Introduction
The lagoon-estuarine systems are transitional environments between rivers and sea. They are characterized by wide variations in their physical and chemical conditions (Day et al., 1989). This spatial and seasonal environmental variability represents a challenge for the local benthic species (Ysebaert et al., 2002). Thus, their assemblages display different compositions and structures, depending on the equilibrium between the seawater and freshwater inputs (Gordo & Cabral, 2001; Rodríguez-Climent et al., 2012).
Coastal lagoons and estuaries are characterized by a high biological productivity and are used by many marine and freshwater organisms as refuges, breeding and feeding grounds (Carvalho et al., 2005). However, these systems are being increasingly modified due to the continuous influence of human activities on abiotic factors and on biodiversity. It has also been shown that the species functional features induce shifts in composition of species and in the ecosystem processes (Villéger et al., 2010). The drastic changes in urban and industrial land uses, acceleration of coastal urbanization, intensive agriculture, tourism and global warming, among the most relevant (Rodríguez-Climent et al., 2012), have notably increased the use and degradation of these natural resources and caused severe changes in their hydrological patterns. The decrease in salinity and the increase in both eutrophication and environmental pollution are the most common consequences of the high nutrient and chemical contaminant inputs (Cañedo-Argüelles et al., 2012; Cloern, 2001; Lucena et al., 2002). Nevertheless, there is no consensus on the actual impact of human activities on the habitat alterations and on the components of biodiversity, including the species functional traits; which is why a multidimensional approach in the assessment of biodiversity changes in communities under stress is needed in order to understand it (Villéger et al., 2010).
Although in the southern Gulf of Mexico the coastal lagoons cover vast areas (623,600 ha), their study has not been a priority for benthic specialists. For their part, the polychaetes are recognized as one of the most important components in the macrofaunal benthic communities inhabiting these ecosystems, but so far, they have only been studied in eleven out of the 174 lagoon-estuarine systems located in this region (Hernández-Alcántara & Solís-Weiss, 1991, 1995; Hernández-Alcántara et al., 2014). In general, the coastal lagoons and estuaries communities show low species diversity but high abundance of organisms (Sarker et al., 2016). These features have been associated with the “minimum species” concept proposed by Remane (1934). He explained that the large environmental variations prevalent in those brackish waters make them hard for many species to survive and many of them are excluded, so that these transitional marine-freshwater areas harbor species-poor communities. In this sense, the diversity patterns of their fauna are essential to understand the functioning of the ecosystems: therefore, and due to the significant importance of the polychaetes in the benthic environments, the biological processes involving them could reflect those of the entire community (Glasby & Read, 1998; Mackie et al., 1997; Olsgard & Somerfield, 2000).
The Términos Lagoon is the largest lagoon-estuarine system in Mexico. It includes several habitats such as fluvial-deltaic systems, wetlands, seagrass beds, mangroves, oyster banks and tropical forests, among the most conspicuous (Ávila et al., 2015; Yáñez-Arancibia & Day, 1982). That is why all the studies aimed at understanding their biodiversity patterns are fundamental to establish monitoring programs that can help mitigate and control the anthropic effects on the native biota. In this lagoon, the polychaetes of the mangroves and seagrass beds have been widely studied (Cruz-Ábrego et al., 1994; Hernández-Alcántara & Solís-Weiss, 1991, 1995; Hernández-Alcántara et al., 2014; Ibáñez-Aguirre & Solís-Weiss, 1986), but their occurrence on soft bottoms and especially their diversity variations along environmental gradients have not yet been explored. Therefore, the aim of the present study was to analyze the faunal changes along 2 transects located on soft bottoms across the lagoon (northern-southern direction), and to examine the role of environmental gradients in the control of the number and diversity of the local polychaete species. The beta-diversity spatial changes, partitioned into its 2 components species turnover and nestedness is also examined.
