Introduction
The Mezquital Valley, a semiarid zone in the state of Hidalgo, Mexico, is a very important agricultural area with about 90,000 ha irrigated with waste water from Mexico City. This area represents the largest scheme of waste water use in Latin America (Braatz & Kandiah, 1996; Prado et al., 2015). The municipality of San Salvador, Hidalgo, has an important agricultural surface where the main crops are alfalfa (Medicago sativa L.), maize (Zea mays L.) and beans (Phaseolus vulgaris L.). According to the “Main Development Plan” of the Municipality (Ayuntamiento de San Salvador, Hgo., 2012) some plots have been irrigated with waste water for more than 100 years (Prado et al., 2015).
The use of waste water in the Mezquital Valley is an undervalued resource for agriculture due to the high concentration of nutrients contained in the water (Romero, 1997; Sagasta et al., 2013; Siebe & Cifuentes, 1995). Nevertheless, the frequent irrigation with such waters for a long period has led to the accumulation of several metals and other elements in the agricultural soils in Mexico. Such accumulation can be toxic for the animals living there (Aktar et al., 2009; Prieto-García et al., 2007; Siebe & Fisher, 1996).
Oribatid mites (Cryptostigmata) are, by their number and ecological role, one of the most dominant groups in most of the organic layers of soil where their densities can reach several hundreds of thousand specimens per m2 (Borah & Kakati, 2013; Norton, 1990). Those mites can usually be found where there is an accumulation of organic matter with certain humidity and temperature ranges. Many oribatid mite species live in natural and altered conditions. Other species have a limited distribution or have a large abundance in places with an anthropogenic impact (Aoki, 1979; Eeva & Penttinen, 2009; Hunter, 2007). These mites are usually characterized by having a low fecundity and a very low capacity to increase their populations in a short time. Very few species have modifications for dispersion, so they cannot escape from the stress of their environments. This characteristic has made them very important as bioindicators due to the incorporation of information about the habitat and niche history and profiles (Behan-Pelletier, 1999; Gulvik, 2007). One example of oribatid mites as bioindicators is Humerobates rostrolamellatus which has a highly restricted relation to heavy metal pollutants (Lebrun & van Straalen, 1995). On the other hand, Al-Assiuty et al. (2000) have shown that species such as Rhysotritia ardua, Oppia bifurcata, Niloppia sticta, Striatoppia niliaca, and Microzetes alces, are restricted to residual muds, pointing out that Scheloribates laevigatus and Epilohmannia cylindrica cylindrica are very tolerant to soils with residual mud with high levels of organic matter, and heavy metals like Fe, Zn, Cu, Mg and Cd mainly.
Studies carried out in the Mezquital Valley on the quality of the irrigation water have shown that after 80 years of use, the content of metals in the plots irrigated with wastewater is 3 to 6 times higher than in the plots irrigated with well water, and particularly Cd, Pb, Cr, and Zn, are incorporated in the soil from the irrigation accumulated in the arable layer (Herre et al., 2004; Siebe, 1994). Among soil invertebrates, oribatid mites have been recorded as the group that can accumulate the highest heavy metal concentrations in different soils (van Straalen et al., 2001). The high concentration of heavy metals in soils can have a negative effect on oribatid mite richness and their reproduction (El-Sharabasy & Ibraim, 2010). Those negative effects can be direct by the poisoning of the specimens or indirect through the disturbance of the trophic web (Parmelee et al., 1993; Vladislav et al., 2015).
Due to the poor knowledge of the ecological function of the oribatid mites in agricultural regions of Mexico, this study of the oribatid mites was done in 2 different parcels with very contrasting irrigation types: one with residual water and the other with well water. In this way, we would be able to detect which species could be used as indicators of the perturbation of the soils. According to the literature, there are some oribatid mite species more tolerant to heavy metal concentrations in soils, thus, we would expect a different composition of oribatid mites in both parcels due to their irrigation type and heavy metal contents, with more species tolerant in the residual water than in the well water parcel, and also more diversity in residual water parcel, since oribatid mites can develop in soils with high contents of heavy metals (van Straalen et al., 2001).
