a coproparasitoscopy and urine studies), prior to the entry of reptiles to the UMA. The captivity of snakes, without the support of preventive medicine, should be considered as a risk factor for the manifestation or exacerbation of metabolic alterations and tissular damages caused by infectious agents. The lack of an adequate management plan, along with environmental conditions, presence of vectors, deficient diet, stress, immunosuppression and coexistence with other animals, favor the accumulation of nitrogen compounds (causative of visceral gout), as well as the transmission of pathogens. The implementation of the management plan proposed for reptiles, will allow to establish the origin of the diagnosed alterations, that is, before being introduced to the UMA or if they were acquired within.











 nova página do texto(beta)
nova página do texto(beta)