Material and methods
The Términos Lagoon is a lagoon-estuarine system located at the southern end of the Gulf of Mexico between latitudes 18°27’37” N - 18°47’36” N and longitudes 91°14’44” W - 91°53’55” W. It is about 70 km long and 30 km wide, elliptically shaped, with a total surface of about 2,500 km2 and an average depth of 3.5 m (Fig. 1). It was declared “Protected Area of Flora and Fauna of Términos Lagoon” (APFFLT, for its initials in Spanish) in 1994, and a Ramsar site in 2004. Its main importance, besides its biological diversity, is its role as a nursery area for many commercially important species, such as shrimps, crabs and fishes. Two ocean inlets, at its eastern and western ends, connect it to the Gulf of Mexico. Variability in environmental features can be explained by tidal and meteorological processes, and previous studies have shown that the seawater enters the lagoon through the Puerto Real Inlet (to the east), while the lagoon waters outflow through the Carmen Inlet (to the west) (David & Kjerfve, 1998). During most of the year the lagoon functions as 2 almost independent systems with limited mixing between the marine and freshwater masses inside the system. However, during the high freshwater discharge, the lagoon functions as a single hydrological unit with a net east to west flow (David & Kjerfve, 1998). The salinity displays a spatial gradient from the Puerto Real Inlet, where the higher salinity waters can vary from 32 to 39 psu, but can drop to 22 psu as a result of the merging of waters coming from 4 large rivers flowing into the lagoon. The Usumacinta-Grijalva is the largest river system in México (2,410 m3 s-1) (SRH, 1970), and as part of it, the Palizada river, located at the southern end of the lagoon, is the main source of freshwater to the lagoon (average discharge: 500 m3 s-1) (Fig.1). It causes drastic reductions in salinity at the local level, even down to 0 psu, but also conveys large amounts of clay and mud to the lagoon (Yáñez-Arancibia & Day, 2005).

Figure 1 Location of the study area showing the sampling stations in Términos Lagoon. Arrows show the water net flow inside lagoon; W: stations from the western transect; E: stations from the eastern transect.
The input of seawater through the Puerto Real inlet and the net water flow in an eastern-western direction, was the basis for the sampling design, i.e. the positioning of transects aimed at examining the response of the benthic polychaetes to environmental gradients. In October 2008, 10 stations were aligned in 2 transects perpendicular to Carmen Island (Fig. 1). Samplings were carried out only in soft bottoms to ensure comparability among sites and to minimize the influence of habitat. The sediments were collected with a Van Veen grab (0.06 m²). Back at the laboratory, the sediments were sieved through a 0.5 mm mesh screen to separate the macrofauna which was then fixed in 4% formaldehyde solution. Then, following separation from the other faunal groups, the polychaetes were preserved in 70% ethanol. In addition, we measured the salinity with an automatic refractometer (± 0.5 psu), the temperature (°C) with a thermometer (± 0.1°C), the dissolved oxygen concentration with the Winkler titration method (Strickland & Parsons, 1977) and the depth (m).
The polychaetes were identified to species, cataloged and deposited in the Colección Nacional de Anélidos Poliquetos at the Instituto de Ciencias del Mar y Limnología (ICML), Universidad Nacional Autónoma de México (UNAM) (CNAP-ICML, UNAM; DFE.IN.061.0598). The validity of names and synonymies of the species identified were verified with recent systematic reviews and with the World Polychaeta database (Read & Fauchald, 2016).
Data analysis. First, line plots and simple linear regression analysis were set up to describe the physical changes among stations belonging to each transect and to verify the presence of an environmental gradient across the lagoon. To analyze the faunal variations along the transects, a database was built, which included, for each species, its density (0.1 m-2) and distribution in the lagoon. Diversity was expressed as the number of species, but since the number of individuals by species widely varied among the sampling stations, individual-based rarefaction curves were created. These rarefaction curves were built taking a common number of 50 individuals per station with the Sanders (1968) modified by Hurlbert (1971) method, which allows direct comparisons of species richness for an equivalent sampling size (Gotelly & Colwell, 2001; Hurlbert, 1971; Sanders, 1968). Then, linear regression analyses were carried out to assess the relationships between the number of species and the spatial variations of the environmental parameters along each transect. A line plot, created with the PRIMER v.7 software (Clarke & Gorley, 2015), was applied to compare the spatial variation of the density of the 25 more abundant species along each transect.