The knowledge of the structure and composition of the population of the oribatid mites and their relationship with edaphic parameters will be a basis for future studies on the environmental impact and can be of use in the agricultural strategies of Mexico and the recognition of oribatid mite species as bioindicators.
Materials and methods
The 2 plots are in the “Distrito de Riego 063” (DDR 063, 1997), in San Salvador Municipality (19o40’-20o29’ N; 99o57’-99o27’ W), situated in the Mezquital Valley, Hidalgo, at an average altitude of 1,985 m (Fig. 1). The weather of the region is semiarid dry, with an annual average temperature of 17 oC and an annual average precipitation of 475 mm (DDR 063, 1997; Ayuntamiento de San Salvador, Hgo., 2012). The municipality is in the Trans-Mexican Volcanic Belt. The soils are derived from basaltic, sedimentary and metamorphic rocks; among the intrusive rocks there are andesitic basaltic, basalts and diabasas; among the sedimentary there are hydroclastic, slates, argillaceous, limestone, limestone slates, marls, conglomerates, breaches, sands and alluviums; among the metamorphic rocks there is only marble (CNA, 1995). The site receives residual water from the most important draining of the river Tula and Mexico Valley. In the natural channel of the Tula River, the Endhó dam was built with agricultural aims. The Tula River starts from the infiltrations of the Requena dam which also stores water draining from the Taxhimay dam of the Tepeji River, the Central emitter and the El Salto River, which contributes to an annual water volume average of 498,3 million of m3 (Conagua, 2015).
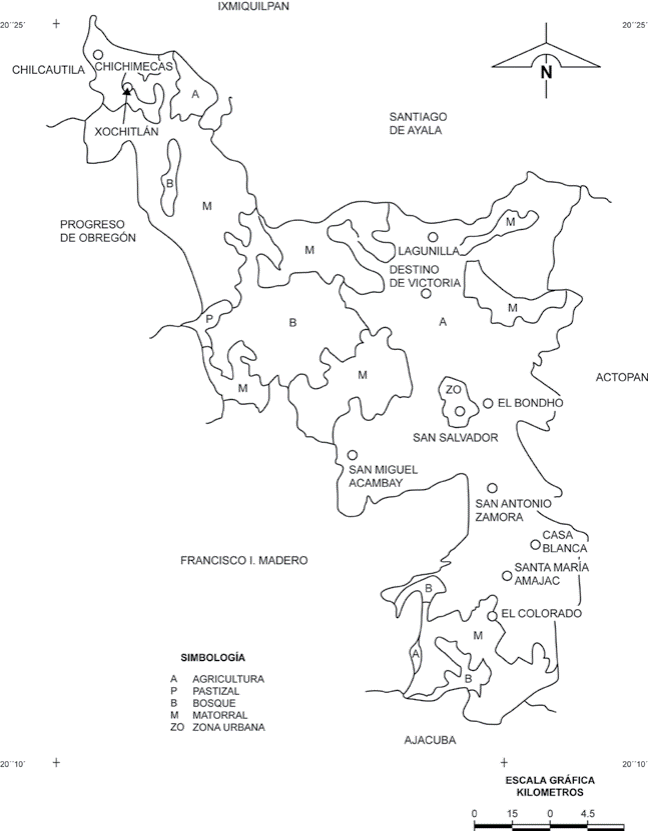
Figure 1 Localization of the plots San Salvador and El Bondho, in Hidalgo, Mexico (modified from INEGI, 1998).