The faunal similarity between transects and among the stations belonging to each transect was calculated with the estimator of shared species Chao’s abundance-based Sørensen’s index, using the EstimateS v.9.1.0 software (Colwell, 2013; Chao et al., 2005). This estimator is based on the number of rare species, which reduces the negative effect associated with incomplete samplings at the stations (Carr, 2012; Chao et al., 2005). Then, the beta-diversity was applied to analyze the changes in species composition among stations from each transect. According to Baselga (2010) and Carr (2012), the Sørensen’s dissimilarity index (βsor) (Sørensen, 1948) provides a comprehensive vision of the beta diversity, since it takes both species loss and replacement into account (Carr, 2012). So, the Sørensen’s dissimilarity index was partitioned into its 2 additive components, which are the basis of the total amount of beta-diversity: the spatial species turnover, assessed with the Simpson’s dissimilarity index (βsim) (Simpson, 1943), and the nestedness, calculated with the Baselga’s nestedness index (βnes) (Baselga, 2010). Dissimilarity values ranged from 0 to 1 with 1 indicating no overlap in species composition (Carr, 2012).
Results
The environmental variations showed that not all the variables considered displayed significant changes from the coasts of Carmen Island towards the continental region of the lagoon, but also that the environmental pattern was different in each transect (Fig. 2). The regression analysis showed that salinity was the most important parameter defining the structure of the environmental gradient at both transects (r 2 = 0.83, p = 0.033 in western zone; r 2 = 0.95, p = 0.005 in eastern zone). On the other hand, the values of dissolved oxygen displayed few changes (mean = 5.30 ml L-1; SD = 1.08 ml L-1) and their spatial pattern along transects did not show significant changes (r2 < 0.35; p > 0.05).
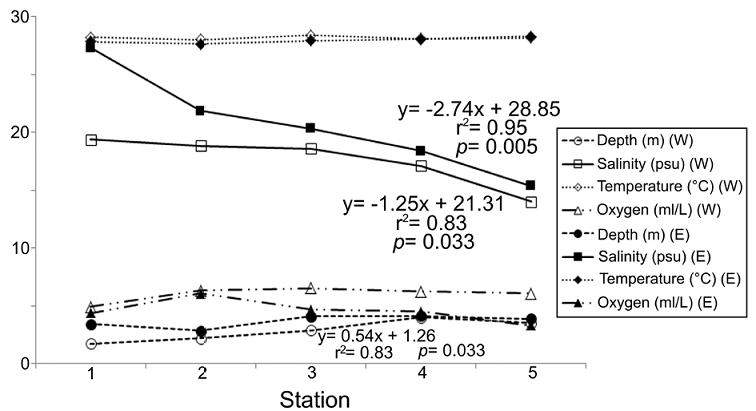
Figure 2 Spatial variation of the environmental factors among the sampling stations. Only the significant correlations and their linear regression equations are shown; clear symbols: western transect; dark symbols: eastern transect.
Depth and temperatures showed different trends at each transect. Depth varied from 1.75 m to 4.12 m, and its changes were not significant along the eastern zone (r 2 = 0.41, p = 0.245). However, in the western transect, together with the salinity factor, depth also contributed to define the environmental structure (r 2 = 0.83, p = 0.030). Temperature showed few changes across the lagoon (mean = 28.06 °C; SD = 0.22 °C), and although in the eastern region the values grew towards the continental margin, their variations were not significant (r 2 = 0.72; p = 0.069). Along the western transect, the temperature was almost constant and its variations were not significant (r 2 = 0.01, p = 0.946) (Fig. 2).
In total, 299 individuals from 51 species and 24 families of polychaetes were identified. The families with the largest number of species were the Lumbrineridae (7 species), followed by the Onuphidae (5 species) and Polynoidae (4 species), which together accounted for 31.4% of the total number of species. On the other hand, 9 families (37.5%) were represented by only 1 species. The fauna was irregularly distributed along transects: in the western one, we found 14 families, a bit more than half the families found in the eastern zone (22 families) (Table 1). The Onuphidae, Syllidae and Polynoidae were the most species-rich families (3 species) in the western transect, while the Lumbrineridae (6 species) and Goniadidae (3 species) dominated the eastern zone.
Table 1 Number of species of polychaetes collected in both transects, ranked by family in decreasing richness order.
| Family | Western transect | Eastern transect | Total |
| Lumbrineridae | 2 | 6 | 7 |
| Onuphidae | 3 | 4 | 5 |
| Polynoidae | 3 | 2 | 4 |
| Syllidae | 3 | 1 | 3 |
| Goniadidae | 1 | 3 | 3 |
| Oenonidae | 1 | 1 | 2 |
| Orbiniidae | 1 | 2 | 2 |
| Cirratulidae | 2 | 1 | 2 |
| Nereididae | 0 | 2 | 2 |
| Oweniidae | 1 | 2 | 2 |
| Capitellidae | 0 | 2 | 2 |
| Serpulidae | 0 | 2 | 2 |
| Pilargidae | 1 | 1 | 2 |
| Sabellidae | 0 | 2 | 2 |
| Paraonidae | 2 | 2 | 2 |
| Eunicidae | 1 | 1 | 1 |
| Cossuridae | 1 | 0 | 1 |
| Trichobranchidae | 0 | 1 | 1 |
| Ampharetidae | 0 | 1 | 1 |
| Spionidae | 1 | 0 | 1 |
| Hesionidae | 0 | 1 | 1 |
| Dorvilleidae | 0 | 1 | 1 |
| Terebellidae | 0 | 1 | 1 |
| Pectinariidae | 0 | 1 | 1 |
The density values were different between transects: the western zone had in average only 21.67 ind. 0.1 m-2, while in the eastern zone, a much more abundant fauna was found (78.0 ind. 0.1 m-2 in average), mainly at the northern stations (> 100 ind. 0.1 m-2) (Fig. 3). Rarefaction curves confirmed that the distribution of the number of species was different along each transect (Fig. 4). In general, stations located in the eastern zone had more species, but in no transect the number of species displayed a northern-southern trend. In the eastern transect, the highest species richness was found in the middle stations (Station 3E = 15 species; Station 2E = 14 species), but towards south, the number of species decreased significantly (Station 4E = 8 species; Station 5E = 7 species). In the western transect the diversity pattern was very different and the richness was almost constant (8-9 species), except for stations 1W and 3W, in which a drastic decrease in the number of species was found (3 and 1 species, respectively).
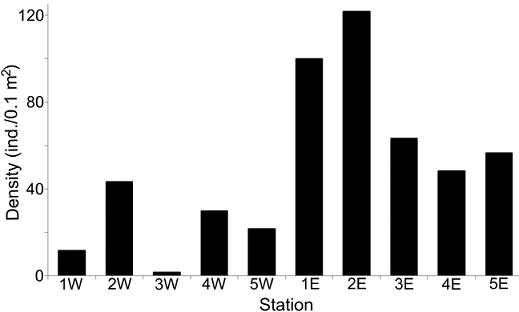
Figure 3 Spatial distribution of density (ind./0.1 m2) per sampling station. W: stations from the western transect; E: stations from the eastern transect.
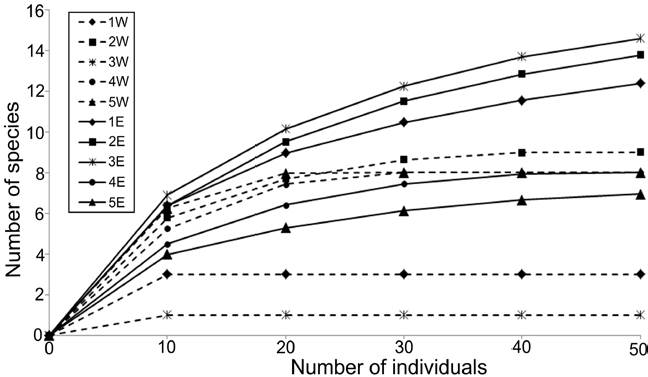
Figure 4 Rarefaction curves of polychaetes by sampling station. Dashed lines: stations from the western transect; full lines: stations from the eastern transect.
Since the number of species did not follow a linear trend, from north to south, in any transect, their correlations with the environmental parameters were largely not significant (r 2 < 0.5), mainly with those variables determining the environmental gradient structure: salinity (r 2 = 0.23 and 0.34) and depth (r 2 = 0.13 and 0.17) (Table 2). Temperature was the only variable significantly associated with the richness changes (r 2 = 0.80 and 0.63), since the higher number of species was found at the stations with lower temperatures and located at the middle-northern zone of the lagoon.