Two plots were selected in the area, each one with a 7,000 m2 extension. The 2 studied plots are similar in their pedogenic characteristics and the distance between them is about 1 km (CNA, 1995). One plot, named San Salvador (SS; 20°17’40” N, 99°00’01” W) has been irrigated with waste water for 80 years, and the other plot, El Bondho (B; 20°17’09” N, 99°00’31” W), has been irrigated with well water for 100 years. In each plot, an area of 50 × 20 m was selected for each sampling data. In that area, 10 samples (95 cm2) were taken randomly bimonthly from December 1998 to October 1999 for a total of 120 samples for all the study. On the same day of the collection, samples were taken to the laboratory where the fauna was extracted by Berlese-Tullgren funnels for 6 days. For 3 days, the samples were kept at room temperature, for the other 3 days, they were kept within the light from a 60-watt lamp. The oribatid mites were isolated, counted and mounted for their identification.
The physical-chemical analysis of the soil-like organic matter, pH, heavy metals, interchanging cations, porosity and electric conductivity (EC) were done in the Laboratorio de Edafología “Nicolás Aguilera” of the Faculty of Sciences, UNAM.
In order to characterize the community structure, the species richness (S), the Shannon diversity index (H’), the Simpson dominance index (λ), the Pielou’s evenness (J’), and the Sörensen similarity index (Ludwig & Reynolds, 1988) were calculated. The Shannon diversity indices between localities were compared by a modified t-student test (Magurran, 1988).
The mean values of the edaphic parameters and the heavy metal contents in the 2 plots were compared with a t-student test for independent samples.
In order to detect the effect of the plot and the collection date on the oribatid mite abundance and the soil parameters (pH, porosity, EC, Mg, Ca, K, Na), a factorial Anova test was performed in order to reduce the effect of pseudoreplication, using the interaction term as an error term (Camus & Lima, 1995; Manly, 1991). A Tukey’s post hoc test was used to compare the months of collecting. The relationship between the edaphic parameters and the density of oribatid mites was evaluated by multiple correlation tests. A principal component test was performed to identify the variables that explain most of the variability observed in the communities of the oribatid mites. The data were normalized by √(X + 0.5), except percentage that was transformed by ARCOSEN √X (Zar, 1984). The analyses were performed using the software Statistica, ver 6.0 (Statsoft, 1999).
Results
A total of 39,487 mites were collected in the 2 plots, the oribatids represented only the 6% of the total (2,448 individuals) including immature stages. In the SS plot, 1,345 oribatid mites were collected, while in the B plot 1,103 oribatids were recorded. A total of 20 species of oribatid mites belonging to 19 genera and 15 families were found in the study (Table 1).
Table 1 Abundance (number of individuals), density (ind/m2) and percentage of oribatid mites density in San Salvador (SS) and El Bondho (B), agricultural plots in Hidalgo, Mexico. H’= Shannon’s diversity index; J’ = Pielou’s evenness index; S = species richness; λ= Simpson’s dominance index.
| Taxa | Abundance | Density | Percentage | |||
| SS B | SS B | SS B | ||||
| Thyrisomidae | ||||||
| Gemmazetes cavatica (Kunst, 1962) | 53 | 4 | 558 | 42 | 4 | 0.40 |
| Oppiidae | ||||||
| Oppiella nova (Oudemans, 1902) | 181 | 37 | 1905 | 389 | 13 | 3 |
| Ramusella sp. | 1 | 116 | 10 | 1221 | 0.01 | 10 |
| Brachioppia sp. | 1 | - | 10 | - | 0.01 | - |
| Microppia sp. | - | 9 | - | 95 | - | 0.80 |
| Oribatulidae | ||||||
| Zygoribatula connexa (Berlese, 1904) | 96 | 814 | 1010 | 8568 | 7 | 74 |
| Z. ca. Bonairensis | 1 | 4 | 10 | 42 | 0.01 | 0.40 |
| Epilohmanniidae | ||||||
| Epilohmannia pallida Balogh et Mahunka, 1980 | 3 | 36 | 31 | 379 | 0.02 | 3 |
| Carabodidae | ||||||
| Carabodes ecuadoriensis Balogh, 1988 | 3 | - | 31 | - | 0.02 | - |
| Euphthiracaridae | ||||||
| Rhysotritia ardua (C. L. Koch, 1841) | 23 | 12 | 242 | 126 | 2 | 1 |
| Scheloribatidae | ||||||
| Scheloribates sp. | 198 | 10 | 2,084 | 105 | 15 | 1 |
| Setobates sp. | 6 | - | 63 | - | 0.40 | - |
| Xylobatidae | ||||||
| Xylobates sp. | 107 | 10 | 1,126 | 105 | 8 | 0.9 |
| Hypochthoniidae | ||||||
| Hypochthonius sp. | 1 | - | 10 | - | 0.01 | - |
| Lohmanniidae | ||||||
| Lohmannia banksi Norton et al., 1978 | 6 | - | 63 | - | 0.40 | - |
| Trhypochthoniidae | ||||||
| Allonothrus sp. | - | - | - | 10 | - | 0.10 |
| Haplozetidae | ||||||
| Rostrozetes sp. | 7 | 24 | 74 | 253 | 0.50 | 2 |
| Tectocepheidae | ||||||
| Tectocepheus velatus elegans Ohkubo, 1981 | 651 | 19 | 6852 | 200 | 48 | 1.70 |
| Ceratozetidae | ||||||
| Ceratozetes sp. | 4 | 7 | 42 | 74 | 0.30 | 1 |
| Galumnidae | ||||||
| Galumna sp. | 3 | - | 31 | - | 0.20 | - |
| Total | 1,345 | 1,103 | 14,157 | 11,610 | 100 | 100 |
| H’ | 1.064 | 1.092 | ||||
| S | 18 | 14 | ||||
| J’ | 0.57 | 0.41 | ||||
| λ | 0.29 | 0.56 | ||||
In the SS plot, 18 species were recorded and 14 in the B plot. The most abundant species in SS was Tectocepheus velatus elegans, while in B, Zygoribatula connexa was the most abundant. The highest values of abundance, diversity, evenness, and species richness were found in SS, only the dominance was higher in B. The exclusive species for SS were Galumna sp., Lohmannia banksi, Hypochthonius sp., Setobates sp., Carabodes ecuadoriensis, and Brachioppia sp., and for B, Microppia sp. and Allonothrus sp. were exclusive (Table 1).
There were variations in the composition and the abundance of the oribatid mites in both plots. The highest species richness values were recorded in December and the lowest values were found in April in both plots. The highest values of Shannon’s diversity index were observed in December and August and the lowest in February and June in B and SS, respectively. Nevertheless, significant differences between the Shanon diversity indices in the 2 localities (t = 1.493, p > 0.05) were not found.
The species O. nova, Z. connexa, and Xylobates sp. were present in all the collecting dates in SS, while in B, Epilohmannia pallida was the most constant species recorded in all the dates except during April. Gemmazetes cavatica was recorded in both plots only during December.
The Sörensen similarity coefficient between both communities was 75%. The highest values registered were in December and August, when more species are shared in both plots and the lowest value was recorded in February. The Anova used to evaluate the effect of the plot on the general density of oribatid mites did not show a statistically significant value. Nevertheless, when the species densities are compared and analyzed, there is a significant effect of the locality, the data and the interaction of both variables on the densities of some species as Oppiella nova, Ramusella sp., Microppia sp., Zygoribatula connexa, Scheloribates sp., and Xylobates sp. (Table 2). For Epilohmannia pallida, only a significant effect of plot on density (F1,108 = 13.21; p < 0.05) was detected. The significant effect of the collecting date was detected on the density of Gemmazetes cavatica (F5, 108 = 2.75; p < 0.05), Carabodes ecuadoriensis (F5,108 = 3.87; p < 0.05), and Rhysotritia ardua (F5,108 = 3.47; p < 0.05). The density of Tectocepheus velatus elegans is affected significantly by the type of plot (F1,108 = 46.39; p < 0.05) and the collecting date (F5,108 = 19.40; p < 0.05), however, the interaction between them is not significant (F5,108 = 1.01; p > 0.05). In most of the species, the differences, according to the post hoc Tukey’s test, were present between December and October (p < 0.05).