Table 2 Relationships between the environmental parameters measured and the number of species at each transect. (* significant value at p < 0.05).
| Western transect | |||
| Variable | m | b | r2 |
| Depth | 1.37 | 1.88 | 0.13 |
| Salinity | -0.79 | 19.62 | 0.23 |
| Temperature | -22.70 | 645.24 | 0.80* |
| Dissolved oxygen | 1.41 | -2.70 | 0.06 |
| Eastern ransect | |||
| Variable | m | b | r2 |
| Depth | -2.68 | 21.03 | 0.17 |
| Salinity | 0.47 | 1.50 | 0.34 |
| Temperature | -12.09 | 349.16 | 0.63* |
| Dissolved oxygen | 2.56 | -0.05 | 0.50 |
The line plots showed a heterogeneous distribution of the polychaete species along the transects, but also showed that in the western zone the fauna was less diverse and less abundant (Fig. 5a, b). The main faunal differences between both transects were associated with the occurrence of Monticellina dorsobranchialis, Scoloplos texana, Aricidea (Acmira) hirsuta and Glycinde multidens, which were widely distributed along the eastern transect. In the eastern zone, Kinbergonuphis cedroensis, Ceratonereis irritabilis and Pionosyllis sp. were mainly located at the northern stations (1E, 2E) (Fig. 5b) and, among the more abundant species in this eastern transect, only the distribution of Malmgreniella sp. 2 (station 4E) and Hydroides protulicola (stations 3E, 5E) was restricted to the area close to the continental region (Fig. 5b).
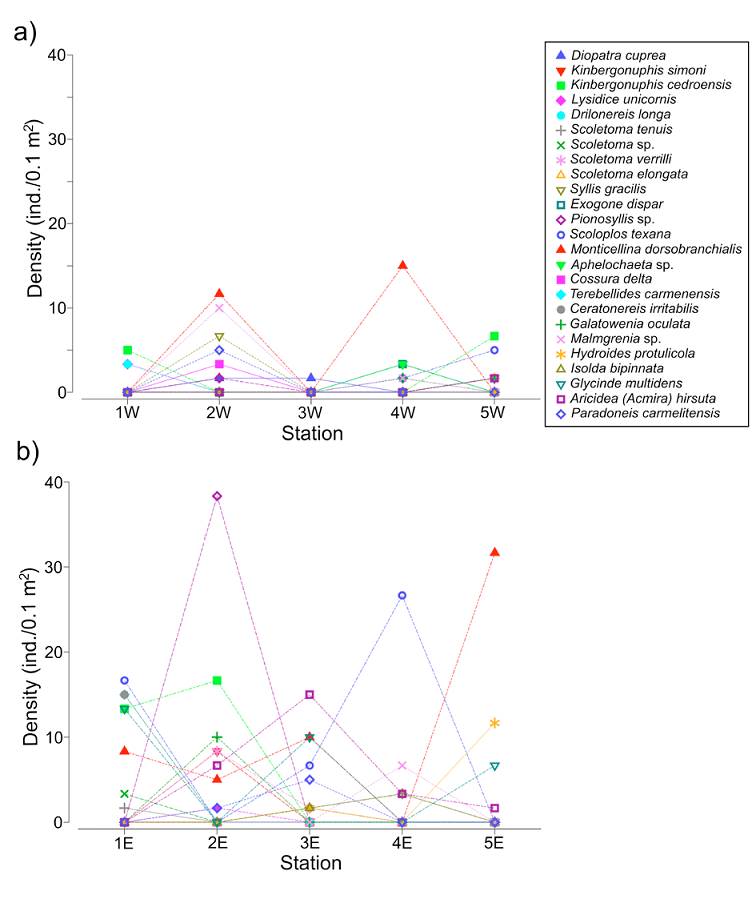
Figure 5 Density line plots of the 25 more abundant species of polychaetes along each transect. a) Western transect; b) eastern transect.
On the other hand, the species better represented in the western transect, Malmgreniella sp. 2, Syllis gracilis, and Diopatra cuprea were mainly located in the middle station (2W), while Kinbergonuphis cedroensis and Nematonereis unicornis were collected at the far northern and southern stations (1W, 5W). Although M. dorsobranchialis was also very abundant in the western zone, it was only collected in 2 middle stations (2W and 4W). Scoloplos texana was distributed in the southern region of the lagoon (stations 4W, 5W), while Drilonereis longa was exclusively located in the northern zone (station 1W) (Fig. 5a).