Table 2 Factorial Anova test results evaluate the effect of plot (P) and collecting date (D) on oribatid mites species in San Salvador and El Bondho, Hidalgo, Mexico. Pt = Plot; D = collecting data; P × D = interaction; * = p < 0.05.
| Effect | df | F value |
|
Oppiella nova P D P × D |
1,108 5,108 5,108 |
12.55* 10.81* 6.81* |
|
Ramusella sp. P D P × D |
1,108 5,108 5,108 |
25.26* 9.24* 8.34* |
|
Microppia sp. P D P × D |
1,108 5,108 5,108 |
12.01* 3.87* 3.87* |
|
Zygoribatula connexa P D P × D |
1,108 5,108 5,108 |
19.30* 25.09* 21.11* |
|
Scheloribates sp. P D P × D |
1,108 5,108 5,108 |
15.80* 7.45* 5.72* |
|
Xylobates sp. P D P × D |
1,108 5,108 5,108 |
12.61* 3.84* 3.79* |
The average of the edaphic parameters and the heavy metals of the 2 plots is shown in table 3. The t-student test results show that there are significant differences in most of the edaphic parameters, except in the percentage of the organic matter and the porosity, and in relation with the heavy metals, only in Cr and Mn differences between plots were not detected (Table 3).
Table 3 Average ± SD and t-student test values for edaphic parameters and heavy metals in San Salvador (SS) and El Bondho (B) plots. Mg = Magnesium; Ca = calcium; K = potassium; Na = sodium; EC = electric conductivity; OM = organic matter percentage; Po = porosity; Fe = iron; Cu = copper; Zn = zinc; Mn = manganese; Cr = chromium; Cd = cadmium; Ni = nickel; Pb = lead. N = 60; df = 118; < 0.05; ns = no significant.
| Parameter | SS | B | t |
| Mg meq/100 g soil | 24.20±12.92 | 44.33±16.39 | 7.47 * |
| Ca meq/100 g soil | 16.85±8.20 | 23.04±14.05 | 2.95* |
| K meq/100 g soil | 3.15±1.20 | 5.56±2.69 | 6.35* |
| Na meq/100 g soil | 2.69±0.91 | 5.12±5.53 | 3.34* |
| pH | 7.58±0.47 | 8.67±0.26 | 15.93* |
| EC ds/m | 2.12±0.76 | 2.97±1.04 | 5.06* |
| OM % | 2.77±3.63 | 2.84±2.72 | 0.11 ns |
| Po % | 49.33±3.54 | 50.13±3.71 | 1.21 ns |
| Heavy metal | |||
| Fe mgKg-1 | 5.76±4.17 | 1.69±1.15 | 7.28* |
| Cu mgKg-1 | 4.57±4.09 | 0.93±1.04 | 6.68* |
| Zn mgKg-1 | 14.13±13.12 | 1.63±1.32 | 7.35* |
| Mn mgKg-1 | 14.27±17.19 | 13.41±20.47 | 0.25 ns |
| Cr mgKg-1 | 0.03±0.05 | 0.03±0.05 | 0.005 ns |
| Cd mgKg-1 | 0.23±0.17 | 0.05±0.06 | 7.86* |
| Ni mgKg-1 | 1.36±0.84 | 0.36±0.41 | 8.25* |
| Pb mgKg-1 | 2.74±2.32 | 0.97±0.76 | 5.61* |
According with the Anova test, we found that in almost all edaphic parameters the plot (irrigation type) and the collection date have a significant effect on values, except on the organic matter; also there is a significant effect of plot to porosity percentage and only for the date for Na (Table 4), and in those parameters we did not find a significant effect with the interaction between the 2 variables. The post hoc Tukey’s test shows that for interchanged cations (Mg, Ca, K and Na), the differences are present in February (p < 0.05), while for the porosity percentage, the differences are detected in April for both plots. For the pH, the most different months are April and December, for the SS and the B plots, respectively; for EC, the differences are detected in December for the SS, and in April for the B plot. In the case of the heavy metals, significant effects of the plot and the collection date were found, and the interaction between the 2 variables on the contents of all elements, except for Mn in which only the collecting date had a significant effect, and for Cr in which no effect was found (Table 5).
Table 4 Factorial Anova test results evaluate the effect of plot (P) and collecting date (D) and interaction between variables (P × D) on edaphic parameters in San Salvador and El Bondho. Mg = Magnesium; Ca = calcium; K = potassium; Na = sodium; EC = electric conductivity; Po = porosity percentage; OM = organic matter percentage. * = p < 0.05, ns = no significant.
| Effect | df | F value |
| Mg P D P × D |
1,108 5,108 5,108 |
108.86* 20.17* 4.23* |
| Ca P D P × D |
1,108 5,108 5,108 |
17.84* 22.40* ns |
| K P D P × D |
1,108 5,108 5,108 |
159.84* 61.04* 10.99* |
| Na P D P × D |
1,108 5,108 5,108 |
11.72* ns ns |
| pH P D P × D |
1,108 5,108 5,108 |
984.7* 61.5* 8.5* |
| EC P D P × D |
1,108 5,108 5,108 |
72.19* 37.62* 7.24* |
| Po P D P × D |
1,108 5,108 5,108 |
ns 5.61* ns |
| OM P D P × D |
1,108 5,108 5,108 |
ns ns ns |
Table 5 Anova two- way test results, evaluate the effect of plot (P) and collecting date (D) on the heavy metals contents in San Salvador and El Bondho. Fe = Iron; Cu = copper; Zn = zinc; Mn = manganese; Cr = chromium; Cd = cadmium; Ni = nickel; Pb = lead. * = p < 0.05, ns = no significant.
| Effect | df | F value |
| Fe P D P × D |
1,108 5,108 5,108 |
121.33* 21.46* 10.90* |
| Cu P D P × D |
1,108 5,108 5,108 |
137.89* 34.91* 16.35* |
| Zn P D P × D |
1,108 5,108 5,108 |
162.61* 27.44* 22.05* |
| Mn P D P × D |
1,108 5,108 5,108 |
ns 3.13* ns |
| Cr P D P × D |
1,108 5,108 5,108 |
ns ns ns |
| Cd P D P × D |
1,108 5,108 5,108 |
123.90* 20.31* 5.47* |
| Ni P D P × D |
1,108 5,108 5,108 |
82.85* 3.59* 3.51* |
| Pb P D P × D |
1,108 5,108 5,108 |
102.41* 43.90* 11.22* |
The correlation analysis between the edaphic parameters, heavy metals, and oribatid mites shows that the most significant relationship in SS was presented by T. velatus elegans (r = 0.80), which was positively correlated with Mn and Cr, and negatively with Zn. Scheloribates sp. had a positive and significant correlation with K (r = 0.74). Rhysotritia ardua also had a negative correlation with K and Mn, and positive with Cu and Ni (r = 0.69). On the other hand, in B, Allonothrus sp. had a positive correlation with the porosity and Cd (r = 0.72). Ramusella was negatively correlated with Ca and Mg, and had a positive correlation with porosity (r = 0.69).