Beta-diversity. The western and eastern zones showed a relatively high faunal similarity (0.70), indicating that the polychaete species found at each transect are very similar. However, the distribution of each species along the northern-southern gradient was very irregular, so that the similarity values among the station pair comparisons at each transect clearly decreased (< 0.54). Except between the 2W/3W and 4W/5W station comparisons, where the nestedness component represented all the beta diversity value (βnes = 0.80 and 0.86, respectively), the species turnover was the dominant factor of the total dissimilarity among the polychaetes inhabiting Términos Lagoon (Fig. 6).

Figure 6 Nestedness (βnes) and turnover (βsim) components of beta-diversity measured by Sorensen’s dissimilarity index (βsor) among stations from the western (W) and eastern (E) transect.
The lowest species overlap occurred among stations from the western transect (0 to 0.10) and was associated with the lower number of species found there, which meant that the polychaetes were practically different at each station. The 1W/2W and 3W/4W pair comparisons showed that the total beta-diversity corresponded to the species turnover (βsim = 1). On the contrary, the similarity value among stations from the eastern transect were clearly higher (0.08 to 0.54), mainly among those stations located in the middle transect. However, the faunal heterogeneity was also the more relevant characteristic of this eastern transect, and its similarity values decreased towards the stations located at the far northern (0.20) and southern (0.08) zones.
In the eastern zone, the turnover component (βsim = 0.38 to 0.86) was at least 66% larger than the nestedness component of the beta diversity (βnes = 0.02 to 0.19). Particularly, the highest number of species found at station 3E and their wide distribution among the neighboring stations, caused that stations 3E/4E were the only pair comparison showing an important increase in the nestedness component of the beta-diversity (βnes = 0.19) (Fig. 6).
Discussion
Despite the lack of accuracy regarding the total number of polychaete species inhabiting Términos Lagoon, we estimate that the 51 species collected in the 10 stations studied, represent approximately 43% of the 119 species reported so far in the lagoon soft-bottoms (Hernández-Alcántara et al., 2014). The polychaete biodiversity observed was difficult to compare to that reported in other lagoon-estuarine environments of the southern Gulf of Mexico, since besides Términos Lagoon, there are few lagoons already studied and from the 10 estuarine systems where data exist, only around 70 species have been recorded in soft bottoms.
The lower biodiversity of polychaetes, but also of invertebrates in general, is a consistent pattern in the lagoon-estuarine habitats, and the 51 species from 10 stations found in this study could even represent a richer fauna than the 58 species of polychaetes from 31 stations reported from an estuary in southern Brazil (Magalhaes & Barros, 2011), the 120 species of polychaetes found in 41 stations from an estuary in Costa Rica (Maurer & Vargas, 1984), or the 77 species from 38 stations collected in 2 different climatic seasons in a polluted estuary of Rio de Janeiro, Brazil (Santi & Tavares, 2009). Nevertheless, only the families Lumbrineridae (7 species) and Onuphidae (5 species) could be considered diverse, since 75% of families were represented by only 1 species (9 families) or 2 species (10 families). Lumbrinerids (36 species) and onuphids (24 species) are also well represented in the adjacent marine region, the Gulf of Mexico continental shelf (Fauchald et al., 2009), but other families very diverse in the Gulf, such as the Eunicidae and Nereididae for example (both with 43 species), were rare in soft-bottoms inside the lagoon. All this suggests that future research efforts, including the description of new species (Arriaga-Hernández et al., 2013), could significantly increase the biodiversity values found inside the lagoon. It is necessary to emphasize that the polychaetes are usually the dominant macrofaunal group in tropical estuaries (Gambi et al., 1997), and are characterized by their high tolerance to severe daily and seasonal environmental variations, allowing them to reach high abundances in those environments (Dittman, 2000; Gambi et al., 1997; Rosa-Filho et al., 2005; Silva et al., 2011).