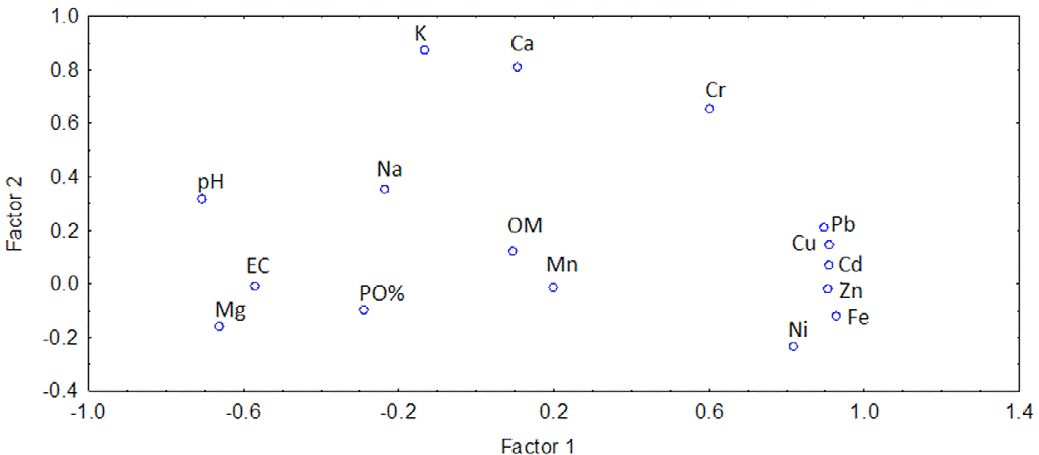
The principal component analysis has shown that there are 2 main factors: the first one is related to the heavy metals (Fe, Cu, Zn, Cd, Ni, Pb) and the pH, and it explains 42.51% of the variation (eigenvalue = 8.80). The second factor is related to the content of K and Ca, and it explains 56.31% of variation (eigenvalue = 2.21), as shown in figure 2 .
Discussion
The densities of oribatid mites recorded in this study were much lower than those found in forest soils and even in those in agricultural soils (Maribie et al., 2011; Yina et al., 2013) although it is known that this group of mites is usually the dominant species in the edaphic environments (Norton, 1990).
These results can be related to the use of pesticides and fertilizers in the agricultural practices which usually have a negative effect on the abundance, distribution and composition of the species (Desmond & Alex, 2013; Minor et al., 2004; Socarrás & Robaina, 2011). In the present study, despite the high concentrations of heavy metals in the parcel watered with residual water, it had the highest values of abundance and diversity. The amount of some oribatid mite species like Tectocepheus velatus elegans, Oppiella nova, Scheloribates sp., Gemmazetes cavatica, and Xylobates sp., seems to increase with the presence of several elements like Cr and Mn. It was observed that the densities of such populations were lower with the diminution of the concentration of the heavy metals in the crops in August and October. These results confirm the resistance that oribatid mites show to concentrations of heavy metals. This resistance was found in other studies in which there are soil invertebrates that can accumulate most of those metals in their bodies (van Straalen et al., 2001). Also, the recorded species, along with Zygoribatula connexa, which was the most abundant in B, have been frequently recorded in agricultural soils (Hubert, 2000; Ivan & Călugăr, 2013); even Scheloribates has been cited from environments polluted with heavy metals and metallurgic accumulations (Al-Assiuty et al., 2000; Corral & Iturrondobeitia, 2012; El-Sharabasy & Ibraim, 2010; Skubala, 1995).
Some studies performed in forest soils in Germany established that Tectocepheus sp. and some members of the family Oppiidae, mainly Oppiella nova, were not affected in their abundance after the soils were perturbed. This species has preference to soils with an acid pH which can indicate a high alteration. It has been suggested that such resistance can be related to its very high reproductive rate, because this species is parthenogenetic (El-Sharabasy & Ibraim 2010; Maraun et al., 2003; Vladislav et al., 2015). According to Aoki (1979), the oribatid mites which are more resistant to different kinds of pollution are members of the families Brachychthoniidae, Oppiidae, Oribatulidae, and Tectocepheidae, which except for Brachychthoniidae, were well represented in this study.
The incorporation of low concentrations of Cu (42-418 mg) in the diet of some oribatid mites can increase their fertility, however, if they exceed certain limits (above 700 mg), their life cycle can be affected and cause their death (Seniczak et al., 1997, 1999). The concentration in both of our plots was lower than 15 mg, so it can be beneficial to those mites (Skubala & Zaleski, 2011).
Skubala and Kafel (2004) have studied the bioaccumulation of metals in oribatid mites in a gradient of forestry ecosystems. They have found that the concentration of Zn and Cu was higher in the mycophagic species (Oppiella nova and Tectocepheus velatus) than in the panfitophagous species (Oribatula tibialis and Pergalumna nervosa) or in the macrofitophagous (Atropacarus striculus). Other studies have demonstrated that the communities of some oribatid mites are almost completely tolerant to the presence of Fe, Zn, Cu, Cd, and Pb in the soil (Vladislav et al., 2015; Zaitsev & van Straalen, 2001). It has been observed that Zn is the metal which accumulates the most in the microfitophagous oribatid mites (Zaitsev & van Straalen, 2001). This may explain the reason for a positive correlation of this metal and the densities of Oppiella nova and Tectocepheus elegans, considered as microfitophagus, in the present work in SS.
As an answer to the effect of the pollutants on populations of oribatid mites, Rusek & Marshall (2000) proposed a classification system: a) sensitive species like Adoristes ovatus, Eporibatula rauscheninsis and Oppiella minus; b) susceptible species such as Carabodes labyrinthicus and Oribatula tibialis, and c) tolerant species like Chamobates schueltzi, Liochthonius sp., Tectocepheus velatus, Trichoribates trimaculatus, and Zygoribatula exilis.
Following the system of Rusek & Marshall (2000), only Tectocepheus velatus elegans and Carabodes ecuadoriensis could be included in our study. However, Oppiella nova and Zygoribatula connexa can also be considered as tolerant species because they are very abundant in SS and B.
After this study, we can say that many species of Tectocepheus, Oppiella, Zygoribatula, and Scheloribates are abundant and constant in those environments where disturbance exists or that have been polluted with heavy metals (Al-Assiuty et al., 2000; Gan, 2013; Vladislav et al., 2015), or in those grounds that have been used for agricultural purposes (Norton & Sillman, 1985; Ruiz et al., 1986).
Studies on the accumulation and presence of heavy metals in soils in the area where the present study was carried out (until 1994) show that metals do not represent a risk for the productive potential and they have not been incorporated to trophic web (Flores-Magdaleno et al., 2011; Siebe, 1994). In view of our results, we believe that those levels of metals are still very similar.
The differences between the population abundance of both parcels may be due to the tolerance (or the intolerance) of some species to the presence of some heavy metals and to the microclimatic conditions of each parcel. Some species do not stand the high concentrations of some metals, however, some other species increase their populations where there are moderate concentrations of those metals (Skubala & Zaleski, 2011) and they most likely use and incorporate them in their metabolism (Corral & Iturrondobeitia, 2012; Skubala & Kafel, 2004).
In this study, Oppiella nova, Rhysotritia ardua, Scheloribates sp., and T. velatus elegans were the most abundant species in the heavy metal polluted parcel. On the other hand, they had a low density in the parcel watered with well water. Epilohmannia pallida, Ramusella sp., and Zygoribatula connexa, were the most abundant species in the less polluted parcel. The species of this last parcel have lower requirements of heavy metals or a low resistance to heavy metals, but they prefer soils with a higher pH.
To confirm the inferred relationships, it is necessary to make ecotoxicological studies at specific level in laboratory conditions (also in the field under natural conditions) to ascertain the accumulation percentage of heavy metals in the tissues of mites, in order to evaluate the impact of some pollutants in the residual waters used for agricultural purposes. We found information on the tolerance of some species such as O. nova, R. ardua, and T. elegans to heavy metals, and low resistance to heavy metals of Epilohmannia pallida and Z. connexa, that are present in the less polluted parcel, but are tolerant to saline soils. Results found in our study may be useful to support the use of some species of oribatid mites as bioindicators in environments with conditions similar to our study area.











 nueva página del texto (beta)
nueva página del texto (beta)