In the benthic communities of the lagoon-estuarine systems, the species composition varies importantly; this is often related with environmental fluctuations. Salinity, dissolved oxygen and sediment type usually regulate the composition and abundance of species in these systems (McLusky & Elliot, 2004). Many studies have shown that sediment is the key factor in controlling the benthic communities in marine and estuarine environments (McLusky & Elliott, 2004; Snelglove & Buttman, 1994). However, in the present study, with the aim to avoid biases caused by the type of habitat, our transects were placed only on soft-bottoms. These sediment types are favorable for the establishment of the benthic fauna, especially the polychaetes, due to their high content of organic matter (Silva et al., 2011), and because their soft consistency facilitates the motility of the infaunal species. So, these conditions can favor dense populations of deposit feeders, as was observed for Monticellina dorsobranchialis, Scoloplos texana, and Aricidea (Acmira) hirsuta, which densities noticeably increased in Términos Lagoon.
The oxygen content in the waters is the single most important abiotic factor responsible for the occurrence or not of any species in a particular locality. The values of 5 ml L-1 of dissolved oxygen are considered as the minimal concentration necessary for fishes to survive, but are probably enough for most polychaetes (Reish, 1979). During the sampling period, the oxygen levels were in average 4.58 ml L-1 in the eastern and 6.01 ml L-1 in the western zone, with few changes along the transects. Thus, the oxygen could not be considered a limiting factor for the polychaetes, and concentrations had no significant effect on the changes in the number of species along any transect. These worms are better adapted to hypoxia levels than other invertebrates such as mollusks, crustaceans or echinoderms (Hendrickx & Serrano, 2014), and they can even tolerate different levels of hypoxia (Lamont & Gage, 2000).
The environmental variations along the transects studied here showed substantial changes from the internal coast of Carmen Island towards the continental region of the lagoon with salinity as the most important parameter structuring the gradient. Large variations in salinity, as a result of freshwater input and water circulation, cause constant adjustments for the fauna to maintain its osmotic and ionic equilibrium (Junoy & Vieitez, 1990; Silva et al., 2011). However, in Términos Lagoon, the local environmental characteristics also modifies the presence and distribution of the fauna at each transect, so that more polychaetes species were found at the eastern zone, almost twice as much as in the western region. This decreasing trend in the number of species on the western region of the lagoon had already been noted in the polychaetes (Hernández-Alcántara & Solís-Weiss, 1991), but also in mollusks (Cruz-Ábrego et al., 1994).
In general, this decreasing pattern in the number of species in Términos Lagoon has mainly been attributed to the influence of river discharges prevalent in the western region, which modify the salinity levels, but also adds large amounts of fine sediments in suspension. Although several studies have shown that detritus is the base of the food webs in the lagoon-estuarine systems of the southern Gulf of Mexico, the productivity, resource abundance and the degree of connectivity are also important factors for small scale habitat variability in the use of resources by aquatic consumers (Sepúlveda-Lozada et al., 2015). Although it is necessary to obtain more information of food availability, nutrient fluxes and hydrological features of the local sites, comparison of the aquatic food web structure within Términos Lagoon showed that the isotopic niche area of the consumers’ communities is different, which suggest that resource availability may greatly differ across the lagoon (Sepúlveda-Lozada et al., 2015). That is why the faunal composition between transects was very different, and this was a very important biotic feature found in this study. The superior positions of the rarefaction curves belonging to stations from the middle-north of the eastern transect, and the fact that the number of species in the southern stations of this same transect was similar to that observed in the intermediate stations of the western zone, indicated that the species richness changes did not follow a linear trend along each transect. Likewise, the eastern-western net flow of water could importantly influence the polychaete distribution inside the lagoon.
The reduction in river inflow and the proximity to the sea have a positive effect on most macrofaunal species, increasing the density and diversity of communities (Silva et al., 2011). In the eastern transect in Términos Lagoon, located close to the water inflow from the Gulf of Mexico, was where the diversity was higher, whereas in the stations of the western transect (subjected to higher river inflow) the lowest number of species were found.
The overall similarity in the polychaete composition showed an overlap between transects, which reflected the high proportion of widespread species in the lagoon. Nevertheless, the large differences in species composition, shown during the pairwise comparisons among stations of each transect, and the non-linear changes in the number of species across the lagoon, suggested that the faunal variations in the study area were not necessarily associated with the observed environmental gradient. This gradient, as mentioned above, was essentially structured by salinity, but in contrast to what we expected, the variations in the number of species were exclusively correlated with the temperature changes. It is true that temperature can reflect the influence of other directly related parameters such as surface heat flux, latitude or season (Mackie et al., 1997). However, in this lagoon, the temperature values varied little, so that their significant correlations with the number of species were the result of the fact that the stations with the higher number of species were located in slightly cooler zones, rather than a direct effect of temperature on the species metabolism.
Therefore, the observed pattern in the number and composition of species could be associated with the water circulation across the lagoon rather than with the roughly northern-southern environmental gradient. This is due to the connection of Términos Lagoon with the Gulf of Mexico by its 2 inlets (Fig. 1), the dominant wind forcing the lagoon, and the tidal phase differences between the 2 main tidal inlets, which together determine the net water flow towards the western region (David & Kjerfve, 1998). It is true that polychaete distribution could be a result of a complex sum of physical, physicochemical and biotic factors, including the littoral current and the river discharges, but the water flow penetrating from the Puerto Real Inlet on the Gulf of Mexico seems to influence the settlement and development of the benthic populations. In fact, Kinbergonuphis cedroensis, Pionosyllis sp., Terebellides carmenensis and Ceratonereis irritabilis in the eastern transect, and Malmgreniella sp. 2, Syllis gracilis and Paradoneis carmelitensis in the western transect, for example, showed a positive relationship with salinity, since they were mainly distributed close to Carmen Island. However, unexpectedly, since they are well represented in the Gulf of Mexico (Fauchald et al., 2009), the abundant M. dorsobranchialis and S. texana were widely distributed towards the continental coasts, where the salinity levels decreased.
Pairwise analyses of the similarity in polychaete species composition revealed that different biological processes could be determining the beta-diversity changes across each transect. Although there are no studies describing the faunal replacement inside the lagoon, the separation of the beta-diversity in its 2 components, the spatial species turnover and nestedness of assemblages (Basselga, 2010) showed that, above all in the eastern zone, the dissimilarity was mainly driven by species turnover. This means that most of their variation was a result of species replacements. Dominance of the species turnover component reflected a spatial separation of the polychaete species, which was not necessarily consistent with the environmental changes along transects, but it could be followed by intermittent opportunities for faunal exchange via the water circulation pattern in eastern-western direction.
The influence of the turnover species component decreased in the western transect, causing the more extreme cases of nestedness in the middle and southern stations. This process occurred because in these sites the smallest number of species was found, and because the fauna is an almost entirely nested subset of polychaete species distributed in the western lagoon. That is, in the western region the low species richness was linked with the near absence of exclusive species. Therefore, the dominance of the nestedness component in the western transect reflected a loss of species that could be attributed to the greater environmental heterogeneity associated with the mixing of waters of the incoming rivers, with their consequent changes in salinity as well as the discharge of fine sediments, nutrients and contaminants.
The tropical lagoon-estuarine systems are transitional environments between rivers and sea waters, characterized by wide variations in their physical and chemical conditions, but along the studied transects in Términos Lagoon, the salinity was the more important factor structuring the environmental gradient (r 2 > 0.83). In contrast to what we expected, variations in the number and composition of the polychaete species did not follow this spatial gradient, since the eastern transect, mainly influenced by marine conditions, showed the highest abundance and species richness, while the western transect located in fluvio-lagoonal environments harbored the less diverse and less abundant fauna.
The overall similarity in the polychaete composition showed an overlap between transects, which reflected the high proportion of widespread species in the lagoon. However, the spatial changes showed that the number and composition of species were mainly associated with the water circulation across the lagoon, rather than with the roughly northern-southern environmental gradient. Studies describing faunal replacements inside the lagoon are lacking, but except in some stations of the western transect, where the nestedness component explained all the beta-diversity value (βnes: 0.80 to 0.88), the species turnover (βsim: 0.38-1.0) was the dominant factor among the polychaete assemblages of Términos Lagoon. Therefore, the beta-diversity pattern in the lagoon could be associated to intermittent opportunities for faunal exchange via the water circulation pattern in eastern-western direction.











 nueva página del texto (beta)
nueva página del texto (beta)


